Abstract
Extracellular lipase was produced from Rhizopus oligosporus NRRL 5905 through solid state fermentation (SSF). To provide an optimum fermentation conditions for maximum lipase yield, five process variables (temperature, liquid–solid ratio, pH, incubation period and spore concentration) were optimized using evolutionary operation (EVOP) factorial design technique taking into account the interaction between the process variables. Optimization through EVOP resulted in around 3 fold increase in lipase activity (77 U gds−1) at a liquid–solid ratio of 1.5:1, fermentation temperature of 35°C, initial fermentation pH 6, incubation period 5 days and a spore concentration of 108 spores ml−1.
Keywords: Lipase, Rhizopus oligosporus, SSF, EVOP, Optimization
Introduction
Lipases (triacylglycerol hydrolases E.C. 3.1.1.3) catalyze hydrolysis of triglycerides into glycerols and fatty acids as well as the reverse reaction of esterification or transesterification. It is a versatile enzyme, which is becoming increasingly important for its immense application in various food, paper pulp [1], detergent, pharmaceuticals [2] and cosmetic industries and in synthesis of enantiopure pharmaceuticals, agrochemicals and flavor compounds [3]. However, the factors posing limitations on use of lipase include its relatively high cost and lack of enzyme with the optimal range of catalytic specificities and properties required in various applications. Though lipases originate from different sources like plants, animals and microorganisms, the microbial lipase is industrially most useful because microbes can be easily cultivated and their lipases can catalyze a wide variety of hydrolytic and synthetic reactions [4]. Among the different microbial sources like fungi, bacteria and yeast, fungi have been chosen for the present study as they are widely recognized as the best lipase sources and are used preferably for industrial applications, especially in the food industry [5]. For the production of enzyme from fungal sources, SSF system has been used as it provides natural growth conditions for fungi [5]. Moreover, SSF produces enzymes at higher concentration [6] and with higher temperature or pH stability [7] compared to submerged fermentation. Although surplus numbers of reports [8–10] are available for the production of both intra and extracellular lipases from Rhizopus sp., there is meager reports available pertaining to the production of lipase by Rhizopus oligosporus [11, 12]. It is well known that the metabolic processes of the microorganisms are very much influenced by the environmental conditions like temperature, pH, incubation period etc. So, it is essential to study the interaction effects of the environmental conditions simultaneously to reach an optimum condition for maximum enzyme production. But previous reports are not available on studies of optimization of process variables for enhancing lipase synthesis from this organism. This study is feasible with the application of a suitable statistical tool like evolutionary operation (EVOP), which will help in studying the combined effect of all individual process variables and their interaction effect on enzyme production.
The EVOP technique was first proposed by Box [13] for studying systematically the effect of two or three process variables and their interactions in a running chemical process plant for improving the quality or productivity. Banerjee and Bhattacharya [14] for the first time reported the application of EVOP methodology to design experiments for optimizing different conditions of a biological process for synthesis of protease. Later this methodology has been effectively employed for optimization of other biological processes [15–17]. EVOP is based on the concept that evolution as a natural process can be significantly accelerated by varying the environmental or operating conditions, measuring the responses and then proceeding towards optimum [14]. However, its limitation to consider at most three variables at a time is overcome by hybridization of the advantages of the two methods, namely EVOP and the factorial technique which provided a new and more effective approach to optimize “n” number of variables [16]. Here, experiments are designed based on the factorial technique and the results are analyzed by EVOP. The major strength of EVOP is its relatively simple and clear-cut decision-making procedure which directs the change of variables to reach the maximum or minimum response.
The objective of the present work was the application of EVOP methodology for the first time to optimize the different fermentation conditions viz. temperature, solid to liquid ratio, spore concentration, pH and incubation period for enhancement of lipase production by Rhizopus oligosporus NRRL5905 under SSF condition.
Materials and Methods
Chemicals
Potato dextrose agar (PDA) (Hi Media Pvt Ltd, Mumbai, India), p-nitro phenyl palmitate (Sigma-Aldrich, USA) were used for the present study. Rest of the chemicals were procured from Merck.
Microorganism
Rhizopus oligosporus NRRL 5905 was exploited for the production of lipase. The organism was maintained on PDA slants and stored at 4°C.
Preparation of Inoculums
The spore suspension was prepared by mixing sterilized distilled water to fully grown microbial culture maintained in slant.
Fermentation Method and Enzyme Extraction
Fermentation was carried out in 250 ml Erlenmeyer flask containing 8 g wheat bran moistened with modified Czapek-dox media (KH2PO4 1.0 gl−1, MgSO4·7H2O 0.5 gl−1, KCl 0.5 gl−1, NaNO3 2.5 gl−1 and glucose 50 gl−1) supplemented with mahua (Madhuka indica) oil (10%). The fermentation medium was sterilized by autoclaving at 15 lb inch−2 for 20 min and was inoculated with 1 ml fungal spore suspension. The flasks were incubated at a relative humidity of 85%. After fermentation the biomass was soaked in 32 ml water and the mixture was agitated at 30°C for 1 h and enzyme was squeezed through wet cheesecloth. The pooled enzyme extract was centrifuged and the supernatant was assayed for lipase activity.
Lipase Assay
Lipase assay was done spectrophotometrically using p-nitro phenyl palmitate as the substrate [18]. One unit (U) of lipase was defined as the amount of enzyme that liberates one micromole of p-nitro phenol, per min under the assay conditions. Enzyme activity was expressed as U gds−1 (gram of dry substrate). The values are mean of three replications.
EVOP-Factorial Design Technique
Experimental Set Up for Optimization of Five Variables
The development and application of the EVOP factorial design technique to maximize the response (lipase activity) of a biological process having five variables has been presented in this section. In the proposed methodology small variations around the initial optimum conditions were endeavored to find out where the actual optimum condition was located with respect to a hypothetical contour map, and in what direction the process variables should be changed to approach the maximum response. Firstly, the control experimental conditions were selected based on the laboratory tests followed by search techniques. The control experimental conditions are called “starting conditions” or “initial conditions” or “search level” for EVOP as shown in E1 and E18 of Table 1. Then new experimental conditions were selected with lower and higher values of the process parameters compared to the initial conditions (E2 to E16 and 19 to E 34 of Table 1). In an attempt to optimize five variables by EVOP, the total number of new experiments to be performed was 25, apart from the 2 control (search level) experiments. The variables were arranged in both higher level (+) and lower level (−) compared to the search level region (0). The variables and the total number of experiments were represented in a [5 × (25 + 2)] matrix which is divided into two blocks as presented in Table 1. Each of the blocks represented 17 trial experiments including the control experiments (E1 in block 1 and E18 in block 2) and the arrangement was such that block 1 had an odd number of lower level parameters such as 1, 3, 5 and block 2 had an even number of lower level parameters, such as 2, 4. As inherent variations occur in a process, too much of confidence could not be placed with results of a single cycle. So all the experiments were repeated twice in cycles I and II.
Table 1.
| Parameters | Experimental (block 1) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | E9 | E10 | E11 | E12 | E13 | E14 | E15 | E16 | E17 | |
| Incubation in days (I) | 0 | + | + | + | + | − | + | + | − | − | − | + | − | + | − | − | − |
| Temperature (T) | 0 | + | + | + | − | + | + | − | + | − | + | − | + | − | − | − | − |
| pH (P) | 0 | + | + | − | + | + | − | + | − | + | − | − | + | − | − | + | − |
| Liquid–solid ratio (R) | 0 | + | − | + | + | + | − | − | + | − | − | − | − | + | + | + | − |
| Spore concentration (S) | 0 | − | + | + | + | + | − | − | − | + | + | + | − | − | + | − | − |
| Response (Lipase activity) | a1 | a2 | a3 | a4 | a5 | a6 | a7 | a8 | a9 | a10 | a11 | a12 | a13 | a14 | a15 | a16 | a17 |
| Parameters | Experimental (block 2) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E18 | E19 | E20 | E21 | E22 | E23 | E24 | E25 | E26 | E27 | E28 | E29 | E30 | E31 | E32 | E33 | E34 | |
| Incubation in days (I) | 0 | + | + | + | + | − | − | + | + | − | − | + | − | − | − | − | + |
| Temperature (T) | 0 | + | − | + | + | − | + | − | − | + | + | − | − | + | − | − | + |
| pH (P) | 0 | + | − | − | + | + | + | + | + | − | + | − | − | − | + | − | − |
| Liquid–solid ratio (R) | 0 | + | + | − | − | + | + | + | − | + | − | − | − | − | − | + | + |
| Spore concentration (S) | 0 | + | + | + | − | + | − | − | + | + | + | − | + | − | − | − | − |
| Response (lipase activity) | a18 | a19 | a20 | a21 | a22 | a23 | a24 | a25 | a26 | a27 | a28 | a29 | a30 | a31 | a32 | a33 | a34 |
(0) search level, (+) higher level, (−) lower level
Calculations of Interaction Effects of Process Parameters
For a five variable system, the effects of individual parameters are termed as zero order interactions and the number of such zero order interactions is estimated as 5C1. For a five variable system, the total number of interactions (N):
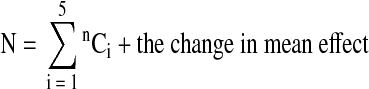 |
The effects of individual as well as interaction parameters were evaluated based on the average values of two-cycle responses. The calculations of the effects were made based on the theory of fractional factorials [17, 19, 20].
Any individual or interaction effect was estimated from the following generalized form:
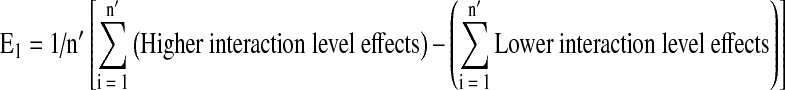 |
The change in mean effects of all the experimental conditions was expressed in generalized form in the following equation:
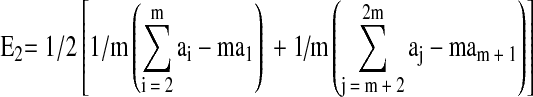 |
where, ai are the responses, E1 is the effect; E2 is change in mean effects; n′ is the number of higher or lower interaction level effects; m is the number of new experiments in block 1 or block 2.
σ (standard deviation) = (σ1 + σ2)/2, where, σ1 = R1f and σ2 = R2f; R1 = (largest difference − smallest difference) in block 1; R2 = (largest difference − smallest difference) in block 2; f (statistical constant) = 0.3 for number of cycles (n″) = 2 and number of new experiments per cycle (k) = 32.
Error limits:
For average: ±2σ/√n″
For effects: ±0.712 × √n″ × σ
For change in mean: ±0.632 × √n″ × σ
Decision Making Procedure
To examine whether any change in the control (search level) experimental conditions would help to improve the response (i.e. lipase activity) and if so, which would be the desired direction of change; the magnitudes of the effects were compared with that of the error limits [15]. The direction of change of a variable has been described in the literature [16] which can be summarized as follows:
To maximize the response, increase of variable would help if effect was positive and large compared to error limit and change in mean effect was small.
To maximize the response, reduction of variable would help if effect was negative and large compared to error limit and change in mean effect was small.
If the effects were negative or positive and smaller than the error limit and the change in mean effect was also small, then it would be advisable to select a new search region and start a new phase of experiments.
If the effects were small compared to error limit while change in mean effect was large and negative, the maximum was reached and the EVOP program ends.
Results and Discussion
EVOP-Factorial Design for Optimization of the Process Parameters
The present investigation deals with optimization of five variables (process parameters) namely, incubation period (I), liquid–solid ratio (R), fermentation temperature (T), initial fermentation pH (P) and spore concentration (S) for maximum lipase production through SSF using wheat bran and locally available mahua oil as substrate and inducer respectively. The average values of lipase activities (response) of the two cycles for each new experiments of the first phase were represented in Tables 2 and 3.
Table 2.
Experimental conditions and results of phase I (block 1: E1–E17)
| Parameters | Experimental block 1 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | E9 | E10 | E11 | E12 | E13 | E14 | E15 | E16 | E17 | |
| Incubation in days (I) | 6 | 7 | 7 | 7 | 7 | 5 | 7 | 7 | 5 | 5 | 5 | 7 | 5 | 7 | 5 | 5 | 5 |
| Temperature in °C (T) | 30 | 35 | 35 | 35 | 25 | 35 | 35 | 25 | 35 | 25 | 35 | 25 | 35 | 25 | 25 | 25 | 25 |
| pH (P) | 5.5 | 6.0 | 6.0 | 5.0 | 6.0 | 6.0 | 5.0 | 6.0 | 5.0 | 6.0 | 5.0 | 5.0 | 6.0 | 5.0 | 5.0 | 6.0 | 5.0 |
| Liquid to solid (R) (x:1) | 1.0 | 1.5 | 0.5 | 1.5 | 1.5 | 1.5 | 0.5 | 0.5 | 1.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1.5 | 1.5 | 1.5 | 0.5 |
| Spore concentration (spores/ml) (S) | 108 | 107 | 109 | 109 | 109 | 109 | 107 | 107 | 107 | 109 | 109 | 109 | 107 | 107 | 109 | 107 | 107 |
| Cycle I–cycle II difference of lipase activity (U/gds) | −1.15 | −2.33 | −2.37 | 2.12 | 0.63 | 2.11 | −2.61 | 3.10 | 3.46 | −2.17 | 1.16 | 1.58 | 1.67 | −3.33 | −3.17 | −2.77 | −3.12 |
| Responses lipase activity (U/gds) | 22.73 | 28.61 | 19.27 | 31.11 | 27.12 | 70.81 | 22.82 | 47.25 | 66.27 | 40.22 | 46.81 | 39.10 | 43.53 | 23.31 | 27.81 | 22.65 | 16.43 |
Table 3.
Experimental conditions and results of phase I (block 2: E18–E34)
| Parameters | Experimental block 2 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E18 | E19 | E20 | E21 | E22 | E23 | E24 | E25 | E26 | E27 | E28 | E29 | E30 | E31 | E32 | E33 | E34 | |
| Incubation in days (I) | 6 | 7 | 7 | 7 | 7 | 5 | 5 | 7 | 7 | 5 | 5 | 7 | 5 | 5 | 5 | 5 | 7 |
| Temperature in °C (T) | 30 | 35 | 25 | 35 | 35 | 25 | 35 | 25 | 25 | 35 | 35 | 25 | 25 | 35 | 25 | 25 | 35 |
| pH (P) | 5.5 | 6.0 | 5.0 | 5.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 5.0 | 6.0 | 5.0 | 5.0 | 5.0 | 6.0 | 5.0 | 5.0 |
| Liquid to solid ratio (R) (x:1) | 1.0 | 1.5 | 1.5 | 0.5 | 0.5 | 1.5 | 1.5 | 1.5 | 0.5 | 1.5 | 0.5 | 10.5 | 0.5 | 0.5 | 0.5 | 1.5 | 1.5 |
| Spore concentration (spores/ml) (S) | 108 | 109 | 109 | 109 | 107 | 109 | 107 | 107 | 109 | 109 | 109 | 107 | 109 | 107 | 107 | 107 | 107 |
| Cycle I–cycle II difference of lipase activity (U/gds) | 1.22 | 2.55 | −2.76 | −2.54 | 3.26 | −2.72 | 1.13 | −2.83 | 2.47 | −2.47 | 3.27 | 2.80 | 3.21 | −2.12 | 1.77 | 2.37 | 3.21 |
| Responses lipase activity (U/gds) | 24.12 | 32.53 | 41.16 | 24.11 | 31.27 | 40.62 | 72.95 | 44.53 | 46.21 | 66.23 | 59.67 | 35.73 | 21.65 | 39.45 | 42.82 | 46.13 | 39.12 |
The effects of individual as well as interaction of the process variables were evaluated from the average values of two-cycle responses. The calculation worksheet for all the individual and interaction effects and change in mean of the five variable system has been given in Table 6 and the standard deviation and error limits for effects is presented in Table 7.
Table 6.
Calculation of effects of phase I and phase II
| Phase I | Phase II | |
|---|---|---|
| Effects of incubation period (I) | −11.92 | 0.32 |
| Effects of temperature (T) | 8.24 | 1.72 |
| Effects of pH (P) | 5.18 | −0.48 |
| Effects of liquid to solid ratio (R) | 6.54 | −0.09 |
| Effects of spore concentration (S) | 0.72 | −0.58 |
| Effects of incubation period × temperature (IT) | −17.68 | 1.27 |
| Effects of incubation period × pH (IP) | −2.63 | 4.52 |
| Effects of incubation period × liquid to solid ratio (IR) | −6.32 | 3.04 |
| Effects of incubation period × spore concentration (IS) | −2.23 | −0.63 |
| Effects of temperature × pH (TP) | −2.34 | 1.80 |
| Effects of temperature × liquid to solid ratio (TR) | 8.55 | −1.24 |
| Effects of temperature × spore concentration(TS) | 0.09 | −0.96 |
| Effects of liquid to solid ratio × pH (RP) | −5.34 | 2.01 |
| Effects of pH × spore concentration (PS) | −0.36 | 0.06 |
| Effects of liquid to solid ratio × spore concentration (RS) | −1.49 | 0.56 |
| Effects of incubation period × temperature × pH (ITP) | −1.57 | −2.30 |
| Effects of incubation period × temperature × Liquid to solid ratio (ITR) | −0.29 | 0.83 |
| Effects of incubation period × temperature × spore concentration (ITS) | −2.29 | −0.27 |
| Effects of pH × liquid to solid ratio × incubation period (PRI) | 2.32 | −0.15 |
| Effects of pH × spore concentration × incubation period (PSI) | −4.76 | 2.24 |
| Effects of liquid to solid ratio × incubation period × spore concentration (IRS) | 2.09 | −1.58 |
| Effects of temperature × pH × liquid to solid ratio (TPR) | 3.04 | −0.82 |
| Effects of temperature × pH × spore concentration (TPS) | 1.03 | −1.26 |
| Effects of liquid to solid ratio × temperature × spore concentration (RTS) | −0.89 | 2.40 |
| Effects of liquid to solid ratio × pH × spore concentration (RPS) | 1.72 | −1.30 |
| Effects of incubation period × temperature × pH × liquid to solid ratio (ITPR) | −3.20 | −0.62 |
| Effects of incubation period × temperature × pH × spore concentration (ITPS) | 3.76 | −1.79 |
| Effects of incubation period × temperature × liquid to solid ratio × spore concentration (ITRS) | 1.95 | −0.61 |
| Effects of incubation period × pH × liquid to solid ratio × spore concentration (IPRS) | −2.43 | −0.05 |
| Effects of liquid to solid ratio × temperature × pH × spore concentration (RTPS) | 0.07 | 2.51 |
| Effects of incubation period × temperature × pH × liquid to solid ratio × spore concentration (ITPRS) | 6.94 | −4.61 |
| Change in mean effect | 15.86 | −50.75 |
Table 7.
Standard deviation and error limits for effects and change in mean of phase I and phase II
| Phase I | Phase II | |
|---|---|---|
| Standard deviation | 2.08 | 2.10 |
| Error limits | ||
| For averages | ±2.95 | ±2.97 |
| For effects | ±2.09 | ±2.11 |
| For change in mean effect | ±1.86 | ±1.87 |
From the differences and averages of lipase activities of the two cycles, standard deviation and error limits (based on a 95% confidence level) were estimated (Table 7) according to the relationship given in the literature [21, 22]. Results of changes made in a judicious manner were analyzed by statistical principles to see if the variation in response i.e. lipase activity was due to the mere change (i.e. inherent variation) or due to significant effect of change in the process variables on the response.
Individual effects, interaction effects and change in mean effects were calculated (according to the relationship given in “Calculations of Interaction Effects of Process Parameters” section) and presented in Tables 6. Analysis of results showed that the change in mean effect in the first phase was large and positive (15.86) (Table 6, phase I) compared to the error limits (Table 7, phase I), which implied that the maximum response was not reached. Further, effects of T, P and R were large and positive while the effect of I was large and negative (Table 7, phase I) compared to the error limits (Table 7, phase I). And the effect of S was within the error limits. So according to the first and the second rules of decision-making procedure (“Decision Making Procedure” section), a second phase of experiments were performed where T, P and R were increased, I was decreased and S was kept unchanged in order to approach the optimum. The experimental conditions and results of phase II have been presented in Tables 4 and 5.
Table 4.
Experimental conditions and results of phase II (block 1: E1–E17)
| Parameters | Experimental block 1 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | E9 | E10 | E11 | E12 | E13 | E14 | E15 | E16 | E17 | |
| Incubation in days (I) | 5 | 6 | 6 | 6 | 6 | 4 | 6 | 6 | 4 | 4 | 4 | 6 | 4 | 6 | 4 | 4 | 4 |
| Temperature in °C (T) | 35 | 40 | 40 | 40 | 30 | 40 | 40 | 30 | 40 | 30 | 40 | 30 | 40 | 30 | 30 | 30 | 30 |
| pH (P) | 6 | 6.5 | 6.5 | 5.5 | 6.5 | 6.5 | 5.5 | 6.5 | 5.5 | 6.5 | 5.5 | 5.5 | 6.5 | 5.5 | 5.5 | 6.5 | 5.5 |
| Liquid to solid ratio (R) (x:1) | 1.5 | 2.0 | 1.0 | 2.0 | 2.0 | 2.0 | 1.0 | 1.0 | 2.0 | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 | 2.0 | 2.0 | 1.0 |
| Spore concentration (spores/ml) (S) | 108 | 107 | 109 | 109 | 109 | 109 | 107 | 107 | 107 | 109 | 109 | 109 | 107 | 107 | 109 | 107 | 107 |
| Cycle I–cycle II difference of lipase activity (U/gds) | −2.14 | 1.12 | −2.88 | 2.32 | 2.3 | 1.41 | 1.60 | 3.57 | 1.22 | 1.38 | 0.94 | 2.94 | −3.37 | 3.18 | 0.64 | 1.08 | 2.36 |
| Responses lipase activity (U/gds) | 78.3 | 34.32 | 28.18 | 27.58 | 31.29 | 28.37 | 27.17 | 19.26 | 23.91 | 25.18 | 32.08 | 21.95 | 33.9 | 27.37 | 33.96 | 28.45 | 33.93 |
Table 5.
Experimental conditions and results of phase II (block 2: E18–E34)
| Experimental block 2 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E18 | E19 | E20 | E21 | E22 | E23 | E24 | E25 | E26 | E27 | E28 | E29 | E30 | E31 | E32 | E33 | E34 | |
| Incubation in days (I) | 5 | 6 | 6 | 6 | 6 | 4 | 4 | 6 | 6 | 4 | 4 | 6 | 4 | 4 | 4 | 4 | 6 |
| Temperature in °C (T) | 35 | 40 | 30 | 40 | 40 | 30 | 40 | 30 | 30 | 40 | 40 | 30 | 30 | 40 | 30 | 30 | 40 |
| pH (P) | 6 | 6.5 | 5.5 | 5.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 5.5 | 6.5 | 5.5 | 5.5 | 5.5 | 6.5 | 5.5 | 5.5 |
| Liquid to solid ratio (R) (x: 1) | 1.5 | 2.0 | 2.0 | 1.0 | 1.0 | 2.0 | 2.0 | 2.0 | 1.0 | 2.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 | 2.0 |
| Spore concentration (spores/ml) (S) | 108 | 109 | 109 | 109 | 107 | 109 | 107 | 107 | 109 | 109 | 109 | 107 | 109 | 107 | 107 | 107 | 107 |
| Cycle I–cycle II difference of lipase activity (U/gds) | 3.12 | −2.14 | 1.80 | −2.86 | 3.71 | 1.86 | 2.17 | −0.78 | 3.24 | −3.33 | 2.32 | 2.24 | 1.31 | −2.80 | 2.42 | −2.46 | 0.80 |
| Responses lipase activity (U/gds) | 75.70 | 27.34 | 17.34 | 22.36 | 27.58 | 16.11 | 20.76 | 29.10 | 27.82 | 23.10 | 21.72 | 22.63 | 31.00 | 29.23 | 16.78 | 24.13 | 26.17 |
In the phase II it was found that the change in mean effect (−50.75) was large and negative (Table 6, phase II). Further, most of the effects (Table 6, phase II) were also less than the error limits (Table 7, phase II) and the five-variable interaction effect (−4.61) was small compared to the error limits. So as per the fourth rule of the decision-making procedure (“Decision making Procedure” section), maximum lipase activity was achieved with the search region as E1 and E18 of phase II. The optimum levels of R, T, I, P and S were 1.5:1, 35°C, 5 days, 6.0 and 108 spores/ml respectively for obtaining a maximum lipase activity of ~77 U/gds. ul-Haq et al. [11] has reported maximumlipase activity of 48 U gds−1 by Rhizopus oligosporus GCBR-3 under SSF using almond meal as the substrate, where as in the present study the activity was increased to 77 U gds−1 by optimizing the physical factors affecting the lipase production by the same organism using EVOP factorial design technique.
Conclusion
The results of present study showed efficient use of wheat bran, a cheap agro-residue as substrate for SSF and locally available mahua oil as an inducer for lipase production, thus contributing to the reduction in the cost of enzyme production. The statistical approach showed significant improvement in lipase activity by optimizing the process variables for maximal lipase production under SSF conditions using R. oligosporus NRRL 5905. Thus, the achievement of the present study is the successful application of EVOP factorial design as a powerful tool for optimization of the multi-variable biological system of lipase synthesis. On optimization of the process parameters using EVOP factorial design technique, lipase activity increased by around 3 fold from 23 to 77 U gds−1. To the best of our knowledge this is the maximum lipase activity ever reported for R. oligosporus.
Acknowledgements
The authors wish to acknowledge Department of Biotechnology and Council of Scientific and Industrial Research for providing financial support to carry out this work.
References
- 1.Jaeger KE, Reetz TM. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 1998;16:396–403. doi: 10.1016/S0167-7799(98)01195-0. [DOI] [PubMed] [Google Scholar]
- 2.Kazlauskas RJ, Bornscheuer UT. Biotransformations with lipases. In: Rehm HJ, Pihler G, Stadler A, Kelly JW, editors. Biotechnology. New York: VCH; 1998. pp. 37–192. [Google Scholar]
- 3.Jaeger KE, Eggert T. Lipases for biotechnology. Curr Opin Biotechnol. 2002;13:390–397. doi: 10.1016/S0958-1669(02)00341-5. [DOI] [PubMed] [Google Scholar]
- 4.Saxena RK, Ghosh PK, Gupta R, Davidson WS, Bradoo S, Gulati R. Microbial lipases: potential biocatalysts for the future industry. Curr Sci. 1999;77:101–115. [Google Scholar]
- 5.Hölker U, Höfer M, Lenz J. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol. 2004;64:175–186. doi: 10.1007/s00253-003-1504-3. [DOI] [PubMed] [Google Scholar]
- 6.Krishna C. Solid-state fermentation systems—an overview. Crit Rev Biotechnol. 2005;25:1–30. doi: 10.1080/07388550590925383. [DOI] [PubMed] [Google Scholar]
- 7.Hölker U, Lenz J. Solid-state fermentation—are there any biotechnological advantages? Curr Opin Microbiol. 2005;8:301–306. doi: 10.1016/j.mib.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Kohno M, Kugimiya W, Hashimoto Y, Morita Y. Purification, characterization and crystallization of two types of lipase from Rhizopus niveus. Biosci Biotechnol Biochem. 1994;58:1007–1012. doi: 10.1271/bbb.58.1007. [DOI] [PubMed] [Google Scholar]
- 9.Kumar KK, Deshpande BS, Ambedkar SS. Production of extracellular acidic lipase by Rhizopus arrhizus as a function of culture conditions. Hindustan Antibiot Bull. 1993;35:33–42. [PubMed] [Google Scholar]
- 10.Hiol A, Jonzo MD, Rugani N, Druet D, Sarda L, Comeau LC. Purification and characterization of an extracellular lipase from a thermophilic Rhizopus oryzae strain isolated from palm fruit. Enzym Microb Technol. 2000;26:421–430. doi: 10.1016/S0141-0229(99)00173-8. [DOI] [PubMed] [Google Scholar]
- 11.ul-Haq I, Idrees S, Rajoka MI. Production of lipases by Rhizopus oligosporus by solid-state fermentation. Process Biochem. 2002;37:637–641. doi: 10.1016/S0032-9592(01)00252-7. [DOI] [Google Scholar]
- 12.Nahas E. Control of lipase production by Rhizopus oligosporus under various growth conditions. Microbiology. 1988;134:227–233. doi: 10.1099/00221287-134-1-227. [DOI] [Google Scholar]
- 13.Box GEP. Evolutionary operation: A method for increasing industrial productivity. Appl Stat. 1957;6:3–23. doi: 10.2307/2985505. [DOI] [Google Scholar]
- 14.Banerjee R, Bhattacharya BC. Evolutionary operation (EVOP) to optimize protease biosynthesis by Rhizopus oryzae. Bioprocess Eng. 1992;8:151–155. doi: 10.1007/BF01254231. [DOI] [Google Scholar]
- 15.Banerjee R, Bhattacharyya BC. Evolutionary operation (EVOP) to optimize three-dimensional biological experiments. Biotechnol Bioeng. 1993;41:67–71. doi: 10.1002/bit.260410109. [DOI] [PubMed] [Google Scholar]
- 16.Tunga R, Banerjee R, Bhattacharyya BC. Optimization of n variable biological experiments by evolutionary operation-factorial design technique. J Biosci Bioeng. 1999;87:224–230. doi: 10.1016/S1389-1723(99)89017-3. [DOI] [PubMed] [Google Scholar]
- 17.Kar B, Banerjee R, Bhattacharyya BC. Optimization of physicochemical parameters for gallic acid production by evolutionary operation—factorial design technique. Process Biochem. 2002;37:1395–1401. doi: 10.1016/S0032-9592(02)00020-1. [DOI] [Google Scholar]
- 18.Kordel M, Hofmann B, Schomburg D, Schmid RD. Extracellular lipase of Pseudomonas sp. strain ATCC-21808, puritication, characterization, crystallization, and preliminary X-ray diffraction data. J Bacteriol. 1991;173:4836–4841. doi: 10.1128/jb.173.15.4836-4841.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis OL. Design and analysis of industrial experiments. New York: Hafner Publishing Co; 1954. pp. 440–480. [Google Scholar]
- 20.Adler YuP, Markos EV, Granovsky YuV. The design of experiments to find optimal conditions. Moscow: Mir Publishers; 1975. pp. 118–144. [Google Scholar]
- 21.Box GEP, Hunter JS. Condensed calculation for evolutionary operation programs. Technometric. 1959;1:77–95. doi: 10.2307/1266311. [DOI] [Google Scholar]
- 22.Pearson ES, Hartley HO. Biometrika tables for statisticians 1. Cambridge: University Press; 1962. p. 46. [Google Scholar]


