Abstract
The present study was performed in order to carefully investigate the interaction of Staphylococcus aureus with murine macrophages and the contribution of catalase and superoxide dismutase in intracellular persistence of Staphylococcus aureus within murine macrophages during in vitro infection. We have reported that Staphylococcus aureus internalized by murine macrophages did not appear to be rapidly killed. Data indicating the contribution of a single catalase and superoxide dismutase in intracellular survival of Staphylococcus aureus were provided using established biochemical assays. The results of the present experiment suggest that the survival of Staphylococcus aureus within phagocytic cells is facilitated by its ability to resist oxidative products. Organisms in the log phase of growth clearly demonstrate a resistance to oxidative products.
Keywords: Intracellular survival, Staphylococcus aureus, Murine macrophages, Anti-oxidant enzymes
Introduction
Staphylococcus aureus (S. aureus) is an extracellular pathogen, which may occasionally survive and even multiply within phagocytes resulting in prolonged and recurrent infections [1]. The infections caused by S. aureus are often acute and pyogenic and if untreated, may spread to surrounding deeper tissues or to metastatic sites involving other organs, resulting in disseminated or deep seated infections which are life threatening [2]. However, S. aureus may occasionally become intracellular, at least within monocytes, macrophages and polymorphonuclear neutrophils (PMN) when host defense mechanisms are activated [3]. Once inside, S. aureus may be exposed to a variety of host killing mechanisms including various reactive oxygen species (ROS) generated by the respiratory burst response [4, 5]. Several mechanisms for resistance to intracellular killing have been proposed, including the scavenging of free radicals produced by macrophages. Catalase, a protein with known free radical scavenging activities, metabolizes hydrogen peroxide (H2O2), a toxic oxygen metabolite. It has been postulated that bacterial factors that inactivate hydrogen peroxide, such as catalase, may interrupt the production of these toxic species and aid persistence and survival within host cells and tissues [6]. In S. aureus and many other organisms, the specific activity of catalase is low during the initial exponential phase of growth and increases steadily with cell growth to a maximal level at onset or during stationary phase [7]. It was also found that log phase S. aureus resisted the antibacterial activity of peritoneal macrophages [8]. Although S. aureus has not been shown to escape from the phagosome, neither the kinetics of escape nor the underlying mechanisms employed by S. aureus have yet been determined [9–11]. S. aureus can survive intracellularly after phagocytosis and this may result in recurrent infections and failure of antibiotic treatment if the drug has a poor penetration into phagocytes [12]. Involvement of superoxide dismutase (SOD) and myeloperoxidase (MPO) in oxygen dependent killing of S. aureus by neutrophils has been reported [13, 14]. The ability of S. aureus to exploit the inflammatory response of the host by surviving inside PMN is a virulence mechanism for this pathogen and the modulation of the inflammatory response is sufficient to significantly alter morbidity and mortality induced by S. aureus infection [15]. These observations suggest that the ability to inactivate H2O2 contributes to the survival in the host [16] and led us to examine the role of staphylococcal catalase in H2O2 resistance and intracellular survival. Additionally, an intracellular lifestyle is thought to shelter microorganism from immune surveillance; however, there is little evidence to support this idea regarding sepsis [17, 18]. This approach was undertaken in the belief that proteins expressed by the pathogen in vivo reflect its expression within the host cell environment, thereby allowing a better understanding of staphylococci-host cell relationship.
The present study was performed in order to carefully investigate the interaction of S. aureus with murine macrophages and the contribution of catalase in intracellular persistence of bacteria within murine macrophages during in vitro infection. In this report we demonstrate that catalase is required for S. aureus hydrogen peroxide resistance as well as persistence within macrophages. We have reported release of catalase and SOD in the exponential phase of growth. On the other hand cells internalized by murine macrophages did not appear to be rapidly killed. The results of the present experiment suggest that the survival of S. aureus within phagocytic cells is facilitated by its ability to resist oxidative products. Organisms in the log phase of growth clearly demonstrate a resistance to oxidative products.
Materials and Methods
Bacterial Strain
Staphylococcus aureus (S. aureus) are pathogenic strain and have hospital origin. CMC-524 was obtained from Calcutta Medical College and Hospital, Calcutta, ICH-629 was obtained from Institute of Child Health, Calcutta and ICH-757 was also obtained from Institute of Child Health, Calcutta. Among these three clinical isolates, CMC-524 and ICH-629 were catalase positive and coagulase positive. ICH-757 was catalase positive [19] and coagulase negative.
Collection of Bacteria During Stage Specific Growth
Cells were collected at each hour during their growth and centrifuged at 10,000 rpm at 4°C for 10 min. The cell pellet thus obtained was resuspended in sterile saline and cell number was adjusted on the basis of the relationship;
A620 0.2 = 5 × 107 cells per ml [20]. These bacterial cells collected during their growth period were now allowed to internalize by murine macrophages.
Isolation of Murine Splenic and Peritoneal Macrophages
Murine splenic macrophages are isolated according to the method of Meltzer [21]. Briefly, spleens excised from immediately sacrificed mice were macerated in Alsever’s solution and the suspension was layered over 3 ml Histopaque-1077 and centrifuged for 30 min. A band with leukocyte enriched fraction at the interface was collected, suspended in RPMI-1640-foetal bovine serum (FBS)(5%) and are allowed to plastic adherence at 37°C for 1 h. Adherent cells were collected, centrifuged and suspended in RPMI-FBS (5%) which contains very high number of macrophages.
Murine peritoneal macrophages were isolated according to the method of Meltzer [21] as described elsewhere. Peritoneal fluids of the mice, previously injected with 3% starch in 0.9% NaCl intraperitoneally, three days prior to the sacrifice, are collected and centrifuged. The cell pellets are re-suspended in RPMI-FBS (5%) and were allowed for plastic adherence. Adherent cells were collected and finally suspended in RPMI-FBS (5%).
Internalization of S. aureus Inside Murine Macrophages Following Infections with Increased Inocula of Clinical Isolates
Internalization of stage specifically grown S. aureus by the murine macrophages was determined according to the method as described by Kahl et al. [22]. Briefly murine splenic or peritoneal macrophages were infected with bacteria collected during their growth in invasion medium (growth medium without antibiotics, antimycotics or fetal bovine serum) for 1 h at 37°C, 5% CO2 incubator. After 1 h incubation, cell culture medium was replaced by RPMI-1640 containing 100 μg of gentamicin/ml to kill extracellular bacteria. Then, infected cells were washed thrice with PBS to remove gentamicin, treated with 200 μl of 0.25% Trypsin, 0.2% EDTA in Hank’s balanced salt solution (HBSS) for 5 min and lysed with 800 μl of 0.025% Triton X100. Cell lysates were diluted, plated in triplicate on nutrient agar and incubated overnight at 37°C and the number of colony forming unit (CFU) per ml were determined.
Phagocytosis Assay
Murine macrophages (5 × 106/ml) were mixed with S. aureus (5 × 106/ml) in a 1:1 cell: bacterium ratio in RPMI-40% Serum and incubated at 37°C cell culture incubator for different times (up to 5 h). Phagocytosis was stopped by adding cold (4°C) RPMI-1640 and extracellular S. aureus were removed by washing the suspension four times in RPMI-1640 by centrifugation at 250g at 4°C for 5 min. Duplicate samples were removed at 1 h interval, centrifuged and the supernatants reserved, the pellets disrupted in sterile water containing 0.01% BSA by vigorously vortexing to release intracellular bacteria in the supernatant. 0.1 ml of the supernatant was serially diluted in sterile distilled water (pH 7.0) and spread on agar plates that were incubated overnight at 37°C for determination of viable S. aureus. The intracellular killing of S. aureus in macrophages was measured by determining the decrease in the number of intracellular viable bacteria [23].
Culture of Intracellular Viable S. aureus and Preparation of Whole Cell Extract (Cell Lysate) for Demonstration of Enzyme Activity
The bacteria that were survived after time dependent phagocytosis as obtained from the plates of respective hours (Plates of intracellular viable S. aureus from the above experiment) were further cultured in nutrient broth.
Cells were washed twice in phosphate buffered saline (PBS), vortexed, passed through a 5 μm pore size filter, diluted to an optical density at 650 nm of 0.4 (106 CFU/ml) and resuspended in lysis buffer.
Bacterial suspensions (10 ml) were washed twice with 25 mM sodium phosphate buffer containing 1 mM disodium EDTA, pH 7.2, 0.5 mM phenylmethylsulfonyl fluoride (PMSF) and finally resuspended in 2 ml buffer. Suspensions were sonicated on ice at 8 μm for 90 s (six 15 s bursts with 15 s cooling periods) and then centrifuged at 16 000 ×g for 45 min at 4°C. Supernatants (lysates) were filtered and assayed for catalase and SOD activity [24].
Preparation of Crude Extracts for Electrophoresis and In Situ Staining
Bacterial lysates were prepared as described above. Lysates were assayed for the presence of catalase and SOD on acrylamide gels. Protein content was determined by using the Bradford assay.
Electrophoresis and In Situ Staining
Electrophoresis was performed on non-denaturing acrylamide gels using the Bio-Rad Mini system with tris/glycine buffer. Samples were applied to 10% bisacrylamide gels prepared with 1.5 M Tris/HCl, pH 8. Electrophoresis was carried out at 200 V.
SOD activity was visualized on gels following the methodology as described earlier [25]. Briefly, gels were washed in distilled water, then soaked in a solution of 2.45 mM nitro blue tetrazolium for 20 min, followed by a 10 min incubation in a solution containing 28 mM tetramethylethylenediamine (TEMED), 0.028 mM riboflavin and 36 mM potassium phosphate buffer, pH 7.8. Gels were illuminated on a light box to obtain a dark background with achromatic bands corresponding to SOD activity.
Catalase activity was visualized on non-denaturing acrylamide gels following the methodology as described earlier [26]. After electrophoresis, gels were washed three times in distilled water for 20 min and soaked in a solution of 0.015% H2O2. Activity was then visualized by transferring the gels to a solution of 1% ferric chloride, 1% potassium ferricyanide. Regions corresponding to catalase activity were determined as yellow bands on a green background.
Assay of Superoxide Dismutase (SOD) Activity:
100 μl of the bacterial lysate was mixed with 1.5 ml of a Tris–EDTA-HCl buffer (pH 8.5), then 100 μl of 7.2 mM pyrogallol was added and the reaction mixture was incubated at 25°C for 10 min. The reaction was terminated by the addition of 50 μl of 1 mM HCl and measured at 420 nm. One unit was determined as the amount of enzyme that inhibited the oxidation of pyrogallol by 50% [27].
The activity was expressed as U/mg protein.
Assay of Catalase Activity
Catalase activity in the cell free lysate was determined spectrophotometrically by measuring the decrease in H2O2 concentration at 240 nm [27]. At time zero, 1.8 ml of each lysate (250 μg/ml) was mixed with 0.2 ml of a phosphate buffer containing 10 mM Hydrogen peroxide. One milliliter of the mixture was immediately added to a disposable cuvette and placed into a spectrophotometer. Measurement of absorbance was taken at 0, 1, 2, 3, 4, 5 min after addition of the lysate to hydrogen peroxide buffer. Units of catalase activity present in 1 ml of lysate were calculated. One unit (U) of catalase activity is defined as the amount of enzyme catalyzing 1 μmol of H2O2 per min at 25°C.
Infection of Animals with S. aureus and Collection of Blood and Lymphoid Organs for Determination of Bacterial Density
Mice were injected intravenously via the tail vein with different clinical isolates of S. aureus (107CFU/mouse) in 0.2 ml saline. At different time intervals after infection, blood was collected from the retro orbital plexus and the mice were sacrificed by cervical dislocation. The blood of each infected mice was plated on nutrient agar. Spleen and lymph nodes were excised, weighed, homogenized, diluted in saline and plated onto nutrient agar. Plates were maintained at 37°C for 48 h and bacterial colonies counted. Results were expressed as the number of CFU/ml of blood and CFU/mg of spleen or lymph node.
Serum TNF-α Assay by ELISA
Blood was collected from the retro orbital plexus of both control and S. aureus infected mice at zero, 24, 48, 72 h post infection. Serum was prepared after clot retraction and kept at −80°C until further use. Serum TNFα level was directly measured by immuno-enzymatic assay method [28] using the TNFα assay kit according to the manufacturer’s instruction.
Results
Internalization of Stage-specifically Grown S. aureus in Murine Macrophages
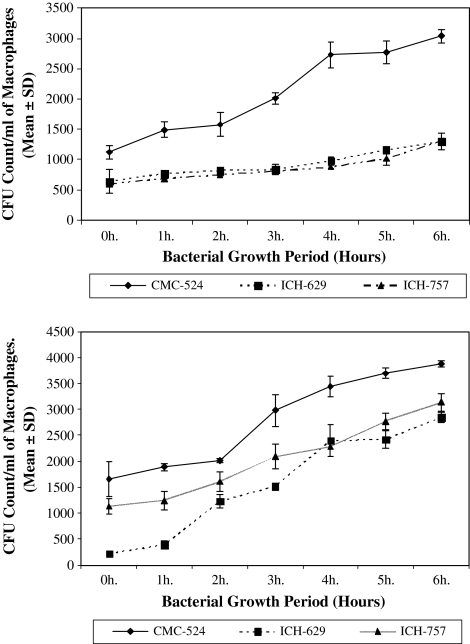
The internalization of different concentrations of S. aureus after 1 h infection to macrophages with different concentrations of S. aureus is shown in Fig. 1a and b. Figure 1a and b showed the number of internalized bacteria, collected up to 6 h of their growth inside macrophages changes significantly with the increase of their growth period.
Fig. 1.
Internalization of stage-specifically grown S. aureus in a murine peritoneal macrophages and b in murine splenic macrophages. The internalization of S. aureus after 1 h infection to macrophages with different concentrations of S. aureus collected during their growth is shown in Fig. 1a. To determine if internalization occurs, we enumerate the number of internalized viable bacteria isolated from different stages of their growth. The results in this figure represent the increase in bacterial count as expressed in CFU/ml of macrophage from different clinical isolates
From the CFU count of the intracellularly viable S. aureus within murine peritoneal and splenic macrophages, increased CFU was obtained with the increase of the bacterial growth period in all the clinical isolates of S. aureus.
Viability of S. aureus Recovered from Macrophage Lysate after 0–5 h In Vitro Infection
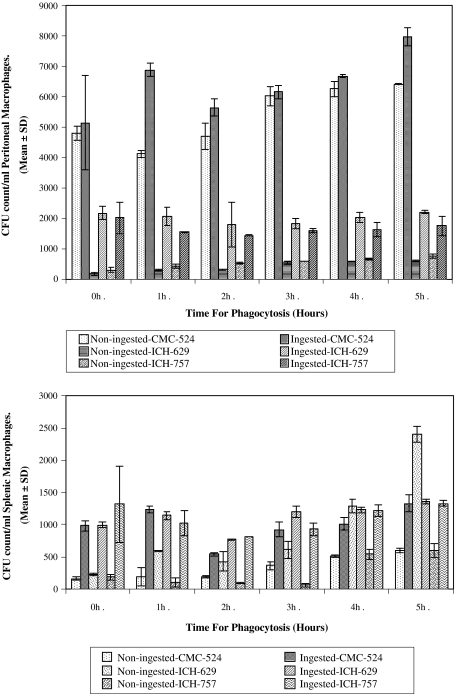
The intracellular survival of S. aureus was substantially higher after 2 h phagocytic time. (Fig. 2a and b). After the 2 h infection period, the CFU of S. aureus from the lysate of murine macrophages was least. Conversely more CFU of S. aureus recovered was viable after 2 h infection period.
Fig. 2.
Phagocytosis of S. aureus by a murine peritoneal macrophages and b murine splenic macrophages. The intracellular killing of S. aureus by murine peritoneal macrophages after time dependent phagocytosis (up to 5 h) is shown in Fig. 2a. The results in this figure represent the gradual increased intracellular viability of S. aureus within murine peritoneal macrophages after 2 h of phagocytosis. The intracellular killing of S. aureus by murine splenic macrophages after time dependent phagocytosis (up to 5 h) is shown in Fig. 2b. The results in this figure represent the gradual increased intracellular viability of S. aureus within murine splenic macrophages after 2 h of phagocytosis
The mean CFU count of the intracellularly viable S. aureus (CMC-524) allowed for 2 h of phagocytic time was found to be lower than that allowed for 0, 1, 3, 4, and 5 h phagocytic time. Mean CFU count for 2 h phagocytic time (5640 ± 282.84) was found significantly lower than the mean CFU count for 1 h (6880 ± 226.27) (P < 0.025), 4 h (6680 ± 56.57) (P < 0.025) and 5 h (7980 ± 311.13) (P < 0.01) phagocytic time, respectively.
The CFU count of the intracellularly viable S. aureus (ICH-757) allowed for 2 h of phagocytic time was found to be lower than that allowed for 0, 1, 3, 4, and 5 h phagocytic time. Mean CFU count for 2 h phagocytic time (1430 ± 14.14) was found significantly lower than the mean CFU count for 1 h (1550 ± 14.14) (P < 0.01), 3 h (1600 ± 56.57) (P < 0.05) phagocytic time, respectively. Similar pattern of results were also observed in case of splenic macrophages for all the strains.
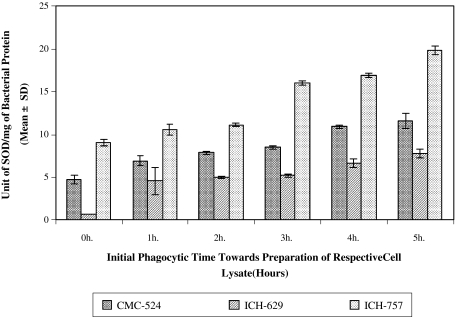
Super Oxide Dismutase (SOD) Release from Intracellularly Viable S. aureus (CMC-524, ICH-629 and ICH-757) Lysate
The bacterial SOD released (in Unit/mg of bacterial protein) from those bacteria (CMC-524) originally allowed for 1 h (6.948 ± 0.561) (P < 0.05), 2 h (7.863 ± 0.061) (P < 0.01), 3 h (8.501 ± 0.221) (P < 0.01), 4 h (10.898 ± 0.182) (P < 0.005) and 5 h (11.563 ± 0.912) (P < 0.01) phagocytic time were significantly higher than that released for 0 h (4.706 ± 0.512) phagocytic time. Identical release of SOD from the intracellularly viable S. aureus was also found in case of ICH-629 and ICH-757 (Fig 3).
Fig. 3.
Super oxide dismutase (SOD) content in intracellularly viable S. aureus (CMC-524, ICH-629 and ICH-757) lysate recovered after time dependent phagocytosis with murine macrophages. The bacteria that were survived after time dependent phagocytosis as obtained from the plates of respective hours (plates of intracellular viable S. aureus from the above experiment) were further cultured in nutrient broth. Results in this figure represent the bacterial SOD content (in Unit/mg of bacterial protein) from the respective group of cell lysate
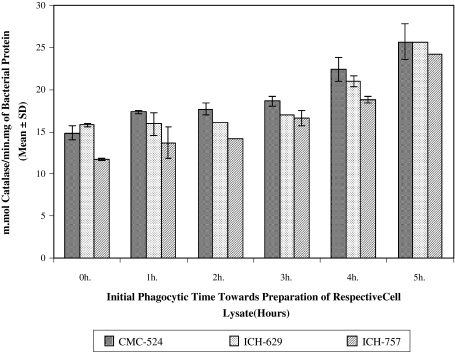
Catalase Release from Intracellularly Viable S. aureus (CMC-524, ICH-629 and ICH-757) Lysate
In case of CMC-524, crude bacterial lysate, bacterial catalase content was found to be increased with the increase of phagocytic time. The bacterial catalase content (in m.mol/min/mg of bacterial protein) from those bacteria originally allowed for 1 h (17.37 ± 0.212) (P < 0.025), 2 h (17.69 ± 0.735) (P < 0.05), 3 h (18.66 ± 0.559) (P < 0.025), 4 h (22.44 ± 1.386) (P < 0.025) and 5 h (25.68 ± 2.136) (P < 0.025) phagocytic time were significantly higher than that released for 0 h (14.88 ± 0.785) phagocytic time. Identical release of catalase from the intracellularly viable S. aureus was also found in case of ICH-629 and ICH-757 (Fig 4).
Fig. 4.
Catalase content in intracellularly viable S. aureus (CMC-524, ICH-629 and ICH- 757) lysate recovered after time dependent phagocytosis with murine macrophages. Preparation of bacterial lysate was done in a way as mentioned in methods. Results in this figure represent the bacterial catalase content (in m.mol/min.mg of bacterial protein) from the respective group of cell lysate
Detection of SOD and Catalase Activity on Acrylamide Gels
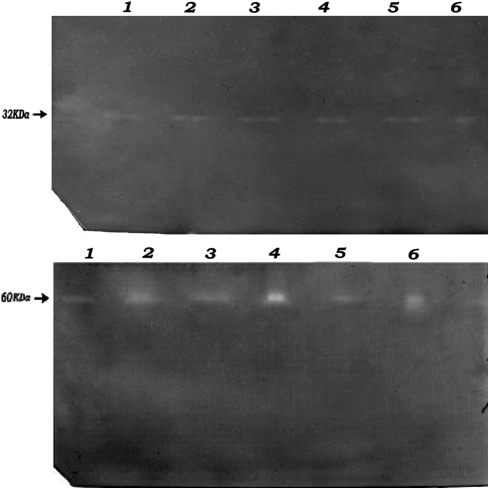
All strains in this study showed a similar clear band of SOD activity on the gels (Fig. 5a). All bands migrated to the same point in acrylamide gels, suggesting a similar enzyme was expressed by all strains. Identical protein concentrations were loaded for all the strains, thus the similar intensity of the SOD bands indicates that the S. aureus strains assayed contained similar levels of SOD activity.
Fig. 5.
Detection of a SOD activity and b catalase activity in the acrylamide gels. Electrophoresis was performed on non-denaturing acrylamide gels using the Bio-Rad Mini system with tris/glycine buffer. Results in this figure represent the non- denaturing polyacrylamide gel electrophoresis (PAGE) analysis of SOD activities in the sonicated extracts of S. aureus (CMC-524, ICH-629 and ICH-757) in 12% native polyacrylamide gels. 1 CMC-524, 2 h lysate, 2 CMC-524, 4 h lysate, 3 ICH-629, 2 h lysate, 4 ICH-629, 4 h lysate, 5 ICH-757, 2 h lysate, 6 ICH-757, 4 h lysate. Electrophoresis was performed in a way as mentioned in methods. Regions corresponding to catalase activity were determined as yellow bands on a green background. Results in this figure represent the non- denaturing polyacrylamide gel electrophoresis (PAGE) analysis of catalase activities in the sonicated extracts of S. aureus (CMC-524, ICH-629 and ICH-757) in 8% native polyacrylamide gels. 1 CMC-524, 2 h lysate, 2 CMC-524, 4 h lysate, 3 ICH-629, 2 h lysate, 4 ICH-629, 4 h lysate, 5 ICH-757, 2 h lysate, 6 ICH-757, 4 h lysate
The mobility of the bands of catalase activity in native gels was similar for all of the strains examined. Although this technique is not accurately quantitative, the difference in intensity of the bands suggests different levels of activity in the extracts of different strains (Fig. 5b).
Bacterial Clearance in Blood, Spleen and Lymph Nodes of Mice Infected with S. aureus (CMC-524, ICH-629 and ICH-757)
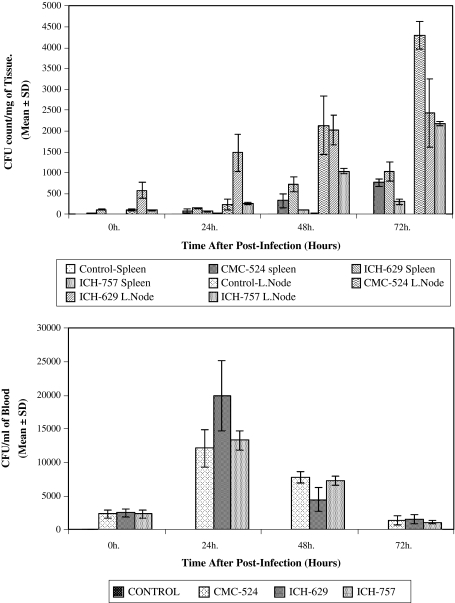
In case of CMC-524, the CFU count of the viable bacteria present within the spleen and lymph node after 0, 24, 48 and 72 h of infection were found to have increased with the increase of in vivo bacterial incubation period (Fig 6).
Fig. 6.
Bacterial density in a the spleen and lymph nodes of mice after intravenous infection with S. aureus (CMC-524, ICH-629 and ICH-757) and b blood following intravenous injection of S. aureus in mice. Spleen and lymph nodes were processed as described in methods. Results were expressed as numbers of bacterial CFU per milligram of spleen or lymph node tissue. The values are the mean ± SD from three different experiments and each experimental group contained at least six mice. Blood samples were collected at different time period from the mice after infection with S. aureus and plated into agar for 48 h. The values are the mean ± SD from three different experiments and each experimental group consist at least six mice
The mean CFU counts of the viable bacteria per ml of blood were found to be maximum at 24 h. post-infection and then decreased gradually.
In CMC-524, infected group, the mean CFU count of the bacteria/ml blood for 24 h post infection (12100 ± 2778.49) was significantly higher than the mean CFU count of bacteria/ml of blood for 0 h (2350 ± 619.14) (P < 0.0005), 48 h (7800 ± 848.53) (P < 0.025) and 72 h (1350 ± 700.00) (P < 0.005) post infection, respectively. Bacterial burden for ICH-629 and ICH-757 were found similar as before.
TNF-α Content in Serum of Mice Infected with S. aureus (CMC-524, ICH-629 and ICH-757) Intravenously for a Varying Period of Time
In case of CMC-524 infected mice, the mean pg TNF-α/ml of serum after 0, 24, 48 and 72 h post infection was found higher than that of the respective control serum. The mean TNF-content (pg/ml) in 0 h serum (1237.5 ± 62.915), 24 h (1343.75 ± 37.5), 48 h (1243.75 ± 42.696) and 72 h (1243.75 ± 42.696) were significantly higher than the control 0 h (442.5 ± 5.0) (P < 0.0005), 24 h (437.5 ± 5.0) (P < 0.0005), 48 h (437.5 ± 5.0) (P < 0.0005) and 72 h (440 ± 8.165) (P < 0.0005), respectively. Among the CMC-524 infected serum TNF-α content of 24 h (1343.75 ± 37.5) serum were significantly higher than the 0 h (1237.5 ± 62.915) (P < 0.025), 48 h (1243.75 ± 42.696) (P < 0.01) and 72 h (1243.75 ± 42.696) (P < 0.01) infected serum, respectively. Similar result on the serum TNF-alpha level in mice after infection with ICH-629 and ICH-757 was observed (Fig 7).
Fig. 7.
Changes in serum TNF-α level after acute infection with S. aureus clinical isolates. Results are expressed as mean ± SD of triplicate experiments (P < 0.0005)
Discussion
This paper describes the survival of three clinical isolates of S. aureus inside murine macrophages in vitro and we have discussed the observation in relation to the activity of S. aureus to produce catalase and SOD. In our study, we provide evidence that S. aureus after internalization can survive inside the macrophages, which is dependent on the size of the inocula. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages has been recently reported [29]. However, we did not use electron microscopy in detecting the intracellular bacteria or elimination of cell surface bacteria by lysostaphin or so on. Also, because of technical limitations in visualizing live intracellular bacteria, it was not possible to determine in these antecedent studies whether the bacteria are actively replicating or are merely by-standers. From the bacteria’s perspectives, there are obvious advantages in maintaining an intracellular location, since the microorganism will be able to avoid many host defense mechanisms as well as to shield themselves from extracellular antibiotics.
This study also demonstrates that following infection, bacteria are not rapidly killed by the phagocytic activity of murine macrophages; they rather survive within macrophages during prolonged infection due to secretion of catalase, which by degrading H2O2 inhibit ROS mediated killing mechanism of the host. Based on the result of in vitro CFU assay within 2 h after infection, we stated that S. aureus survived intracellularly. However, we are not sure whether the bacteria survive or not intracellularly based on further longer period (up to 5 h) of assay. Since these three clinical isolates possess catalase-producing activities, therefore, involvement of catalase can be suggested for contributing intracellular survival. Since all the standard strains of S. aureus produce catalase there is no particular significance to look at the catalase activity in bacterial culture. Oxidative products were clearly implicated as the antibacterial agents for this system, since antibacterial activity was inhibited with catalase (H2O2 inhibition). Survival studies with these three catalase positive S. aureus clearly indicates that the bacterial catalase protects against host H2O2.
In the context of superoxide having a direct role in killing results with S. aureus suggest that bacteria with SOD production would be more resistant to oxidative killing by macrophages. It has been reported that killing of S. aureus was impeded when SOD-coated latex beads were co-ingested with the bacteria [30]. It is possible that this bacterial associated SOD could slow down intra-phagosomal killing and be a factor in their pathogenecity. Regulation of this enzyme is presumably one aspect of the adaptation process that allows the bacteria to survive under hostile condition.
The relevance of these observations to S. aureus infection in vivo is unknown. We speculate that natural infection involves organisms in both exponential and stationary phases of growth. Such organisms are readily phagocytosed by PMN, monocytes and macrophages resident in the liver and spleen. These organisms in the stationary phase of growth may be quickly killed by murine macrophages since they are susceptible to concentrations of OH− and OCl−, which can be easily achieved. Organisms in the exponential phase of growth are also taken up by macrophages but are more likely to resist intracellular killing. Since the ability of macrophages to produce OCl− and OH− is limited, the organism may thus survive and replicate in these cells.
As early factors in inflammation and immunity, TNF-α and IL-1β modulate host reactions to foreign antigens. Probably in relation to this modulation of phagocytic function, these cytokines have been implicated in increased nonspecific resistance to infection [31]. TNF-α was found in the lungs of mice experimentally infected with Chlamydia trachomatis and showed that exogenous TNF-α, induced by lipopolysaccharide provided protection from death caused by the pathogen. The presence of TNF-α whether of host or pathogen origin was required [32].
Acknowledgments
This study was carried out with support of an unrestricted research grant from Indian Council of Medical Research (ICMR), New Delhi, Government of India, provided to Dr. Biswadev Bishayi, Senior Lecturer, Department of physiology, University of Calcutta, Kolkata. We are deeply indebted to Prof. (Mrs.) Manjusree Bal, Department of Physiology, University of Calcutta, for supplying us with S. aureus (CMC-524, ICH-629 and ICH-757). We remain thankful to Prof. Sanghamitra Raha, Saha Institute of Nuclear Physics, Kolkata, India, for their kind help and guidance in the in situ staining techniques.
References
- 1.Pailard D, Grellet J, Dubois V, Saux CM, Quentin C. Discrepancy between uptake and intracellular activity of moxifloxacin in a staphylococcus aureus-Human THP-1 monocytic cell model. Antimicrobiol Agents Chemother. 2002;46(2):288–293. doi: 10.1128/AAC.46.2.288-293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowy FD. Staphylococcus aureus infection. N Eng J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein SC, Steinbery TH. Host defense against bacterial and fungal infections. In: Davis BD, Dulbecco R, Eisen HN, Ginsberg HS, editors. Microbiology. Philadelphia: J.P Lippincott Company; 1990. pp. 485–505. [Google Scholar]
- 4.Stor ZG, Tartaglia LA, Farr SB, Ames BN. Bacterial defense against oxidative stress. Trends Genet. 1990;6:363–368. doi: 10.1016/0168-9525(90)90278-E. [DOI] [PubMed] [Google Scholar]
- 5.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants myeloperoxidase and bacterial killing. Blood. 1998;92:3013–3017. [PubMed] [Google Scholar]
- 6.Day WA, Sajecki JL, Pitts TM, Joens LA. Role of catalase in Campylobacter jejuni intracellular survival. Infect Immun. 2002;68:6337–6345. doi: 10.1128/IAI.68.11.6337-6345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin SE, Chaven S. Synthesis of catalase in Staphylococcus aureus MF-31. Appl Environ Microbiol. 1987;53:1207–1209. doi: 10.1128/aem.53.6.1207-1209.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington-Fowler L, Henson PM, Wilder MS. Fate of Listeria monocytogenes in resident and activated macrophages. Infect Immun. 1981;33:11–20. doi: 10.1128/iai.33.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayles KW, Wesson CA, Liou LF, Fox LK, Bohach GA, Trumble WR. Intracellular S. aureus escapes the endosome and reduces apoptosis in epithelial cells. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balwit JM, Langevelde PV, Vann JM, Proctor RA. Gentamicin resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J Infect Dis. 1994;170:1033–1037. doi: 10.1093/infdis/170.4.1033. [DOI] [PubMed] [Google Scholar]
- 11.Proctor RA, Balwit JM, Vesga O. Variant subpopulation of Staphylococcus aureus as cause of persistent and recurrent infections. Infect Agents Dis. 1994;3:302–312. [PubMed] [Google Scholar]
- 12.Yancey RJ, Sanchez MS, Ford CW. Activity of antibiotics against Staphylococcus aureus within polymorphonuclear neutrophils. Eur J Clin Microbiol Infect Dis. 1991;10:107–113. doi: 10.1007/BF01964421. [DOI] [PubMed] [Google Scholar]
- 13.Clement MO, Watson SP, Foster SJ. Characterization of the major superoxide dismutase of S.aureus and its role in starvation, survival, stress resistance and pathogenicity. J Bacteriol. 1999;181:3898–3903. doi: 10.1128/jb.181.13.3898-3903.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamton MB, Kettle AJ, Winterbourn CC. Involvement of superoxide and myeloperoxidase in oxygen dependent killing of S. aureus by neutrophils. Infect Immun. 1996;64:3512–3517. doi: 10.1128/iai.64.9.3512-3517.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contribute to infection. J Immunol. 2000;164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 16.Beaman L, Beaman BL. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Ann Rev Micobiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- 17.Verdrengh M, Tarkowski A. Role of macrophages in Staphylococcus aureus induced arthritis and sepsis. Arthritis Rheum. 2000;43:2276–2282. doi: 10.1002/1529-0131(200010)43:10<2276::AID-ANR15>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JC. Staphylococcus. In: Gyles CL, Thunen CD, editors. Pathogenesis of bacterial infections in animals. Ames: Iowa State Univ Press; 1986. p. 14. [Google Scholar]
- 19.Collins CH, Lyne PM. Microbiological methods. 4. Boston: Butterworths; 1976. p. 170. [Google Scholar]
- 20.Yao L, Berman JW, Stephen MF, Franklin DL. Correlation of histopathologic and bacteriologic changes in experimental murine model of bacteremic Staphylococcus aureus infection. Infect Immun. 1997;65:3889–3895. doi: 10.1128/iai.65.9.3889-3895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meltzer MS. In: Methods for studying mononuclear phagocytes. Adams DO, Edelson PJ, Koren HS, editors. New York: Academic Press; 1981. p. 133. [Google Scholar]
- 22.Kahl BC, Goulian M, Wamel W, Herrmann M, Simon SM, Kaplan G, Peters G, Cheung AL. Staphylococcus aureus RN6390 replicates and induces apoptosis in pulmonary epithelial cell line. Infect Immun. 2000;68:5385–5392. doi: 10.1128/IAI.68.9.5385-5392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielson SL, Black FT, Storgaard M, Obel N. Evaluation of a method for measurement of intracellular killing of Staphylococcus aureus in human neutrophil granulocytes. APMIS. 1995;103:460–468. doi: 10.1111/j.1699-0463.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 24.Barnes AC, Balebona MC, Horne MT, Ellis AE. Superoxide dismutase and catalase in Photobacterium damselae subsp. Piscicida and their roles in resistance to reactive oxygen species. Microbiology. 1999;145:483–494. doi: 10.1099/13500872-145-2-483. [DOI] [PubMed] [Google Scholar]
- 25.Beauchamp C, Fridovich Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 26.Woodbury W, Spencer AK, Stahmann MA. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971;44:301–305. doi: 10.1016/0003-2697(71)90375-7. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, Bok SH, Park YB, Lee MK, Choi MS. 4-Hydroxycinnamate lowers plasma and hepatic lipids without changing antioxidant enzyme activities. Ann Nutr Metab. 2003;47:144–151. doi: 10.1159/000070037. [DOI] [PubMed] [Google Scholar]
- 28.Soop M, Duxbury H, Agwunobi AO, Agwunobi AO, Gibson JM, Hopkins SJ, et al. Euglycemic hyperinsulinemia augments the cytokine and endocrine responses to endotoxins in humans. Am J Physiol Endocrinol Metab. 2002;282:E1276–E1285. doi: 10.1152/ajpendo.00535.2001. [DOI] [PubMed] [Google Scholar]
- 29.Macay MB, Seral C, Leclercq MP, Tulkens PM, Bambeke VF. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother. 2006;50:841–851. doi: 10.1128/AAC.50.3.841-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston RB, Jr, Keele BB, Jr, Misra HP, Lehmeyer JE, Webb LS, Bachner RL, Rajagopalan KV. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975;55:1357–1360. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez MS, Ford CW, Yancey RJ. Effect of tumor necrosis factor alpha, interleukin 1 beta and antibiotics on the killing of intracellular Staphylococcus aureus. J Dairy Sci. 1994;77:1251–1258. doi: 10.3168/jds.S0022-0302(94)77064-8. [DOI] [PubMed] [Google Scholar]
- 32.Bekker LG, Freeman S, Murray P, Ryffel B, Kaplan G. Tumor necrosis factor alpha controls intracellular mycobacterial growth by both inducible nitric oxide synthase dependent and inducible nitric oxide synthase independent pathways. J Immunol. 2001;166:6728–6734. doi: 10.4049/jimmunol.166.11.6728. [DOI] [PubMed] [Google Scholar]