Abstract
A simple, inexpensive and effective genomic DNA isolation procedure for Lactobacillus isolates from traditional Indian fermented milk (dahi) is described. A total of 269 Lactobacillus isolates from fermented milk collected from four places in North and west India were tested for lysis by an initial weakening of the Gram positive cell wall with Ampicillin followed by Lysozyme treatment. The average genomic DNA yield was ~50 μg/ml log phase culture. Quality and repeatability of the method was found to be adequate for subsequent molecular applications. The quality of the genomic DNA isolated by this method was verified by restriction digestion and polymerase chain reaction (PCR). No inhibition was observed in subsequent PCR amplification and restriction digestion. The presented method is rapid, cheap and useful for routine DNA isolation from gram positive bacteria such as Lactobacillus.
Keywords: Lactobacillus, Indian fermented milk (dahi), Ampicillin, Lysozyme, Genomic DNA isolation
Introduction
Prominent microorganisms present in fermented dairy food are the Lactic Acid Bacteria. Lactic acid fermentations were first observed by Pasteur in 1857, and ever since, extensive work has gone into studying the microorganisms responsible for such fermentations. A century and a half later, lactic acid bacteria still hold a prominent place in the food microbiologist’s routine work. The first ever classification system for lactic acid bacteria was advanced by a scheme first proposed by Sigurd Orla-Jensen in 1909. This ubiquitous and prominent group of food based bacteria includes various divergent and similar microorganisms spread over a broad range of morphologies and functions, and contains morphologically variant bacteria including bacilli (Lactobacillus), cocci (Pediococcus, Lactococcus, Enterococcus, Streptococcus) and bacteria of variant morphology, such as ovoid cocci (Leuconostoc). According to the classification advanced by Orla-Jensen, the genus Lactobacillus was further divided into three subgenera—Thermobacterium, Streptobacterium and Betabacterium in accordance with the observed optimal growth temperature characteristics and hexose fermentation pathways [1]. Lactobacilli are gram positive, rod shaped, catalase negative bacteria and could safely be considered the most important of all lactic acid bacteria owing to their role in various food and feed fermentations, production of many important metabolites, and owing to their role in the prevention of food spoilage, intoxication and infection by acting as antagonists against other pathogens by the production of antimicrobials [2–4]. With the growing population, and the demand exerted on the quality fermented dairy products, Lactobacilli have increasingly been under the research scanner for the last two decades. The present effort in India focuses on determining the diversity of Lactobacillus species in dairy products such as traditional fermented milk (dahi) and cheeses to result in a formidable repertoire of diverse Lactobacillus strains that could be functionally and genetically explored in the immediate and near future to reveal strains that have strong functional and probiotic attributes, and various industrially important uses. These strains could then be exploited to produce various useful compounds [low-molecular mass compounds such as hydrogen peroxide (H2O2) [5], carbon dioxide (CO2) [6], diacetyl (2,3-butanedione) [7]; high molecular mass compounds such as bacteriocins [8] and other uncharacterized compounds; commercial Lactic Acid [9]; exopolysaccharides [10]; low calorie sweeteners [11]; alcohols for industrial, transportation and beverage use [12]; oils and fats [13]; and by the use of genetically modified strains, vitamins [14], enzymes [15] and hormones [16].
Genetic characterization of Lactobacillus isolates from various environmental samples (Indian traditional fermented milk, or dahi) is an important tool to understand the microbial biodiversity of this genus. The first step in genetic characterization is the isolation and purification of quality DNA from any organism. The presence of a high peptidoglycan content in the cell walls of Gram positive bacteria, however, is a major hurdle in the isolation of DNA due to it’s role in the rigidity of the cell wall, and it’s resistance to conventional methods of lysis [17]. Lysozyme has conventionally been used, in conjunction with detergents such as SDS to ensure better lysis of the Gram positive cell wall [18, 19]. The development of a simplified protocol that involves a primary weakening component for the Gram positive cell wall before the use of a conventional lysis approach was tested. Penicillins have been known to interfere in the assembly of N-Acetylglucosamine (NAG) and N-Acetylmuramic acid (NAM) moieties during Gram positive cell wall synthesis [20, 21]. Our hypothesis was to test the action of an aminopenicillin, namely, ampicillin for its role in weakening the cell wall during growth and subsequent efficient lysis of the Lactobacillus cell by lysozyme and SDS treatment. Hence, a simplified Ampicillin-Lysozyme tandem lysis protocol was developed for genomic DNA isolation and tested on 269 isolates of lactobacilli from Indian fermented milk (dahi).
Materials and Methods
Collection of Traditional Indian Fermented Milk (dahi) Samples
Indian fermented milk (dahi) samples were obtained from four places in Northern India (Haryana and Gujrat, Table 1). Samples were collected in a sterile container and aseptically transferred. A short summary of the sample was made on the spot and a temporary code was assigned to each sample which was then immediately stored on ice and transferred to the laboratory.
Table 1.
Collection schema for traditional Indian fermented milk (Dahi)
| S. no. | Sample code | Isolate numbers | Date of collection | Place from where the sample was collected |
|---|---|---|---|---|
| 1 | Sample C | C1-C27 | 26/10/06 | Jagadhri, Haryana, India |
| 2 | Sample C | C28-C55 | 4/11/06 | Jagadhri, Haryana, India |
| 3 | Sample D | D1-D17 | 16/11/06 | Karnal, Haryana, India |
| 4 | Sample F | F11-F112 | 7/12/06 | Karnal, Haryana, India |
| 5 | Sample G | G1-G146 | 11/12/06 | Shyamgarh, Haryana, India |
| 6 | Sample H | H1-49 | 25/1/07 | Gujarat, India |
Isolation of Lactic Acid Bacteria
One gram of each fermented milk (dahi) sample was aseptically transferred into a sterile flask, serial dilutions of the samples were made in sterile physiological saline and pour plated into MRS Agar [22] (BD Biosciences, DE, USA). After solidification of the agar, another layer of molten MRS agar was added on top to ensure microaerophilic conditions suitable for the growth of Lactobacillus species. The plates were incubated at 37°C for 24–48 h. Colonies showing characteristic morphology such as color (buff, pale-yellow, white), size (less than 1 mm diameter), and shape (biconvex) were selected, numbered and inoculated into sterile MRS broth without aeration. After incubation for 24–48 h, the MRS broth cultures were examined microscopically for purity, subjected to the catalase test and gram stained. Pure gram positive, catalase negative bacilli were coded, preserved in Lactobacilli MRS broth containing 15% (v/v) glycerol and stored at −86°C till future processing.
Genomic DNA Isolation from Lactobacilli
Frozen isolates were revived from the glycerol stock, thawed and reinoculated into freshly prepared sterile MRS lactobacilli broth and incubated at 37°C for 24 h. After confirmation of purity, 10 μl of active broth culture was reinoculated into 10 ml sterile MRS broth and incubated at 37°C for 10 h. Two milliliter aliquots of active log phase cultures from this broth were then used to isolate genomic DNA.
To the 2 ml culture, 2–3 μl of a sodium salt of ampicillin solution (50 mg/ml) was added and the mixture incubated at 37°C for 1 h. Post incubation, the bacteria were harvested by centrifugation at 5000 rpm for 5 min in a refrigerated centrifuge. The media supernatant was decanted and the pellet washed thrice with 1 ml of NaCl-EDTA (30 mM NaCl, 2 mM EDTA, pH 8.0). The washed bacterial pellet was resuspended in 100 μl of NaCl-EDTA (30 mM NaCl, 2 mM EDTA, pH 8.0) and 100 μl of freshly prepared lysozyme solution (concentration 10 mg/ml in NaCl-EDTA) was added to it and mixed. The mixture was incubated at 37°C for 1 h with intermittent shaking. To remove RNA, 4 μl of Rnase-A solution (Stock 10 mg/ml, working concentration 100 μg/ml,) was also added to the mixture before incubation. The volume of the mixture was then made up to 500 μl with additional NaCl-EDTA and 50 μl of a 10% SDS solution followed by 10 μl of proteinase K solution (20 mg/ml) were added to the mix. The contents were mixed thoroughly and incubated at 55°C for 1 h. After incubation, an equal volume of Tris-saturated phenol (pH 8.0) was added and mixed thoroughly. The resultant mixture was centrifuged at 10,000 rpm at 22°C for 10 min and the upper aqueous phase was separated without disturbing the interphase containing cell debris and proteins. This step was repeated once with a fresh aliquot of Phenol–Chloroform mixture (1:1) and the supernatant was collected in a sterile eppendorf tube. DNA in the supernatant was precipitated out with 0.8 volumes of Isopropanol in the presence of 0.3 M Sodium Acetate (pH 5.2). The precipitated DNA was pelleted by centrifugation at 10,000 rpm at 4°C for 5 min. The supernatant was discarded and the DNA pellet was washed once with freshly prepared 70% ethanol and air-dried. The final pellet thus obtained was dissolved in 50 μl Tris–EDTA (10:1, pH 8.00) and stored frozen at −20°C till further analysis. In about ten isolates of the total 269 isolates tested, DNA was also isolated by using a commercial kit following the protocol suggested by the manufacturer and the DNA obtained was stored frozen at −20°C till further analysis.
Qualitative and Quantitative Assessment of DNA
All samples of the preliminary study were analyzed by spectrophotometry. DNA concentration was determined by recording the absorbance at 260 nm (A260) using a Nanodrop spectrophotometer (Bio-Tek instruments, inc.). The purity of the DNA was determined from the A260/A280 ratio. The quality of the isolated DNA was also evaluated by (0.9% agarose) gel electrophoresis using 2 μl of isolated DNA. The type of band pattern indicates the quality of the DNA isolated. A known amount of bacteriophage lambda DNA was used to compare the intensity and approximate size of the isolated DNA.
PCR Using Isolated DNA
DNA integrity was evaluated by PCR analysis. The DNA sample was used as a template for selective amplification of a single-copy house keeping gene; the elongation factor Tu gene (tufB gene) by PCR. The selected primers used were forward 5′ATGGACGGTGCGATCTTAGTT3′ and reverse 5′ACTTGACCACGAACAACTTGTTCA3′. Expected size of the amplified fragment corresponds to 584 bp. The PCR master mix was as follows: 10 μl of 10× reaction buffer (100 mM Tris [pH 8.3], 500 mM KCl, 9 mM MgCl2), PCR primers (final concentration, 40 μM each), and deoxynucleoside triphosphates (final concentration, 200 μM) in a final volume of 100 μl with 2.5 U Taq DNA polymerase. Water was then added to bring the volume to 100 μl. The PCR reaction was performed with 25 μl of the reaction mix containing ~100 ng of isolated Lactobacillus genomic DNA, in a hot lead thermocycler (Eppendorf). The reaction mixture was subjected to an initial heating at 95°C for 2 min. The temperature was cycled through 94°C for 1 min, 55°C for 1 min, and then a final extension at 72°C for 2 min. The cycle was repeated 30 times.
Restriction Digestion of the DNA Sample
DNA samples from five Lactobacillus isolates obtained by this protocol were assayed by restriction enzyme digestion. Eight micrograms of purified Lactobacillus DNA were subjected to restriction digestion with EcoRI, HindIII and PvuII enzymes according to the procedure recommended by the manufacturer (Promega corporation). 5 U of the enzyme were added per microgram of DNA in 60 μl reaction buffer. The solution was incubated at 37°C for 1 h, and the reaction stopped by addition of 0.1 vol of 0.1 M EDTA. Ten microliters (~1.5 μg DNA per lane) were combined with 2 μl of 6× Gel loading buffer (0.25% bromphenol blue, 0.25% xylene cyanol, 30% glycerol) and loaded into a 0.9% agarose gel containing ethidium bromide. The samples were electrophoresed at 60 V for 1.5 h in a horizontal mini-gel apparatus.
Results
DNA Yield and Quality
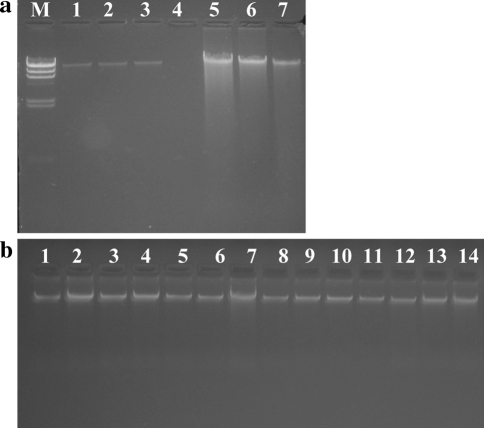
Chromosomal DNA was isolated from Lactobacillus isolates by the Ampicillin-Lysozyme tandem lysis method and estimated spectrophotometrically by measuring the Absorbance at 260 nm (A260). A total of 269 Lactobacillus isolates were tested for this approach and the mean amount of DNA isolated from these isolates was 2187.654 ng/μl, with a range of 293.58–5353.9 ng/μl. The variation in the DNA yield was due to handling errors while isolating DNA from a large number of isolates (chiefly due to pipetting error) and differences in the number of bacteria in a fixed volume of inoculum. The number of PCR reactions that could be performed using DNA extracted from 2 ml of bacterial culture, thus, was around 1500 (an estimate from amount of DNA, about 50–100 ng, required for each PCR reaction). Ten isolates from the 269 isolates were randomly chosen and their DNA was also isolated by a commercial DNA isolation kit. The spectrophotometic reads from these isolates estimated DNA with a mean of 760.5 ng/μl. Lesser yield from the commercial kit is clearly evident from the Fig. 1a. The average DNA yield from the presented protocol was approximately 1093.8 μg/ml of log phase culture compared to 380.2 μg/ml from the commercial kit. The amount of culture used for this protocol is 2 ml, but we could scale up the amount of active culture accordingly without any reduction in DNA quality and quantity.
Fig. 1.
Agarose gel Electrophoresis pattern of isolated Lactobacillus DNA. Two microliter DNA samples were run in each lane of a 0.9% Agarose gel. a Genomic DNA isolated by the present protocol and by a commercial kit using a similar volume of isolates grown in MRS broth (C1, C2 and C3). Lanes 1–3 Lactobacillus DNA isolated by the commercial kit; Lanes 5–7, DNA isolated by the present protocol; Lane M, HindIII digested Lambda DNA fragments (23.13, 9.4, 6.5, 4.3, 2.3, 2.0, 0.5 and 0.12 kb) b Agarose gel electrophoretic profile of Lactobacillus genomic DNA isolated using the present protocol
The purity of DNA isolated by both these methods was measured spectrophotometrically and by running on 0.9% agarose gel. The purity of DNA is estimated by the ratio of absorbances at 260 nm (A260) and 280 nm (A280), A260/A280. The average value was higher than 1.8, which clearly demonstrates that the purified genomic DNA is of high quality. The upper and lower extremes were deviant from the mean values mainly due to the handling of a large number of isolates while isolation (Table 2).
Table 2.
Yields of DNA isolated from Lactobacillus isolates by the Ampicillin-Lysozyme tandem lysis method and commercial kit (average values only)
| Amount of DNA in ng/μl | Absorbance at 260 nm (A260) | Absorbance at 280 nm (A280) | Purity (Absorbance at 260 nm/Absorbance at 260 nm [A260/A280]) | |
|---|---|---|---|---|
| Average | 2187.654 | 37.878 | 19.234 | 1.862 |
| Maximum | 5353.9 | 107.078 | 72.612 | 2.27 |
| Minimum | 293.58 | 5.86 | 3.16 | 0.85 |
| Standard deviation | 1126.509 | 25. 977 | 14.935 | 0.135 |
| Commercial kit (average of 10 isolates) | 760.5 | 15.21 | 8.75 | 1.738 |
The integrity of the purified genomic DNA was also analyzed by agarose gel electrophoresis (Fig. 1). Intact high molecular weight (~20 kb) DNA was observed in all samples. This confirms that the purified genomic DNA from Gram-positive Lactobacillus isolates is of a high quality and can be used in downstream applications. Excessive shearing of DNA was not observed proving the fact that the method was gentle enough to generate isolated DNA of ~20 kb size.
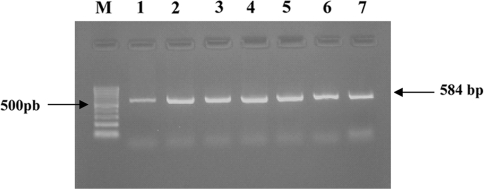
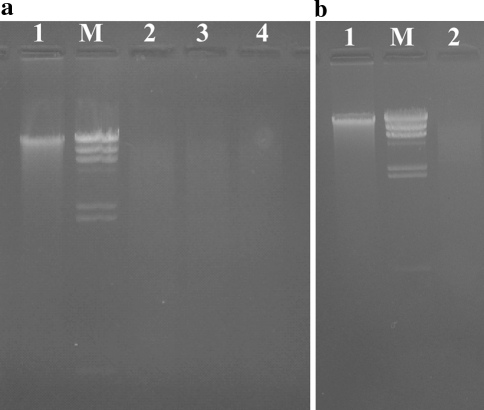
To check the applicability of the present method for downstream applications such as the Polymerase Chain Reaction (PCR) and Restriction Digestion, the DNA isolated from a few random isolates was checked for these applications. DNA from these isolates was used to perform amplification of a single copy gene, elongation factor Tu gene (tufB), by PCR to identify the presence of this gene in these isolates at the reaction conditions detailed above. In all the samples tested, a single band of the amplicon at ~584 bp was observed, and proved the applicability of the isolated DNA to standard PCR based techniques (Fig. 3). DNA from a few random isolates was also used for a standard restriction digestion experiment and the resultant digest showed a complete cleavage of the isolated DNA yielding an expected continuous fragment pattern on 0.9% agarose gel (Fig. 2). The results prove that the purity of the genomic DNA is sufficiently high for a sensitive PCR analysis and restriction enzyme digestion.
Fig. 3.
Agarose gel electrophoresis of a PCR-amplified Lactobacillus elongation factor Tu gene (tufB gene). Forward Primer 5′ATGGACGGTGCGATCTTAGTT3′; Reverse Primer 5′ACTTGACCACGAACAACTTGTTCA3′; Lanes M 100-bp DNA size ladder; Lanes 1–7, PCR product (584 bp) generated from Lactobacillus isolates
Fig. 2.
Lactobacillus genomic DNA restriction endonuclease digestion patterns in an agarose gel. Wells were loaded with approximately 1.5 μg of the DNA digest, which was electrophoresed in 0.9% agarose gels. aLane 1 Undigested Lactobacillus DNA (~1 μg); Lanes 2–4 Lactobacillus DNA digested with EcoRI, HindIII and PvuII; and Lane MHindIII digested lambda DNA fragments. bLane 1, Undigested Lactobacillus DNA (~1 μg); Lane 2, Lactobacillus DNA digested with EcoRI and Lane M, HindIII digested lambda DNA fragments
The time taken for isolation of DNA by the Ampicillin-Lysozyme method was slightly longer than the commercial kit tested, due to the incubation times required for Ampicillin and Lysozyme action. However, considering the yield, purity and economy of the presented method, longer isolation times were a relatively small price to pay. Hence, the Ampicillin-Lysozyme tandem lysis DNA isolation method is a simple, economical and efficient method for the routine isolation of high quality DNA from Gram positive bacteria such as Lactobacillus.
Discussion
The first step in the genetic characterization of any isolate of Lactobacillus from Indian fermented milk (dahi) is the isolation of moderately pure, PCR amplifiable DNA from the cells by the best possible isolation process, whether chemical, mechanical or a combination of chemical and mechanical methods. Various protocols have been suggested in the past and many commercial kits for the rapid isolation of DNA rely on proven combinations of chemicals and mechanical steps such as bead beating, etc. [23–26]. The problem in isolation of chemically pure DNA from Gram positive bacteria arises from the difficulty in lysis of bacterial cells and associated secondary metabolites or PCR inhibitors. However, in some cases the DNA can be obtained after prolonged heating at 95°C (boiling) of a bacterial aqueous suspension. The DNA isolated with these procedures (short-cut methods, boiling etc.) found best use the day it was prepared as the quality of DNA dropped after storage. Therefore, it was necessary to evaluate the efficiency of each preparation from this method in terms of providing quality template DNA for subsequent analyses like PCR and Restriction digestion. The average size of the isolated DNA was ~20 kb and DNA from almost all isolates was found to be in an undegraded condition (Fig. 1). The process also could eliminate cellular proteins and metabolites that would inhibit the PCR reaction either by damaging the DNA or interfering with the polymerization process.
The failure of complete lysis of Gram positive bacteria such as Lactobacillus is due to the inherent nature of the cell wall, which, in such bacteria, contains a high concentration of peptidoglycan. Peptidoglycan contains β-(1-4)-N-Acetyl-D-glucosamine subunits polymerized via relatively strong covalent linkages such as the pentapeptide pentaglycine bridges [27]. Among the many approaches the molecular microbiologist uses against the inherent stability of the Gram positive cell wall is the use of a class of enzymes which serve to disrupt the cell wall by enzymatic cleavage of the covalent cross-links in the peptidoglycan moieties. Various enzymes have been discovered over the years and utilized with varying success by different groups. The most commonly used cell wall disrupting enzyme is a hydrolase, lysozyme; which disrupts the β-(1-4)-linkages between N-acetylmuraminic acid and N-acetyl-D-glucosamine residues in peptidoglycans. Endopeptidases such as achromopeptidase [28] and lysostaphin [29] are generally directed at the tetramer and poly-glycine cross-links and serve to disrupt them thus attacking the core of the gram positive cell wall. Hence, these enzymes are suitable for bacteria that are generally resistant to lysis by lysozyme. Mutanolysin is another cell wall degrading enzyme with muralytic action which also cleaves the β-(1-4) linkages between N-acetylmuraminic acid and N-acetyl-d-glucosamine residues in peptidoglycans, similar to lysozyme [30]. However, this enzyme, like the endopeptidases described above, is costly and not generally available in routine laboratories. A more recently described cell wall degrading enzyme is Labiase, isolated from Streptomyces fulvissimus acting by a combination of the β-N-acetyl-d-glucosaminidase and lysozyme activities to disrupt the gram positive cell wall with a higher efficiency [31]. However, this enzyme is extremely costly and thus unsuitable for routine use in a project like ours, which requires the handling of a large number of Lactobacillus isolates.
According to our own experience (unpublished data), the use of lysozyme alone is insufficient for the lysis of Lactobacillus isolates, resulting in a low yield of DNA. Thus, we looked for a system where the cell wall could be weakened before the lysis step to ensure a high degree of cellular disruption and a high yield of pure, amplifiable and restriction-digestible DNA for downstream processing. Beta-lactam antibiotics such as penicillins have been used for a long time as agents against Gram-positive bacterial infections. The antibacterial activity of ampicillin results from the disruption of Gram positive call wall synthesis by inhibiting the action of a family of glycosyl-transferases, chief among them; the enzyme, murein transferase, which the catalyzes the transfer of sugar moieties from activated donor molecules to specific acceptor molecules on polysaccharide-protein moeties such as N-acetylglucosamine and N-acetylmuramic acid, forming glycosidic bonds, and hence, the ultrastructure of the Gram positive cell wall. The inhibition of glycosyltranferases by penicillins such as ampicillin is the direct consequence of their binding to cell wall proteins called Penicillin-binding-proteins or PBP’s [32, 33] and these PBP’s are the sites of action for these enzymes. Ampicillin may also have a role to play in the disinhibition of autolysins in Gram positive bacteria, either directly or as a downstream consequence of their binding to PBP’s [34–36]. Ampicillin, therefore, weakens the Lactobacillus cell wall and thus reduces the rigidity and mechanical resistance inherent to this bacterium making it suitable for a higher degree of lysis by simple cell wall degrading enzymes such as lysozyme.
An initial 2 ml log phase culture of each Lactobacillus isolate was used to estimate the amount of DNA isolated using this method. The amount of DNA isolated was measured by its absorbance at 260 nm (A260). The average yield of the isolation process is presented in Table 2. However, the large disparity in DNA yields across isolates could be due to any of the following reasons: (1) the number of lactobacilli in each aliquot of inoculum used for this culture may have varied, and thus could have resulted in varying biomass yields over isolates. As the amount of DNA is a direct consequence of the microbial biomass, the disparity in yields could have been due to this aspect of culturing induced error, (2) various steps in the DNA isolation protocol involve repetitive pipetting of different chemicals. Pipetting error may have resulted in a small, yet functionally important change in the chemical composition of the extracting solutions, resulting downstream in different DNA yields, (3) the DNA yield is also influenced by the age and phase of growth of the microorganism. Though the control in incubation times resulted in bacteria with a probable similar early phase of growth, different isolates, and thus different species and strains of Lactobacillus, owing to differences in cell cycle, may have had different growth rates, viz., while one isolate may have been in the early log phase, another isolate may have been in the late log phase, while other isolates may have reached the stationary or early lag phase at the time of DNA isolation and (4) it has been reported that gram positive bacteria have evolved different strategies to combat ampicillin action [37] thus resulting in lessened cellular disruption rates but would have to be proven by a different set of experiments.
Conclusion
The goal of the outlined investigation was to propose a simple, inexpensive but effective genomic DNA isolation procedure for Lactobacillus isolates. These results show that the DNA produced by our universal, simple, low cost, fast and safe protocol is of high quality and can be used reliably in DNA manipulation and PCR-based techniques on a wide range of organisms even in low technology laboratories. The average amounts of DNA recovered by the method described above for 269 isolates ranged from 293–5354 ng/μl. The average A260/A280 ratio was 1.862, indicating pure DNA. Each of the DNA preparations generated was run in 0.9% agarose gels, and all were found to be in an undegraded condition. This procedure was found to produce reasonably pure genomic DNA from Lactobacillus isolates and had no interference with other procedures, such as restriction digestion and PCR. There was no RNA contamination in the isolated DNA preparation as it was treated with RNase A. The RNase A treatment however, had not degraded the isolated genomic DNA during preparation.
Acknowledgments
This work was supported by a grant for the “Microbial Diversity and Identification Project” funded by the National Bureau of Agriculturally Important Organisms, Mau, Uttar Pradesh, India. The authors would like to thank Dr. D. K. Arora (Director, NBAIM) for the help in supporting this work.
References
- 1.Holt JG et al (ed) (1994) Bergey’s manual of determinative bacteriology, 9th edn. Williams & Wilkins, Baltimore
- 2.Niku-Paavola M-L, Laitila A, Mattila-Sandholm T, Haikara A. New types of antimicrobial compounds produced by Lactobacillus plantarum. J Appl Microbiol. 1999;86:29–35. doi: 10.1046/j.1365-2672.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- 3.Lavermicocca P, Valerio F, Evidente A, Lazzaroni S, Corsetti A, Gobbetti M. Purification and characterization of novel antifungal compounds from the Sourdough Lactobacillus plantarum Strain 21B. Appl Environ Microbiol. 2000;66:4084–4090. doi: 10.1128/AEM.66.9.4084-4090.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vescovo M, Torriani S, Orsi C, Macchiarolo F, Scolari G. Application of antimicrobial producing lactic acid bacteria to control pathogens in ready-to-use vegetables. J Appl Bacteriol. 1996;81:113–119. doi: 10.1111/j.1365-2672.1996.tb04487.x. [DOI] [PubMed] [Google Scholar]
- 5.Tomas MS, Claudia-Otero M, Ocana V, Elena Nader-Macias M. Production of antimicrobial substances by lactic acid bacteria I: determination of hydrogen peroxide. Methods Mol Biol. 2004;268:337–346. doi: 10.1385/1-59259-766-1:337. [DOI] [PubMed] [Google Scholar]
- 6.Dodds KL, Collins-Thompson DL. Production of N2O and CO2 during the reduction of NO2 by Lactobacillus lactis TS4. Appl Environ Microbiol. 1985;50:1550–1552. doi: 10.1128/aem.50.6.1550-1552.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartowsky EJ, Henschke PA. The ‘buttery’ attribute of wine-diacetyl-desirability, spoilage and beyond. Int J Food Microbiol. 2004;96:235–252. doi: 10.1016/j.ijfoodmicro.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez JM, Martinez MI, Horn N, Dodd HM. Heterologous production of bacteriocins by lactic acid bacteria. Int J Food Microbiol. 2003;80:101–116. doi: 10.1016/S0168-1605(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 9.Panesar PS, Kennedy JF, Knill CJ, Kosseva MR. Applicability of pectate-entrapped Lactobacillus casei cells for L (+) lactic acid production from whey. Appl Microbiol Biotechnol. 2007;74:35–42. doi: 10.1007/s00253-006-0633-x. [DOI] [PubMed] [Google Scholar]
- 10.Arskold E, Svensson M, Grage H, Roos S, Radstrom P, van-Niel EW. Environmental influences on exopolysaccharide formation in Lactobacillus reuteri ATCC 55730. Int J Food Microbiol. 2007;116:159–167. doi: 10.1016/j.ijfoodmicro.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Di-Cagno R, De-Angelis M, Limitone A, Minervini F, Carnevali P, Corsetti A, Gaenzle M, Ciati R, Gobbetti M. Glucan and fructan production by sourdough Weissella cibaria and Lactobacillus plantarum. J Agric Food Chem. 2006;54:9873–9881. doi: 10.1021/jf061393+. [DOI] [PubMed] [Google Scholar]
- 12.Narendranath NV, Power R. Relationship between pH and medium dissolved solids in terms of growth and metabolism of lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl Environ Microbiol. 2005;71:2239–2243. doi: 10.1128/AEM.71.5.2239-2243.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa J, Kishino S, Ando A, Sugimoto S, Mihara K, Shimizu S. Production of conjugated fatty acids by lactic acid bacteria. J Biosci Bioeng. 2005;100:355–364. doi: 10.1263/jbb.100.355. [DOI] [PubMed] [Google Scholar]
- 14.Hugenholtz J, Sybesma W, Groot MN, Wisselink W, Ladero V, Burgess K, van-Sinderen D, Piard JC, Eggink G, Smid EJ, Savoy G, Sesma F, Jansen T, Hols P, Kleerebezem M. Metabolic engineering of lactic acid bacteria for the production of nutraceuticals. Antonie Van Leeuwenhoek. 2002;82:217–235. doi: 10.1023/A:1020608304886. [DOI] [PubMed] [Google Scholar]
- 15.Ikram-ul-Haq Mukhtar H. Biosynthesis of protease from Lactobacillus paracasei: kinetic analysis of fermentation parameters. Indian J Biochem Biophys. 2006;43:377–381. [PubMed] [Google Scholar]
- 16.Matsuzaki T, Yamazaki R, Hashimoto S, Yokokura T. Antidiabetic effects of an oral administration of Lactobacillus casei in a non-insulin-dependent diabetes mellitus (NIDDM) model using KK-Ay mice. Endocr J. 1997;44(3):357–365. doi: 10.1507/endocrj.44.357. [DOI] [PubMed] [Google Scholar]
- 17.Wintzingerode FV, Gobel UB, Stackebrandt F. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev. 1997;21:213–229. doi: 10.1111/j.1574-6976.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 18.Chassy BM, Giuffrida A. Method for the lysis of Gram-positive, asporogenous bacteria with lysozyme. Appl Environ Microbiol. 1980;39:153–158. doi: 10.1128/aem.39.1.153-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klaenhammer TR. A general method for plasmid isolation in lactobacilli. Curr Microbiol. 1984;10:23–28. doi: 10.1007/BF01576043. [DOI] [Google Scholar]
- 20.Blumberg PM, Strominger JL. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Microbiol Mol Biol Rev. 1974;38:291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghuysen J-M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968;32:425–464. [PMC free article] [PubMed] [Google Scholar]
- 22.Man JC, Rogosa M, Scharpe ME. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 23.Bürgmann H, Pesaro M, Widmer F, Zeyer J. A strategy for optimizing quality and quantity of DNA extracted from soil. J Microbiol Methods. 2001;45:7–20. doi: 10.1016/S0167-7012(01)00213-5. [DOI] [PubMed] [Google Scholar]
- 24.Frostegard A, Courtois S, Ramisse V, Clerc S, Bernillon D, Le Gall F, Jeannin P, Nesme X, Simonet P. Quantification of bias related to the extraction of DNA directly from soils. Appl Environ Microbiol. 1999;65:5409–5420. doi: 10.1128/aem.65.12.5409-5420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller DN, Bryant JE, Madsen EL, Ghiorse WC. Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl Environ Microbiol. 1999;65:4715–4724. doi: 10.1128/aem.65.11.4715-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roose-Amsaleg CL, Garnier-Sillam E, Harry M. Extraction and purification of microbial DNA from soil and sediment samples. Appl Soil Ecol. 2001;18:47–60. doi: 10.1016/S0929-1393(01)00149-4. [DOI] [Google Scholar]
- 27.Braun V, Hantke K. Biochemistry of bacterial cell envelopes. Ann Rev Biochem. 1974;43:89–121. doi: 10.1146/annurev.bi.43.070174.000513. [DOI] [PubMed] [Google Scholar]
- 28.Ezaki T, Suzuki S. Achromopeptidase for lysis of anaerobic gram-positive cocci. J Clin Microbiol. 1982;16:844–846. doi: 10.1128/jcm.16.5.844-846.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schindler CA, Schuhardt VT. Lysostaphin: a new bacteriolytic agent for the staphylococcus. Proc Natl Acad Sci USA. 1964;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fliss I, Emond E, Simard RE, Pandian S. A rapid and efficient method of lysis of Listeria and other gram-positive bacteria using mutanolysin. Biotechniques. 1991;11(453):456–457. [PubMed] [Google Scholar]
- 31.Niwa T, Kawamura Y, Katagiri Y, Ezaki T. Lytic enzyme, labiase for a broad range of Gram-positive bacteria and its application to analyze functional DNA/RNA. J Microbiol Methods. 2005;61:251–260. doi: 10.1016/j.mimet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Wise EM, Jr, Park JT. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci USA. 1965;54:75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strominger JL, Park JT, Thompson RE. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959;234:3263–3268. [PubMed] [Google Scholar]
- 34.Kitano K, Tomasz A. Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob Agents Chemother. 1979;16:838–848. doi: 10.1128/aac.16.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buist G, Karsens H, Nauta A, van-Sinderen D, Venema G, Kok J. Autolysis of Lactococcus lactis caused by induced overproduction of its major autolysin, AcmA. Appl Environ Microbiol. 1997;63:2722–2728. doi: 10.1128/aem.63.7.2722-2728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch AL. Autolysis control hypotheses for tolerance to wall antibiotics. Antimicrob Agents Chemother. 2001;45:2671–2675. doi: 10.1128/AAC.45.10.2671-2675.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baquero F. Gram-positive resistance: challenge for the development of new antibiotics. J Antimicrob Chemother. 1997;39:1–6. doi: 10.1093/jac/39.suppl_1.1. [DOI] [PubMed] [Google Scholar]