Abstract
Rubella Virus (RUBV) is a common cause of childhood rash and fever in non-immunized populations, and its public health importance relates to teratogenic effects of primary rubella infection in women with early pregnancy. Infection of the fetus may lead to congenital rubella syndrome (CRS). This work aimed to assess the degree of risk associated in acquiring rubella virus infection by the women during pregnancy and developing CRS among their children in Bangladesh. The study population (n = 275) included pregnant mothers (15–38 years) from various socioeconomic backgrounds attending a women health care based hospital. All subjects were personally interviewed, clinically examined and a standardized questionnaire was filled up for each of them. From each participant 3 ml blood was taken and serum was separated. Commercially available ELISA kit was used for the qualitative and quantitative determination of IgM and IgG class antibodies against RUBV in collected serum samples. 209 women were found to contain detectable level of antiRUBV IgG antibodies, but did not possess IgM antibodies against rubella. Only 9% participants were vaccinated previously against rubella virus among the whole antenatal population studied. Ninety-two percent of these vaccinated pregnant women contained serum anti-rubella IgG antibody which was significantly (P = 0.05) higher than that of the nonvaccinated study population (75%). Pregnant women from lower middle and poor socioeconomic class had significantly (P = 0.05) more intra uterine growth retardation (IUGR) of fetus than the upper middle class. 20% of the women of child bearing age examined in this work were not yet exposed to RUBV and at risk of acquiring this virus during pregnancy and subsequently transmitting the virus to the fetus. Our work demonstrates rubella attack rate among antenatal population in Bangladesh as 14.5 in 1000 during pregnancy. A proper and reliable vaccination policy against rubella virus is not yet adopted at the national level in many developing countries including Bangladesh. This work identifies the requirement of detailed study for the identification of intrauterine rubella infection and its related influence on perinatal morbidity and mortality. Thorough epidemiological studies are also considered necessary prior to the development and acceptance of national immunization program against rubella virus in Bangladesh.
Keywords: Rubella virus, Pregnancy, Congenital rubella syndrome
Introduction
Rubella virus infection appears to be endemic throughout most of the world. Postnatal rubella and congenital rubella present different scenario in context of mode of transmission, clinical manifestation, severity of illness, immunity developed, and recovery pattern. Postnatal rubella could be acquired through the mucosa of the upper respiratory tract among neonates, children and adults. This infection usually results in a mild disease that can rarely produce significant sequelae. However, primary RUBV infection during early stage of pregnancy may result in the transmission of virus through the placenta and infection of the fetus. This may in turn lead to congenital rubella syndrome (CRS), the most common manifestations of which are blindness, mental retardation, and deafness [1]. Congenital rubella infection can also result in miscarriages, stillbirths, fetal anomalies and therapeutic abortions [2].
Diagnosis of rubella virus infection is essential to assess the state of exposure of a woman in the first trimester of pregnancy, is necessary for confirmation of potential cases of congenital rubella syndrome (CRS) in newborns and young children, and is important as the surveillance component of rubella vaccination programs [3].
Classically, serological diagnosis of primary rubella virus infection has relied on the detection of rubella virus-specific IgM or the demonstration of a four-fold increase in the IgG titer to rubella virus antigens in serum samples that have been taken sequentially and then assayed in pairs. Detection of antiRUBV IgG is verification of immunity, as there is only one serotype of rubella virus [4].
An estimated 20,000 cases of CRS occurred in the US in 1964–1965 during rubella epidemic before the vaccine available [2]. A reduction of cases of CRS was noted in Western Australia and Taiwan after proper introduction of rubella immunization policy there [5]. Therefore, prevention of CRS becomes the major objective of rubella vaccination program. In Bangladesh, like other developing countries, there is no accepted national policy for rubella immunization or screening of women non immune for RUBV in their reproductive years [6]. This work aimed to assess the degree of risk associated in acquiring rubella virus infection by the women during pregnancy and developing CRS among their children in Bangladesh.
Materials and Methods
Study Population
The study population (n = 275) included pregnant mothers (15–38 years) from various socioeconomic backgrounds attending a women health care based hospital in Dhaka for antenatal check up from September 2006 to October 2007.
Questionnaire
All subjects were personally interviewed and a standardized questionnaire was filled up for each of them to obtain relevant demographic, anthropometric, socioeconomic, clinical and health related information. Each participant was provided with an identification (ID) number which was subsequently used for the serodiagnosis, data entry and analysis.
Sample Collection and Serum Separation
From each participant approximately 2–3 ml blood was collected. Serum was separated from blood sample using standard method.
ELISA
Commercially available ELISA kit (NOVATECH, Immundiagnostica GMSH; product number RUBM0400 and RUBG0400; Dietzenbach, Germany) was used for the qualitative and quantitative determination of anti rubella IgM and IgG antibodies in human serum, respectively. Detection of these two classes of antibodies was carried out and result was interpreted according to the protocols provided with the kits.
Data Analysis
The data was analyzed using SPSS (statistical package for social services) version 11.5. The data was loaded numerically. Frequency was analyzed and cross sectional study was undertaken to determine the seroprevalence of antiRUBV IgG and IgM antibodies among the study population. Chi-square tests were used to compare the differences among the groups. P-value of <0.05 (2-tailed) was considered to comprehend significant relationship between the two selected variables.
Result and Discussion
A large extent of population may get infected by RUBV before puberty; still around 20% of adults remain susceptible [7]. The global prevalence of anti rubella antibodies was reported as 98.1% previously. The prevalence of rubella antibodies was higher in women than men (98.8 vs 92.2%, respectively) [8]. Among the studied antenatal population (n = 275; 15–38 years), 209 women contained detectable level of antiRUBV IgG antibodies (Table 1), but did not possess IgM antibodies against rubella when their serum samples were examined by commercial ELISA kit. The absence of rubella virus-specific IgG and IgM antibodies signifies susceptibility to infection whereas the presence of RUBV specific IgG but not IgM antibodies signifies past infection [2]. Accordingly, 76% women of this study population might have been infected by or vaccinated against RUBV in past (Table 2) and most of them could have developed protective immunity against reinfection [9].
Table 1.
Seroprevalence of rubella virus infection among pregnant mothers of different age groups (n = 275)
| Age of the pregnant women (years) | Presence of anti-rubella IgG antibodies in serum samples | Total (%) | (χ2 test) P-value | ||
|---|---|---|---|---|---|
| Negative (%) | Positive (%) | Equivocal (%) | |||
| 15–20 | 09 (19) | 37 (77) | 02 (04) | 48 (17) | |
| 21–25 | 19 (22) | 61 (72) | 05 (06) | 85 (31) | |
| 26–30 | 21 (20) | 81 (78) | 02 (02) | 104 (38) | |
| 31–35 | 03 (10) | 27 (90) | 00 (00) | 30 (11) | 0.018 |
| >36 | 04 (50) | 03 (38) | 01 (12) | 08 (03) | |
| Total/Average | 56 (20) | 209 (76) | 10 (04) | 275 (100) | |
Table 2.
Seroprevalence of anti rubella IgG antibodies among rubella vaccinated and nonvaccinated pregnant women
| Rubella vaccination status of pregnant women | Presence of anti-rubella IgG antibody in serum samples | Total (%) | (χ2 test) P-value | ||
|---|---|---|---|---|---|
| Negative (%) | Positive (%) | Equivocal (%) | |||
| Non vaccinated | 54 (21) | 187 (75) | 10 (04) | 251 (91) | |
| Vaccinated | 02 (08) | 22 (92) | 00 (00) | 24 (09) | 0.05 |
| Total/average | 56 (20) | 209 (76) | 10 (04) | 275 (76) | |
Seventeen percent of the examined population conceived while they were teen aged which indicates the still present custom of too early marriage for girls in Bangladeshi society. The pattern of seropositivity of RUBV infection change with the increasing age during the reproductive years of the women population is shown in Table 1. Production of anti rubella IgG antibody increased with the age up to 35 years (90%) and then there was a sudden decrease to 38% observed in the age group of >36 years. The P value was 0.018 which indicated a strong correlation between the acquisition of rubella virus infection and subsequent development of antiRUBV Ig G antibody with the age of the pregnant population. Previous surveys [10, 6] have demonstrated a similar pattern of seroprevalence of RUBV infection among the women population. Seth et al. [11] reported a gradually increasing seropositivity with an increase in age, reaching a maximum value (88%) for the age group of 25–34 years. Furthermore, Table 1 demonstrates that 20% of the women of child bearing age in Bangladesh are not yet exposed to RUBV and are at risk of acquiring this virus during pregnancy and subsequently transmitting the virus to the fetus.
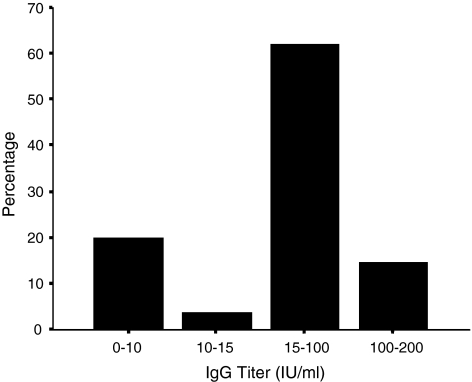
Figure 1 demonstrates the anti rubella IgG antibody titer determined among the pregnant women. Twenty percent of the pregnant women had a negative antibody titer (<10 IU/ml). Four percent of the pregnant women contained a doubtful antibody titer (10–15 IU/ml) which were considered as equivocal according to the guideline provided with the commercial ELISA kit and excluded from the further analysis. The remainder of the women (76%) possessed anti rubella Ig G antibody (>15 IU/ml) titer at various levels and all of these were marked as of positive category. Our data shows similarity with the study conducted in Thailand in 2004 where 75% of the studied pregnant women (n = 150; 15–40 yrs) were antirubella IgG positive [12]. From December 2000 to 31 December 2002 an surveillance for CRS was conducted among children aged 0–17 months at 13 hospitals and 2 private clinics in Myanmar. Blood samples from children with suspected CRS (n = 81) were tested for rubella-specific IgM; selected samples were tested for rubella-specific IgG and for rubella RNA by reverse transcriptase-polymerase chain reaction (RT-PCR). Of these, 18 children had laboratory-confirmed CRS (7 were IgM positive; 7 were RT-PCR positive; and 10 were IgG positive at >6 months of age) [13].
Fig. 1.
Titer (%) of anti rubella IgG antibody among the antenatal population
Among this studied population, 52% of women belonged to the later stage of pregnancy (Table 3), whereas 103 women were with gestational periods of 13–24 weeks. Timing of the fetal infection by RUBV determines the extent of teratogenic effect; the earlier in pregnancy infection occurs, the greater the damage to the fetus done. Maternal infection during the first trimester of pregnancy is most critical for the risk of the fetus in developing CRS. Inapparent maternal infections are equally potent to produce anomalies as well [9]. Ten percent of the participants in this research belonged to the early gestational period group that is duration of their pregnancy was within 12 weeks of time. Sixty-eight percent (Table 3) among this category were exposed naturally to or vaccinated against RUBV in past as they contained detectable level of serum antiRUBV IgG. However, 25% of these women with first trimester of pregnancy were unexposed to RUBV and hence at great risk of acquiring RUBV any time and transmitting this virus to their fetus. Birth defects are uncommon if maternal infection occurs after the 18th week of pregnancy [4].
Table 3.
Seroprevalence of rubella infection among pregnant women of different gestational periods (n = 275)
| Duration of pregnancy | Presence of anti-rubella IgG antibody in serum samples | Total (%) | (χ2 test) P-value | ||
|---|---|---|---|---|---|
| Negative (%) | Positive (%) | Equivocal (%) | |||
| 1–12 weeks | 07 (25) | 19 (68) | 2 (7) | 28 (10) | |
| 13–24 weeks | 20 (20) | 81 (79) | 2 (1) | 103 (37) | |
| 25–38 weeks | 29 (21) | 109 (76) | 6 (3) | 144 (52) | 0.70 |
| Total/average | 56 (20) | 209 (76) | 10 (4) | 275 (76) | |
a1–12 weeks = first trimester
b13–24 weeks = second trimester
c25–38 weeks = third trimester
Only 9% participants were vaccinated previously against rubella virus among the whole antenatal population studied. Ninety-two percent of these vaccinated pregnant women contained serum anti-rubella IgG antibody which was significantly (P = 0.05) higher than that of the nonvaccinated study population (75%). Although the vaccination did not produce protective anti-rubella IgG antibody for all the immunized women in this research, it was found protective for majority of the cases. Furthermore, the all four serum anti-rubella IgM antibody positive cases (Table 4) found in this work were from nonvaccinated group which clearly shows the potential risk of RUBV acquisition by the pregnant mother if they are left unvaccinated. This observation states the significance of probable inclusion of anti-rubella virus vaccination programme in Bangladesh. However, a large number of nonvaccinated women (75%) also produced protective anti-rubella IgG antibody by getting naturally exposed to RUBV from the surrounding infected persons.
Table 4.
Rubella attack rate among antenatal population (n = 275) and the relevant features
| Parameters | Rate in 1000 or % |
|---|---|
| Prevalence rate of women positive for RV specific IgM | 14.5 in 1000 |
| Gestational Agea | 1–12 weeks 75% 13–24 weeks 25% |
| Socioeconomic statusa | LMC 75% Poor 25% |
| Clinical manifestationa | Morbilliform rash 75% Fever 25% Arthralgia 50% Fever and Arthralgia 25% |
| Prevalence of anti rubella IgGa antibodies | 100% |
aAccording to the number of RV specific IgM positive cases
Socioeconomic status of the participants was categorized on the basis of their reported monthly family income. Women from the families with income of below Tk. 5000 per month was graded as poor, whereas Tk. 5,000–12,000 income holders belonged to lower middle class, and Tk. 12,000–20,000 income holders to upper middle class, and above Tk. 20,000 to rich. Most (79%) of the studied women came from lower middle class (Table 5) and least (9%) from upper middle class category.
Table 5.
Intra uterine growth retardation (IUGR) of fetus among pregnant women (n = 275) from different socioeconomic backgrounds
| Socioeconomic status of pregnant women | Occurrence of IUGR in pregnant women | Total (%) | (χ2 Test) P-Value | |
|---|---|---|---|---|
| No (%) | Yes (%) | |||
| Poor | 29 (85) | 05 (15) | 34 (12) | |
| LMC | 179 (82) | 37 (18) | 216 (79) | |
| UMC | 23 (92) | 02 (08) | 25 (09) | 0.05 |
| Total/average | 231 (82) | 44 (18) | 275 (76) | |
LMC Lower middle class, UMC Upper middle class
Pregnant women from lower middle and poor socioeconomic class had significantly (P = 0.05) more intra uterine growth retardation of fetus than the upper middle class (Table 5). This observation can be explained by the probable lack in adequate nutrient uptake by and deficit in proper antenatal care taken for the women in these two (poor and LMC) categories. Besides, child bearing women belonging to low socioeconomic group in developing countries may be exposed to a variety of infections due to the poor environment and hygiene status they live in. Maternal infections could be considered as significant factors in the causation of poor pregnancy outcome as well [14]. Intra uterine growth retardation of fetus may affect the future physical and mental development of the children in various degrees. This study clearly recommends that steps should be taken to upgrade the nutritional uptake and antenatal care of the child bearing women among poor and lower middle class for achieving physically and mentally healthy new generation.
Table 1 demonstrates that 20% of the women of reproductive age in Bangladesh are not yet exposed to RUBV and are at risk of acquiring this virus during pregnancy and subsequently transmitting the virus to the fetus. Among these 20% susceptible population, four pregnant women (Table 4) have been shown to contain serum antiRUBV IgM antibodies. The presence of RUBV specific IgM antibodies with or without virus specific IgG antibodies identifies the infection as current or very recent [9]. Yasodhara et al. [14] investigated antenatal population (n = 422) in India and had reported prevalence rate of serum IgM specific for RUBV as 65 in 1000 during pregnancy. Our work demonstrates lower attack rate (14.5 in 1000) by rubella among antenatal population in Bangladesh than India [14] and agrees with some other documents where the overall rubella attack rate has been reported as 1 in 1,000, rising to 22 in 1,000 during pregnancies [6].
The four IgM positive pregnant mothers identified in this work were 21, 22, 26 and 30 years of age, respectively. The gestational age for the three women among this RUBV specific IgM antibody positive category (Table 4) was within 12 weeks. Therefore, fetuses of these three pregnant women were at high risk of acquiring rubella infection and developing congenital rubella syndrome afterwards [15].
Among these four IgM positive pregnant women, morbilliform rash on three and fever with moderate temperature for one were noted when examined. Postnatal rubella frequently commences with malaise, low grade fever, and a morbilliform rash appearing on the same day [2]. The rash starts on the face, extends over the trunk and extremities, and seldom continues for more than 3 days. Two women among these four cases had reported arthralgia only whereas one woman was suffering from both fever and arthralgia. Unless an epidemic occurs, the rubella disease to diagnose clinically is unreliable; as the rash and the additional relevant symptoms here are caused by other viruses could be similar. However, the clinical features (Table 4) presented by these women are undoubtedly associated with their very recent or current rubella infection since they have been clearly found as serum anti rubella IgM antibody positive. One limitation of this study was the consideration of the clinical picture based on just one contact between the participants and the evidence collector, as new signs and symptoms developing later were not available.
Only 57% of the countries around the world currently practice rubella vaccination programs, and it is estimated that more than 100,000 cases of CRS occur each year in developing countries [16]. A proper and reliable vaccination policy against rubella virus is not yet adopted at the national level in many developing countries including Bangladesh. This is probably due to non-focusing on rubella related problems or numerous other health related issues keeping this important morbidity related issue out of sight [6]. Current research identifies rubella as an endemic condition in Bangladesh like many other countries. This work also notifies the requirement of detailed study for the identification of intrauterine rubella infection and its related influence on perinatal morbidity and mortality. Thorough epidemiological studies are also considered necessary prior to the development and acceptance of national immunization program against rubella virus in Bangladesh. Although these strategies may lead to high expenditure, the benefits of interrupting the widespread circulation of rubella virus and preventing the incidence of CRS should pay off.
Acknowledgments
We are grateful to the participants of this study, and to the laboratory staff of Ad-din Hospital, Dhaka for their help and very kind cooperation.
Contributor Information
Hasan Imam, Phone: 8801911519741, Email: imam_019@yahoo.com.
Mahmuda Yasmin, Phone: 9661920-73/7743, Email: yasmin962001@yahoo.com.
Chowdhury Rafiqul Ahsan, Phone: 9661920-73/7738, Email: crahsan@yahoo.com.
Jamalun Nessa, Phone: 02-9661900-73, Email: jamalun_nessa@hotmail.com.
References
- 1.Lee JY, Bowden DS. Rubella virus replication and links to teratogenicity. Clin Microbiol Rev. 2000;13:571–587. doi: 10.1128/CMR.13.4.571-587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng H, Chang C, Tan H, Yang S, Chang H. Seroepidemiology study of rubella antibodies among pregnant women from seven Asian countries: evaluation of the rubella vaccination program in Taiwan. Vaccine. 2006;24:5772–5777. doi: 10.1016/j.vaccine.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 3.Tzeng W, Zhou Y, Icenogle J, Frey TK. Novel replicon-based reporter gene assay for detection of rubella virus in clinical specimens. J Clin Microbiol. 2005;43:879–885. doi: 10.1128/JCM.43.2.879-885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks FG, Butel JS, Morse SA, Melnick JL, Jawetz E, Adelberg EA (2004). Rubella virus. In: Medical Microbiology. Chapter 40, 23rd (edn). McGraw-Hill professional, New York, pp 566–568
- 5.Menser MA, Hudson JR, Murphy AM, Upfold LJ. Epidemiology of congenital rubella and results of rubella vaccination in Australia. Rev Infect Dis. 1985;7:37–41. doi: 10.1093/clinids/7.Supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafunnessa, Khatun S, Islam MN, Chowdhury S. Seroprevalence of rubella antibodies among antenatal population attending a tertiary level hospital in Dhaka city. Bangladesh Med Res Counc Bull. 2000;26:75–81. [PubMed] [Google Scholar]
- 7.Assaad F, Jungars-Esteves KL. Rubella world impact. Rev Infect Dis. 1985;7:29–36. doi: 10.1093/clinids/7.Supplement_1.S29. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez A, Plans P, Costa J, Torner N, Cardenosa N, Batalla J, Plasencia A, Salleras L. Seroprevalence of measles, rubella, and mumps antibodies in Catalonia, Spain: results of a cross-sectional study. Eur J Clin Microbiol Infect Dis. 2006;25:310–317. doi: 10.1007/s10096-006-0133-z. [DOI] [PubMed] [Google Scholar]
- 9.Hobman TC, Chantler JK. Rubella Virus. In: Knipe DM, Howley PM, editors. Fields Virology. 5. USA: Lippincott Williams and Wilkins; 2007. pp. 1069–1101. [Google Scholar]
- 10.Mathur A, Chaturvedi UC, Mehrotra RML. Serological study for the prevalence of rubella at Lucknow. Indian J Med Res. 1974;62:307–312. [PubMed] [Google Scholar]
- 11.Seth P, Balaya S, Mohapatra LN. Seroepidemiological study of rubella infection in female subjects of Delhi and its surrounding villages. India J Med Res. 1971;59:190–194. [PubMed] [Google Scholar]
- 12.Boonruang S, Buppasiri P. Rubella antibodies in normal pregnant women at Srinagarind Hospital, Khon Kaen, Thailand. J Med Assoc Thai. 2005;8(4):455–459. [PubMed] [Google Scholar]
- 13.Thant KZ, Oo WM, Myint TT, Shwe TN, Han AM, Aye KM, Aye KT, Moe K, Thein S, Robertson SE. Active surveillance for congenital rubella syndrome in Yangon, Myanmar. Bull World Health Organ. 2006;84(1):12–20. doi: 10.2471/BLT.05.022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasodhara P, Ramalakshmi BA, Naidu AN, Rahman L. Prevalence of specific IgM due to Toxoplasma, Rubella, CMV and C. trachomatis infections during pregnancy. Indian J Med Microbiol. 2001;19:52–56. [PubMed] [Google Scholar]
- 15.Wilson KM, Camillo CD, Doughty L, Dax EM. Humoral immune response to primary rubella virus infection. Clin Vaccine Immunol. 2006;13:380–386. doi: 10.1128/CVI.13.3.380-386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization Rubella vaccines: WHO position paper. Wkly Epidemiol Rec. 2000;75:161–169. [Google Scholar]