Abstract
Two hundred and seven bacteria were isolated from composts and macrofauna and screened for plant growth promoting and antagonistic traits. Seven of the 207 isolates showed antagonistic activity against Sclerotium rolfsii in plate culture. Inhibition of S. rolfsii by the bacterial isolates ranged between 61 and 84%. Two of the seven isolates were Bacillus sp. and rest belonged to Pseudomonas sp. Two isolates, Pseudomonas sp. CDB 35 and Pseudomonas sp. BWB 21 was compatible with chickpea Rhizobium sp. IC 59 and IC 76 in plate culture conditions. Increase in plant biomass (dry weight) ranged between 18 and 30% on application of these bacteria by seed coating and seed priming methods. However, by seed-priming there was an increase in plant biomass by 5–7% compared to seed coating. Number of nodules and the nodule weight was similar by both seed coating and seed priming methods. Disease incidence was reduced up to 47% in treatments where captan (fungicide) or antagonistic Pseudomonas sp. CDB 35 was applied. Increase in shoot weight was 36% by seed coating with Rhizobium sp. IC 59 and Pseudomonas sp. CDB 35 when compared to captan application. Whereas by seed priming with IC 59 and CDB 35 increased shoot weight by 3 and 39% increase in nodulation was observed.
Keywords: Seed priming, Rhizobium, Antagonistic bacteria, Pseudomonas, Sclerotium rolfsii
Chickpea (Cicer arietinum L.) is an important legume crop in the semi arid tropical countries and its production is second to cereals. Among the biotic factors contributing towards low production of chickpea, the collar rot disease caused by Sclerotium rolfsii Sacc., is a major cause for 55–95% mortality of chickpea seedlings. There are no substantial levels of host plant resistance for collar rot in chickpea but the disease can be minimized by fungicides and appropriate crop rotation [1, 2]. However environmental concerns of usage of chemicals have led to the alternatives such as the use of bacteria as biofertilizers and biopesticides for sustainable agriculture. Several mechanisms has been suggested by which bacteria can promote plant growth including phytohormone production, nitrogen fixation, stimulation of nutrient uptake and biocontrol of pathogenic fungi [1]. Beneficial microorganisms, including antagonistic bacteria and fungi, applied as seed treatment provide unique benefits for crop protection.
Seed priming is now a widely used method to help accelerate germination and improve seedling uniformity in many crop and ornamental plants [2]. There are four technologies to achieve priming, i.e., osmoconditioning or osmopriming, solid-matrix priming, hydropriming and drum priming [3]. This simple procedure of seed priming—soaking seeds in water followed by air-drying before sowing is possible in a range of tropical and subtropical crops [4]. It is observed that by priming seeds for 8 h before sowing has been particularly effective in yields of legumes like chickpea (C. arietinum) and mungbean (Vigna radiata) were substantially increased. Seed treatment with Pseudomonas fluorescens decreased tomato damping off [5]. Priming also significantly reduced the damage caused by collar rot (S. rolfsi) in Bangladesh in two contrasting seasons [6]. In bio-priming, the inoculum applied as antagonist is in close proximity to the sites of pathogen entry into the seed and the emerging seedling. In such application, much less antagonist inoculum is needed than for soil treatment, which will reduce crop production costs [7]. In the present study, we report the coinoculation of antagonistic bacteria and compatible Rhizobium for control of chickpea collar rot under glasshouse conditions.
Materials and Methods
Characterization of Bacteria for Antagonistic Activity
Two hundred and seven bacteria isolated from different composts (24 from farm waste compost, 55 from rice straw compost and 50 from vermicompost) and 78 from macrofauna (earthworms, centipedes, slugs and snails) were characterized for different traits such as phosphate solubilization, IAA, ACC deaminase, phytase, siderophore and HCN production. All the isolates were screened for antagonistic activity against soil-borne plant pathogenic fungi, S. rolfsii, Macrophomina phaseolina, Fusarium solanii and Fusarium oxysporum by dual plate method [8]. Seven of the 207 isolates inhibited S. rolfsii on Kings B medium in petri dishes. Per cent inhibition of fungi (growth reduction over control) was calculated by the following equation:
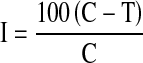 |
where I is the % inhibition of mycelial growth, C the radial growth of fungus in control plate (mm), and T is the radial growth of fungus on the plate inoculated with bacteria (mm). All the seven were tested for their interaction with two different Rhizobium strains (IC 59 and IC 76).
Compatibility of Plant Growth Promoting Bacteria with Rhizobium Strains
Two rhizobial strains, IC 59 and IC 76 were obtained from the microbial culture collection at ICRISAT [9] and grown on yeast extract mannitol agar (YEMA) [10]. Rhizobia were streaked horizontally on YEMA medium in 10 cm diameter plate and antagonistic bacteria were streaked perpendicular to the rhizobium on either side of the plate 2 cm away from Rhizobium [11]. Rhizobia were inoculated 24 h prior to the inoculation of antagonistic bacteria because of their slow growth. The plates were incubated further for 96 h and “interaction distance” (towards Rhizobium), “spreading capacity” (away from Rhizobium) of antagonistic bacteria was measured.
Interaction of Rhizobium IC 59 and Antagonistic Pseudomonas sp. CDB 35 in Glasshouse Conditions
Based on the compatibility of Pseudomonas sp. CDB 35 with Rhizobium IC 59 in plate culture, they were evaluated in glasshouse conditions using chickpea (ICCV2) as host plant. Bacterial inoculation was done by seed coating and seed priming methods. For seed coating, peat (Biocare Technology Pvt. Ltd, Australia) based inoculum of Rhizobium sp. IC 59 (108–109 CFU g−1 peat), antagonistic Pseudomonas sp. CDB 35 (108–109 CFU g−1 peat) and 1% carboxymethylcellulose (CMC) was used as adhesive and the seeds were air-dried before sowing. For seed priming, 200 g of chickpea seeds were soaked in 200 ml of water for 5–6 h along with peat-based inoculum of Rhizobium sp. IC 59 and Pseudomonas sp. CDB 35, 1% CMC as adhesive and air-dried before sowing. Five seeds were sown in 15 cm diameter plastic pots using unsterilized soil of BR1D field at International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) as potting mix. Thinning was done to three plants per pot on fifth day of emergence. Plants were irrigated once every 2 days with 50 ml deionized water. Temperature in the glasshouse ranged from 18 to 24°C during the experiment period. The treatments were arranged in a randomized complete block design with three replications and repeated twice. Harvesting was done 45 days after sowing (DAS) and the parameters measured were plant biomass (dry weight), number of nodules, nodule weight and nitrogenase activity was measured by method of Hardy et al. [12].
Efficacy of Antagonistic Bacteria against Collar Rot Disease Caused by S. rolfsii
Antagonistic Pseudomonas sp. CDB 35 was tested against collar rot disease caused by S. rolfsii in glasshouse conditions using chickpea (ICCV 2) as host. Soil from a collar rot sick plot at ICRISAT infested with S. rolfsii having population of about 10 × 10−2 CFU g−1 soil was used for this experiment. The soil mix consisted of RP as the P source, as Pseudomonas sp. CDB 35 was known to solubilize P from our previous study [13]. Inoculation of Rhizobium IC 59 and antagonistic bacterial isolate Pseudomonas sp. CDB 35 was done as mentioned above. Seeds were allowed to dry in air and ten seeds were sown in 21 cm diameter plastic pots for each treatment. Thinning was done to five plants per pot on fifth day of emergence. The treatments were control (infested with S. rolfsii), fungicide (Captan: 0.3%), Rhizobium sp. IC 59, Pseudomonas sp. CDB 35 with seed priming and seed coating methods. Treatments were arranged in a randomized complete block design with five replications and harvested at 45 DAS. Disease incidence (based on germination percent and survival of plants till the harvesting period) and plant growth parameters such as shoot and root weight, number of nodules and nodule weight were recorded.
Statistical Analysis
Glasshouse experiments were arranged in completely randomized block design with three replications in each treatment and repeated twice. The data were subjected to analysis of variance using Genstat 6.1 statistical package (Lawes Agricultural Trust, Rothamsted, UK). Mean values in each treatment were compared using least significant differences at 5% probability (P = 0.05).
Results and Discussion
Compatibility of Antagonistic Bacteria with Rhizobium (Plate Culture Conditions)
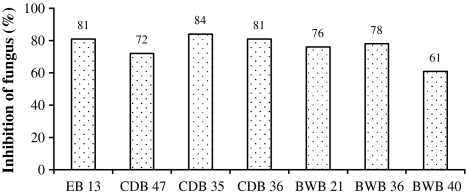
Seven isolates showed antagonistic activity against S. rolfsii and highest inhibition was by Pseudomonas sp. CDB 35 (84%) followed by Bacillus licheniformis EB 13 and Pseudomonas sp. CDB 36 (81%). Least activity was shown by Pseudomonas sp. BWB 40 (61%) (Fig. 1). All the seven strains studied for antagonistic activity were tested for their interaction with the rhizobial strains IC 59 and IC 76 of chickpea. Growth towards the Rhizobium sp. was measured as interacting distance and growth away from Rhizobium sp. was measured as spreading capacity. It was observed that two strains, CDB 35 and BWB 21 showed maximum interaction with the tested rhizobial strains, followed by EB 13, CDB 36, CDB 47, BWB 36 and BWB 40 (Table 1). However, there was no apparent sign of suppression of rhizobia by any of the antagonistic bacteria that were studied.
Fig. 1.
Inhibition (%) of growth of S. rolfsii in presence of antagonistic bacteria (B. licheniformis EB 13, B. licheniformis CDB 47, Pseudomonas sp. CDB 35, Pseudomonas sp. CDB 36, Pseudomonas sp. BWB 21, Pseudomonas sp. BWB 36, Pseudomonas sp. BWB 40) on Kings B medium
Table 1.
Interaction between antagonistic bacteria and Rhizobium on YEMA medium
| Isolates | Source | Spreading capacity (mm) (growth of antagonistic bacteria away from Rhizobia) | Interaction distance (mm) (growth of antagonistic bacteria towards Rhizobia) | |||||
|---|---|---|---|---|---|---|---|---|
| IC 59 | IC 76 | Mean | IC 59 | IC 76 | Mean | |||
| B. licheniformis EB 13 | Earth worm body | 11 | 11 | 10 | 8 | 8 | 8 | |
| Pseudomonas sp. CDB 35 | Rice straw compost | 23 | 19 | 20 | 26 | 25 | 26 | |
| Pseudomonas sp. CDB 36 | Rice straw compost | 11 | 10 | 11 | 8 | 4 | 6 | |
| B. licheniformis CDB 47 | Rice straw compost | 4 | 4 | 4 | 4 | 5 | 5 | |
| Pseudomonas sp. BWB 21 | Vermicompost | 19 | 19 | 17 | 24 | 27 | 26 | |
| Pseudomonas sp. BWB 36 | Vermicompost | 6 | 6 | 6 | 5 | 4 | 5 | |
| Pseudomonas sp. BWB 40 | Vemicompost | 7 | 7 | 6 | 5 | 5 | 5 | |
| Mean | 12 | 11 | 11 | 11 | 11 | 11 | ||
| LSD (P = 0.05) | 1.4 | |||||||
| CV% | 3 | |||||||
IC (Strain number given for Rhizobium isolates from ICRISAT)
Effect of Rhizobium sp. and Antagonistic Bacteria on Growth of Chickpea
The antagonistic bacterium Pseudomonas sp. CDB 35 was compatible with the Rhizobium sp. IC 59 and IC 76 in plate culture. Rhizobium sp. IC 59 (that nodulates chickpea) was selected and evaluated along with antagonistic bacteria to see their dual effect of plant growth and biological control activity under glasshouse conditions with chickpea as host plant (Table 2). Increase in plant biomass (dry weight) of chickpea by seed coating was 20% with Rhizobium sp. IC 59 alone and 30% with Rhizobium sp. IC 59 and Pseudomonas sp. CDB 35. Whereas by seed priming method, the increase in plant biomass was 18% with Rhizobium sp. IC 59 alone and 29% with Rhizobium sp. IC 59 and Pseudomonas sp. CDB 35. The nodule number, nodule weight and nitrogenase activity increased 2–3 times when Rhizobium and antagonistic bacteria were applied. It was observed that inoculation by seed priming method increased the plant biomass by 5–7% compared to seed priming (Table 2). Nitrogenase activity was highest in treatment with CDB 35 and Rhizobium by seed priming method.
Table 2.
Effect of dual inoculation of Rhizobium IC 59 and Pseudomonas sp. CDB 35 on growth, nodulation and nitrogenase activity of chickpea (ICCV 2)
| Treatments | Plant biomass (dry weight (mg) | Number of nodules | Nodule weight (mg) | Nitrogenase activity μM of C2H4 plant−1 h−1 |
|---|---|---|---|---|
| Control* + SC | 529 | 7 | 15 | 1.3 |
| Control + SP | 741 | 7 | 15 | 1.5 |
| Control + RP + SC | 721 (0) | 14 | 26 | 3.4 |
| Control + RP + SP | 769 (0) | 14 | 37 | 3.9 |
| IC 59 + RP + SC | 867 (20)a | 19 | 35 | 3.9 |
| IC 59 + RP + SP | 907 (18)a | 20 | 34 | 4.4 |
| IC 59 + CDB 35 + RP + SC | 939 (30)a | 25 | 45 | 4.8 |
| IC 59 + CDB 35 + RP + SP | 989 (29)a | 28 | 51 | 4.9 |
| Mean | 808 | 17 | 32 | 3.5 |
| LSD | 8.5 | 216 | 12.4 | 3.45 |
| CV% | 30 | 16 | 22 | 40 |
Values in parentheses are per cent increase over control by the respective treatments SC/SP
Control* where only sterilized peat was applied without any bacterial inoculum, IC strain name of rhizobia used in the study, SC seed coating, SP seed priming, RP rock phosphate
aMeans are significantly different by SC and SP method at P = 0.05 when compared by LSD
Efficacy of Pseudomonas sp. CDB 35 against Collar Rot (S. rolfsii) Disease of Chickpea
Antagonistic Pseudomonas sp. CDB 35 was evaluated against collar rot disease caused by S. rolfsii in glasshouse conditions by seed biopriming and seed coating methods and compared with fungicide captan. It was observed that inoculant applied by seed priming method was better than seed coating method. Disease incidence observed was similar with captan and CDB 35 application. Increase in shoot weight was around 31% on inoculation with Rhizobium sp. IC 59 and Pseudomonas sp. CDB 35 by seed coating when compared to fungicide captan application (Table 3). Where as, inoculation with IC 59 and CDB 35 by seed priming method showed increase in shoot weight by 3% when compared with captan application. It was observed that highest number of nodules were present where inoculation was done with Rhizobium sp. IC 59 and Pseudomonas sp. CDB 35 by seed priming method.
Table 3.
Evaluation of Rhizobium sp. IC 59 and Pseudomonas sp. CDB 35 on disease incidence, growth and nodulation of chickpea (ICCV2) infected with S. rolfsii
| Treatments | Disease incidence (%) | Shoot weight (mg) | Root weight (mg) | Nodules (per plant) | Nodule weight (mg) (per plant) |
|---|---|---|---|---|---|
| Control* + SC | 83 | 396 | 145 | 2 | 2 |
| Control + SP | 80 | 286 | 121 | 2 | 1 |
| Captan + SC + IC 59 | 67 | 688 (0) | 285 | 11 | 4 |
| Captan + SP + IC 59 | 47 | 1038 (0)a | 293 | 13 | 7 |
| Pseudomonas sp. CDB 35 + IC 59 + SC | 53 | 939 (31) | 207 | 7 | 5 |
| Pseudomonas sp. CDB 35 + IC 59 + SP | 47 | 1070 (3)a | 261 | 20 | 34 |
| Mean | 63 | 767 | 193 | 9 | 10 |
| LSD | 43 | 631.5 | 219 | 7.56 | 30.9 |
| CV% | 24 | 63.6 | 58.4 | 64 | 352 |
Values in parentheses are per cent increase over fungicide treatment captan by SC/SP methods
Control* where only sterilized peat was applied without any bacterial inoculum, IC strain name of rhizobia used in the study, SC seed coating, SP seed priming, RP rock phosphate
aMeans are significantly different by SC and SP method at P = 0.05 when compared by LSD
In this study bacteria isolated from composts and macrofauna inhibited collar rot pathogen S. rolfsii (Fig. 1). Previous study indicated that bacteria inhabiting composts inhibited plant pathogenic fungi such as S. rolfsii, F. oxysporum, Pythium aphanidermatum and M. phaseolina [14]. All the seven antagonistic bacterial isolates studied here were compatible with Rhizobium of chickpea in plate culture (Table 1). In the previous study it was observed that bacterial isolates from termitaria soil and composts showed commensal behaviour towards groundnut rhizobia, under in vitro conditions [4]. Pseudomonas sp. CDB 35 inoculated along with Rhizobium showed growth promotion of chickpea in pots having unsterilized soil (Table 2). The plants inoculated by seed priming method showed significant difference in biomass (dry matter) and nitrogenase activity of chickpea than seed coating method (Table 2). The increase in nitrogenase activity was highest in inoculation of Rhizobium sp. IC 59 and Pseudomonas sp. CDB 35 by seed priming. In earlier studies of on-farm participatory trials in Western India by ICRISAT, it was observed that seed priming increased yields of chickpea, maize, wheat and upland rice [15]. Seed priming is also done to alleviate stress conditions for in vitro tissue-propagated plants [16]. Co-inoculation of fluorescent Pseudomonas and Rhizobium improved plant growth of Pisum sativum [17]. Siderophores produced by Pseudomonas could increase the level of flavonoid-like compounds in the root which in turn increased total plant nitrogen in chickpea [18]. It is also known that plant root flavonoids are inducers of nodulation gene (nod genes) expression in Rhizobium. It was observed in our studies that Pseudomonas sp. CDB 35 showed siderophore production and inhibited the soil-borne plant pathogenic fungi [19].
In this study Pseudomonas sp. CDB 35 suppressed S. rolfsii and decreased disease incidence (Table 3). Seed bio-priming with Rhizobium and antagonistic bacteria showed enhancement in growth of chickpea and reduced the collar rot disease. Seed bio-priming is reported to induce resistance against downy mildew and enhance growth of pearl millet [20]. Fungicide and biocontrol bacterium, P. fluorescens, in combination reduced the incidence of collar rot of chickpea [21]. Previous studies revealed that seed treatment by bio-priming resulted in enhanced germination, increased microbial population which inhibit the pathogen propagules on seeds thereby protecting the plant [22].
Seed priming is an effective method for resource poor farmers to increase the yields in a range of tropical and subtropical crops. However, since no negative effects of seed priming are observed, this low-cost technology can be adopted by resource-poor farmers to mitigate the effects of suboptimal management or adverse physical conditions.
Acknowledgment
The authors thank ICRISAT Patancheru for providing glasshouse and other facilities and financial assistance by Jawaharlal Nehru Memorial Fund, New Delhi is gratefully acknowledged.
References
- 1.Kumar J, Singh NB, van Rheenen HA, Johansen C, Asthana AN, Ali M, Agrawal SC, Pandey RL, Verma MM, Gaur RB, Satyanarayana A, Patil MS, Rahman MM, Saxena NP, Haware MP, Wightman JA (1997) Growing chickpea in India. International Crops Research Institute for the Semi-Arid Tropics, Patancheru; Indian Council of Agricultural Research, New Delhi, 60 pp
- 2.Azhar H, Muhammad Iqbal SH, Najma A, Zahid AM. Factors affecting development of collar rot disease in chickpea. Pak J Bot. 2006;38(1):211–216. [Google Scholar]
- 3.Rokhzadi A, Asgharzadeh A, Darvish F, Nour-Mohammadi G, Majidi E. Influence of plant growth promoting rhizobacteria on dry matter accumulation of chickpea under field conditions. J Agric Environ Sci. 2008;3:253–257. [Google Scholar]
- 4.Amanda JB, Whipps JM. Dual application of beneficial microorganisms to seed during drum priming. Appl Soil Ecol. 2008;38(1):83–89. doi: 10.1016/j.apsoil.2007.08.001. [DOI] [Google Scholar]
- 5.Harris D, Breese WA, Kumar Rao JVDK. The improvement of crop yield in marginal environments using ‘on-farm’ seed priming: nodulation, nitrogen fixation and disease resistance. Aust J Agric Res. 2005;56:1211–1218. doi: 10.1071/AR05079. [DOI] [Google Scholar]
- 6.Jayaraman J, Parthasarathi T, Radhakrishnan NV. Characterization of a Pseudomonas fluorescens strain from tomato rhizosphere and its use for integrated management of tomato damping-off. Biocontrol. 2007;52:683–702. doi: 10.1007/s10526-006-9046-0. [DOI] [Google Scholar]
- 7.Musa AM, Harris D, Johansen C, Kumar JVDK. Short duration chickpea to replace fallow after aman rice: the role of on-farm seed priming in the High Barind Tract of Bangladesh. Exp Agric. 2001;37:509–521. doi: 10.1017/S0014479701000448. [DOI] [Google Scholar]
- 8.Hameeda B, Rupela OP, Reddy G, Satyavani K. Application of plant growth-promoting bacteria associated with composts and macrofauna for growth promotion of Pearl millet (Pennisetum glaucum L.) Biol Fertil Soils. 2006;43:221–227. doi: 10.1007/s00374-006-0098-1. [DOI] [Google Scholar]
- 9.Rupela OP, Kumar Rao JVDK, Sudarshana MR, Usha Kiran M, Anjaiah V (1991) Rhizobium germplasm resources at ICRISAT center. Patancheru, AP, India. Research Bulletin no. 15
- 10.Dalton H. The cultivation of diazotrophic microorganisms. In: Bergersen FJ, editor. Methods for evaluating biological nitrogen fixation. Brisbane: Wiley; 1980. pp. 13–64. [Google Scholar]
- 11.Sriveni M, Rupela OP, Gopalakrishnan S, Krajewski M. Spore-forming bacteria a major group among potential antagonists isolated from natural sources such as termitaria soil and composts used by organic farmers. Indian J Microbiol. 2004;44:95–100. [Google Scholar]
- 12.Hardy TWF, Holsten RD, Jackson EK, Burns RC. The acetylene reduction assays for N2 fixation: laboratory and field evaluation. Plant Physiol. 1968;43:1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hameeda B, Harish Kumar Reddy Y, Rupela OP, Kumar GN, Reddy G. Effect of carbon substrates on rock phosphate solubilization by bacteria from composts and macrofauna. Curr Microbiol. 2006;53:298–302. doi: 10.1007/s00284-006-0004-y. [DOI] [PubMed] [Google Scholar]
- 14.Muhammad S, Amusa NA. In vitro inhibition of growth of some seedling blight inducing pathogens by compost-inhabiting microbes. Afr J Biotechnol. 2003;2:161–164. [Google Scholar]
- 15.Harris D, Joshi A, Khan PA, Gothkar P, Sodhi PS. On-farm seed priming in semi-arid agriculture: development and evaluation in maize, rice and chickpea in India using participatory methods. Exp Agric. 1999;35:15–29. doi: 10.1017/S0014479799001027. [DOI] [Google Scholar]
- 16.Nowak J, Shulaev V. Priming for transplant stress resistance in in vitro propagation. In vitro cell. Dev Biol Plant. 2003;39:107–124. [Google Scholar]
- 17.Dileep Kumar BS, Berggren I, Mårtensson AM. Potential for improving pea production by co-inoculation with fluorescent Pseudomonas and Rhizobium. Plant Soil. 2001;229:25–34. doi: 10.1023/A:1004896118286. [DOI] [Google Scholar]
- 18.Parmer N, Dadarwal KR. Stimulation of nitrogen fixation and induction of flavonoid like compounds by rhizobacteria. J Appl Microbiol. 1999;86:36–44. doi: 10.1046/j.1365-2672.1999.00634.x. [DOI] [Google Scholar]
- 19.Hameeda B, Rupela OP, Reddy G. Evaluation of bacterial isolates from composts and macrofauna for their biocontrol activity against soil-borne plant pathogenic fungi. Indian J Microbiol. 2006;46:389–396. [Google Scholar]
- 20.Niranjan SR, Shetty NP, Shetty HS. Seed bio-priming with Pseudomonas fluorescens isolates enhances growth of pearl millet plants and induces resistance against downy mildew. Int J Pest Manag. 2004;50:41–48. [Google Scholar]
- 21.Singh SD, Girish AG, Rupela OP, Gopalakrishnan S, Anitha K, Rao PJM. Synergism between Pseudomonas fluorescens Migula and Thiram for the control of collar rot of chickpea. Ind J Plant Prot. 2003;31:40–42. [Google Scholar]
- 22.Dijk K, Nelson EB. Inactivation of seed exudate stimulants of Pythium ultimum sporangium germination by biocontrol strains of Enterobacter cloacae and other seed associated bacteria. Soil Biol Biochem. 1998;30:183–192. doi: 10.1016/S0038-0717(97)00106-5. [DOI] [Google Scholar]