Abstract
Removal of heavy metals (Pb2+, Zn2+) from aqueous solution by dried biomass of Spirulina sp. was investigated. Spirulina rapidly adsorbed appreciable amount of lead and zinc from the aqueous solutions within 15 min of initial contact with the metal solution and exhibited high sequestration of lead and zinc at low equilibrium concentrations. The specific adsorption of both Pb2+ and Zn2+ increased at low concentration and decreased when biomass concentration exceeded 0.1 g l−1. The binding of lead followed Freundlich model of kinetics where as zinc supported Langmuir isotherm for adsorption with their r2 values of 0.9659 and 0.8723 respectively. The adsorption was strongly pH dependent as the maximum lead biosorption occurred at pH 4 and 10 whereas Zn2+ adsorption was at pH 8 and 10.
Keywords: Biosorption, Spirulina sp., Lead, Zinc
Introduction
Environmental pollution due to toxic heavy metals is a significant worldwide problem due to their incremental accumulation in the food chain and continued persistence in the ecosystem [1]. Efforts have been made to remove toxic heavy metal contamination from the environment by using conventional technologies such as ion exchange or limit precipitation, which are sometimes inefficient and expensive, particularly for removal of heavy metal ions lower than 100 mg l−1, and also leads to generation of toxic sludge which further adds burden on the techno-economic feasibility of the treatment process [2, 3].
In recent years, role of microbes in removal of toxic metal ions from the polluted effluent have taken more importance in this area as microorganisms due to their small size have a high surface area-to-volume ratio and therefore provide a large contact area for metal binding [4, 5]. In this race bacteria, algae, fungi, actinomycetes, yeast, activated sludge and various other biopolymers have been well recognized for heavy metal removal directly or indirectly [4, 6–12]. In this context the role of algae is known for a few decades, but has received increased attention only in recent years because of its potential for application in environmental protection or recovery of precious or strategic metals [13–16]. The affinity of various algal species for binding of metal ions shows different hierarchies whereas, in general, metal ions with greater electronegativity and smaller ionic radii are preferably sorbed by algal biomass [16]. Metal accumulation capacity of algal biomass is either comparable or sometimes higher than chemical sorbents therefore algal biomass may serve as an economically feasible and efficient alternative to the existing physicochemical methods for metal removal and recovery from wastewaters. The major challenge in biosorption studies is to select the most promising types of biomass from an extremely large pool of readily available and inexpensive biomaterials [17]. Contribution of Spirulina (family Oscillatoriaceae) in the metal sorption is of considerable importance with adherent advantage of mass cultivation [18, 19].
In the present work, non-living biomass of Spirulina sp. was characterized for its Zn2+ and Pb2+ removal potential from synthetic metal solution whereas, the effect of pH, biomass concentration, initial metal concentration and time was studied for metal sorption process along with equilibrium sorption kinetics.
Materials and Methods
Biosorbent Material
The dry biomass of Spirulina sp. was procured from Global Green Company, Bangalore (India).
Lead and Zinc Sorption Experiments
Metal ion solutions were prepared by diluting 1.0 g l−1 of stock solutions, which were obtained by dissolving weight quantity of zinc sulphate heptahydrate (4.39 gm) and lead nitrate (1.59 gm) of analytical grade in double distilled water. Metal sorption studies were carried out to evaluate the capacity of non-living dry biomass of Spirulina to adsorb metal ions form solution. In batch process 100 ml of synthetic metal solution having different concentrations (20–100 mg l−1) of zinc or lead were taken in 250 ml Erlenmeyer flasks with a range of biomass concentrations (0.1, 0.4, 0.8, 1.6 and 3.2 g) as biosorbent. Erlenmeyer flasks were kept under shaking condition at 100 rpm at ambient temperature. Samples were drawn after 5, 15, 30, 60, 120 and 240 min and filtered and analysed for residual metal concentration (Cf) using atomic absorption spectrophotometer (GBC 932 AA; GBC Scientific Equipment Pvt. Ltd. Australia).
To see the effect of pH on Pb2+ and Zn2+ removal, a range of pH (2, 4, 6, 8 and 10) was adjusted with 0.1 M NaOH and 0.1 M H2SO4 in 100 ml metal synthetic solutions containing fixed concentration of metal at 50 mg l−1 and biomass of 0.1 g followed by contact time of 30 min at 100 rpm.
The metal adsorption (q) by the alga and bioremoval efficiency (R) were calculated by the following formulae [20, 21].
 |
1 |
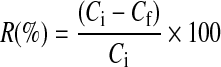 |
2 |
Where q = metal adsorption (mg/g); M = dry mass of alga (g); V = volume of initial metal ion solution used (L); R = bioremoval efficiency (%); Ci = initial concentration of metal in aqueous solution (mg l−1); Cf = final concentration of metal in aqueous solution (mg l−1).
Adsorption Isotherm
During biosorption, a rapid equilibrium is established between absorbed metal ion on the algal cell (q) and unabsorbed metal ions in the solution (Cf). This equilibrium represented by Langmuir and Freundlich adsorption isotherms, are widely used to analyse data for wastewater treatment application [22, 23]. Langmuir equation, which is valid for monolayer sorption onto a surface, a finite number of identical sites are given by Eq. 3.
 |
3 |
Where qmax is the maximum amount of the metal ion per unit weight of alga to form a complete monolayer on the surface bound at high Cf (mg l−1), and b is a constant related to the affinity of the binding sites (l mg−1), qmax represents a practical limiting adsorption capacity when the surface is fully covered with metal ions and assists in the comparison of adsorption performance, particularly in cases where the sorbent did not reach its full saturation in experiments. qmax and b can be determined from the liner plot of Cf/q versus Cf. The empirical Freundlich equation based on sorption on a heterogeneous surface is given below by Eq. 4.
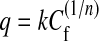 |
4 |
where k and n are the constants, characteristic of the system. k and n are indicators of adsorption capacity and adsorption intensity, respectively Eq. 4 can be linearized in logarithmic form and Freundlich constants can be determined. Freundlich isotherm is also more widely used but provides no information on the monolayer adsorption capacity, in contrast to the Langmuir model.
Results
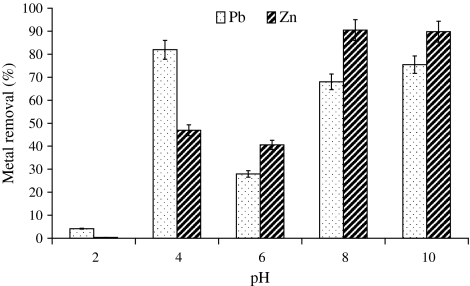
pH is one of the important parameters in metal sorption by Spirulina sp. [24] therefore metal sorption studies were carried out at different pH values. Results revealed maximum biosorption of Pb2+ at pH 4 (82%) and 10 (75%) whereas, Zn2+ demonstrated the same at pH of 8 (90%) and 10 (89%) from synthetic solution containing 50 mg l−1 of metal concentration (Fig. 1). It was observed that Pb2+ and Zn2+ adsorption was less than 5% at pH 2. Biosorption studies carried out for both Pb2+ and Zn2+ in 100 ml solutions containing metals varying from 20 to 100 mg l−1 by 3.2 g of biomass exhibited effective role of initial metal concentration on metal removal. A consistent decrease in metal removal was observed by increasing external metal concentration (Fig. 2) where 90–91% Pb2+ removal efficiency was reported from 20 to 40 mg l−1 Pb2+ containing solution followed by 5% decline in solution containing 100 mg l−1 Pb2+ (Fig. 2). Similarly 35% decrease in Zn2+ removal efficiency was observed by increasing external metal concentration from 40 to 100 mg l−1 Zn2+ with 90% removal from solution containing 20 mg l−1 of Zn2+ (Fig. 2).
Fig. 1.
pH-dependent sorption of Pb2+ and Zn2+ by Spirulina biomass (Ci Pb2+: 20 mg l−1, Zn2+: 20 mg l−1)
Fig. 2.
Initial concentration dependent sorption of Pb+2 and Zn+2 by Spirulina biomass
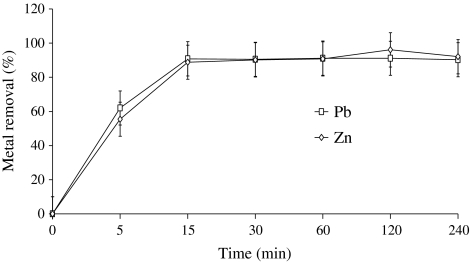
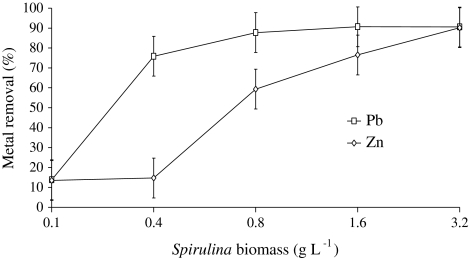
Rapid metal adsorption profile of Spirulina sp. was obtained for both Pb2+ and Zn2+, which is important when the material is to be used for bioremediation. It exhibited rapid biosorption in first 0–15 min by removing 90% Pb2+ and 89% of Zn2+ from metal solutions thereafter increase in metal removal was marginal. Equilibrium was established between absorbed metal ions and the metal ions in the solution after 30 min with maximum removal of 90 and 93% of Pb2+ and Zn2+ (Fig. 3). Increase in metal removal efficiency from 20 mg l−1 synthetic metal solution for both Pb2+ and Zn2+ was observed on increasing biomass concentration (Fig. 4). Pb2+ adsorption was increased by 76.71% by increasing biomass concentration from 0.1 to 3.2 g l−1 from 20 mg l−1 of lead containing synthetic solution whereas, same trend was observed in case of Zn2+ with the increase of 76.73%. Spirulina biomass displayed its equilibrium for Pb2+ removal at 0.8 g l−1 of biomass concentration whereas, a continuous increase in Zn2+ removal on increasing biomass concentration was observed till 3.2 g l−1. To the contrary of metal removal, metal adsorption studies from solution mass balance revealed a decline by 75 and 80% in sorption of Pb2+ and Zn2+ respectively by increasing biomass from 0.1 to 3.2 g l−1 after 15 min of contact time. The results showed that amount of metal ion adsorbed per unit mass of Spirulina (mg g−1), at equilibrium was 48.21 mg g−1 Pb2+ and 8.75 mg g−1 Zn2+ by 0.1 g l−1 of biomass (Table 1).
Fig. 3.
Time dependent removal of Pb2+ and Zn+2 by Spirulina biomass (Ci Pb2+: 20 mg l−1, Zn+2: 20 mg l−1)
Fig. 4.
Effect of Spirulina biomass concentration on Pb2+ and Zn2+ removal. (Ci Pb2+: 20 mg l−1, Zn2+: 20 mg l−1)
Table 1.
Langmuir and Freundlich parameters for the sorption of the test metals by Spirulina sp
| Metal | Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|
| b (1 mg−1) | r2 | qmax (mg g−1) | k(mg1−n/g ln) | n | r2 | |
| Pb2+ | 1.237 | 0.649 | 48.21 | 2.738 | 4.11 | 0.965 |
| Zn2+ | 1.57 | 0.872 | 8.75 | 1.073 | 7.31 | 0.743 |
A linear regression of the experimental results for Pb2+ and Zn2+ differed in terms of Pb2+ sorption fitted better to Freundlich isotherm and Zn2+ to Langmuir isotherms with r2 values of 0.9659 and 0.8723 respectively (Table 1). The maximum adsorption per unit mass of Spirulina (mg g−1), at equilibrium (qmax) for Pb2+ and Zn2+ were calculated to be 48.21 and 8.75 mg g−1 respectively (Table 1).
Discussion
Enhanced adsorption of Pb2+ and Zn2+ ions at higher pH was observed which coincides with earlier findings where in most cases the removal efficiency increased steadily with rise in pH [9]. The adsorption of metal ions was lower at low pH because of high concentration of protons in the solution which competed with metal ions in forming a bond with the active sites on the surface of the algal biomass [25]. Selective sorption of specific metals due to distinct pH optima for their sorption may be due to differences in chemical composition of cell surface [16, 26]. A distinct relationship between pH of aqueous metal solution and involvement of functional group in binding of Pb2+ onto Spirulina maxima was observed at pH range of 2–5, 5–9 and 9–12 with the involvement of functional groups such as carboxyl, carboxyl and phosphate and carboxyl, phosphate and hydroxyl respectively [27]. Rapid metal adsorption profile of Spirulina sp. was obtained for both Pb2+ and Zn2+, which is important when the material is to be used for bioremediation. It exhibited rapid biosorption in first 0–15 min by removing 90% Pb2+ and 89% of Zn2+ from metal solutions thereafter increase in metal removal was marginal [19]. Decrease in metal removal on increasing initial metal concentration was supported by the findings of Mehta and Gaur [28] who observed that the removal of metal generally decreases with increasing concentration of metals in the solution. Algal cell surface has different functional groups with varying affinity for ionic species, where low and high affinity of functional groups in sorption of metal ions also depends upon concentration of metal ions therefore decline in metal removal is largely attributed to saturation of adsorption sites [19].
Spirulina biomass showed rapid biosorption in first 15 min, similar to the findings reported by Incharoensakdi and Kitjaharn [6] for the rapid adsorption of Zn+2 by Aphanotheca halophytica from aqueous solution. It has been reported that the sorption of heavy metal ions by algae followed a two-step mechanism where the metal ion is physically or chemically taken up onto the surface of the algal cell before being taken up biologically into the cell [29, 30]. The first step, known as a passive uptake which occurs rapidly, while the second biological step or active transport could take much longer time to complete. In this case, since the alga was dried and biological functions were no longer active, the sorption could only take place on the cell surface. The increase in Pb2+ adsorbed by increasing biomass was also expected as a result of increase in available area-to-volume ratio and therefore providing a large contact area for metal binding [4, 5]. In a similar study, 82.64% decrease in Pb2+ sorption capacity of Spirulina maxima with increasing biomass from 0.1 to 20 g l−1 was reported although this is generally attributed to a shift in the sorption equilibrium [18]. The other probable explanation for such a relationship between biomass concentration and adsorption may be limited availability of metal, increased electrostatic interactions between binding sites and reduced mixing at higher biomass concentration [16].
The sorption isotherm is the relationship between equilibrium concentration of solute in the solution and equilibrium concentration of solute in the sorbent at constant temperature where either Freundlich or Langmuir model can describe the passive biosorption equilibrium of zinc and lead [22, 23]. An extremely high r2 value of Freundlich isotherm for Pb2+ sorption indicated that ion exchange interaction takes place between metal ion and the biosorbent where as, Zn2+ follows Langmuir isotherm and thus supported physico–chemical interactions [12]. qmax for Pb2+ and Zn2+ adsorption by Spirulina sp. were 48.21 and 8.75 mg g−1 respectively (Table 1), which is higher than earlier report of Sandau and his co-workers who observed 16.98 and 9.58 mg g−1 of Pb2+ and Zn2+ adsorption respectively by Spirulina platensis [31].
Acknowledgments
The authors thank the Director, Thapar University, Patiala, India, for providing the infrastructure and facilities and to University Grant Commission for providing the financial support vide Approval F-3-16/2002 (SR-II), dated March 16, 2002.
List of symbols
Variables
- Q
Metal ion adsorbed per unit mass of Spirulina (mg g−1), at equilibrium
- V
Volume of solution (l)
- Ci
Initial concentration of metal ions in solution (mg l−1)
- Cf
Final concentration of metal ions in solution (mg l−1)
- M
Mass of biomass (g)
- qmax
Langmuir parameter, maximum theoretical adsorption upon complete saturation of the surface (mg g−1)
- b
Langmuir constant related to the energy of adsorption desorption (l mg−1)
- k
Freundlich constant related to the strength of the adsorptive bond (mg1−n/g ln)
- n
Freundlich constant related to bond distribution
References
- 1.Dudka S, Miller WP. Accumulation of potentially toxic elements in plant and their transfer to human food chain. J Environ Sci Heal B. 1999;34:681–708. doi: 10.1080/03601239909373221. [DOI] [PubMed] [Google Scholar]
- 2.Volesky B. Advances in biosorption of metals: selection of biomass types. FEMS Microbiol Rev. 1994;14:291–302. doi: 10.1111/j.1574-6976.1994.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 3.Ahluwalia SS, Goyal D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol. 2007;98:2243–2257. doi: 10.1016/j.biortech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Kim DW, Che DK, Wang J, Huang CP. Heavy metal removal by activated sludge: influence of Nocardia amarae. Chemosphere. 2002;46:137–142. doi: 10.1016/S0045-6535(00)00598-1. [DOI] [PubMed] [Google Scholar]
- 5.Zouboulis AI, Loukidou MX, Matis KA. Biosorption of toxic metals from aqueous solution by bacterial strains isolated from metal-polluted soils. Process Biochem. 2004;39:909–916. doi: 10.1016/S0032-9592(03)00200-0. [DOI] [Google Scholar]
- 6.Incharoensakdi A, Kitjaharn P. Zinc biosorption from aqueous solution by halotolerant cyanobacterium Aphanotheca halophytica. Curr Microbiol. 2002;45:261–264. doi: 10.1007/s00284-002-3747-0. [DOI] [PubMed] [Google Scholar]
- 7.Yan G, Viraraghavan T. Heavy-metal removal from aqueous solution by fungus Mucor rouxii. Water Res. 2003;37:4486–4496. doi: 10.1016/S0043-1354(03)00409-3. [DOI] [PubMed] [Google Scholar]
- 8.Oswald WJ. My sixty years in applied algology. J Appl Phycol. 2003;15:99–106. doi: 10.1023/A:1023871903434. [DOI] [Google Scholar]
- 9.Pavasant P, Apiratikul R, Sungkhum V, Suthiparinyanont P, Wattanachira S, Marhaba TF. Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour Technol. 2006;97:2321–2329. doi: 10.1016/j.biortech.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Polti MA, Amaroso MJ, Abate CM. Chromium(IV) resistance and removal by actinomycete strains isolated from sediments. Chemosphere. 2007;67:660–667. doi: 10.1016/j.chemosphere.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Xiong J, He Z, Liu D, Mahmood Q, Yang X. The role of bacteria in the heavy metals removal and growth of Sedum alfredii Hance in an aqueous medium. Chemosphere. 2008;70:489–494. doi: 10.1016/j.chemosphere.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Ahluwalia SS, Goyal D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol. 2007;98:2243–2257. doi: 10.1016/j.biortech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Dönmez G, Aksu Z. Removal of chromium(VI) from saline wastewaters by Dunaliella species. Process Biochem. 2002;38:751–762. doi: 10.1016/S0032-9592(02)00204-2. [DOI] [Google Scholar]
- 14.Davis TA, Volesky B, Mucci A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003;37:4311–4330. doi: 10.1016/S0043-1354(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 15.Chojnacka K, Chojnacki A, Górecka H. Trace element removal by Spirulina sp. from copper smelter and refinery effluents. Hydrometallurgy. 2004;73:137–153. doi: 10.1016/j.hydromet.2003.10.003. [DOI] [Google Scholar]
- 16.Mehta SK, Gaur JP. Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit Rev Biotechnol. 2005;25:113–152. doi: 10.1080/07388550500248571. [DOI] [PubMed] [Google Scholar]
- 17.Kratochvil D, Volesky B. Advances in the biosorption of heavy metals. Trends Biotechnol. 1998;16:291–300. doi: 10.1016/S0167-7799(98)01218-9. [DOI] [Google Scholar]
- 18.Gong R, Ding Y, Liu H, Chen Q, Liu Z. Lead biosorption and desorption by intact and pretreated Spirulinamaxima biomass. Chemosphere. 2001;58:125–130. doi: 10.1016/j.chemosphere.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 19.Hong C, Shan-shan P. Bioremediation potential of Spirulina toxicity and biosorption studies of lead. J Zhejiang Univ Sci B. 2005;6:171–174. doi: 10.1631/jzus.2005.B0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volesky B. Removal of heavy metals by biosorption. In: Ladisch MR, Bose A, editors. Harnessing biotechnology for the 21st Century. Washington DC: ACS Publications; 1992. pp. 462–466. [Google Scholar]
- 21.Zhang L, Zhao L, Yu Y, Chen C. Removal of Pb2+ from aqueous solution by non-living Rhizopus nigricans. Water Res. 1998;32:1437–1444. doi: 10.1016/S0043-1354(97)00348-5. [DOI] [Google Scholar]
- 22.Langmuir I. The adsorption of gases in plain surface of glass, mica, and platinum. J Am Chem Soc. 1918;40:1361–1403. doi: 10.1021/ja02242a004. [DOI] [Google Scholar]
- 23.Freundlich H. Uber die adsorption in lousumgen. Z F Phys Chem. 1907;57:385–471. [Google Scholar]
- 24.Solisio C, Lodi A, Soletto D, Converti A. Cadmium biosorption on Spirulina platensis biomass. Bioresour Technol. 2008;99:5933–5937. doi: 10.1016/j.biortech.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Parent L, Campbell PGC. Aluminium bioavailability to the green alga Chlorella pyrenoidosa in acidified synthetic soft water. Environ Toxicol Chem. 1994;13:587–598. [Google Scholar]
- 26.Aksu Z, Kutsal T. Comparative study for biosorption characteristic of Zn ion with C. vulgaris. Environ Technol. 1990;11:979–987. doi: 10.1080/09593339009384950. [DOI] [Google Scholar]
- 27.Chojnacka K, Chojnacki A, Górecka H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue-green algae Spirulina sp: kinetics, equilibrium and the mechanism of the process. Chemosphere. 2005;59:75–84. doi: 10.1016/j.chemosphere.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Mehta SK, Gaur JP. Removal of Ni and Cu from single and binary metal solution by free and immobilized Chlorella vulgaris. Eur J Protistol. 2001;37:261–271. doi: 10.1078/0932-4739-00813. [DOI] [Google Scholar]
- 29.Volesky B. Removal and recovery of heavy metals by biosorption. In: Volesky B, editor. Biosorption of heavy metals. Boca Raton: CRC Press; 1990. pp. 7–43. [Google Scholar]
- 30.Kojima H, Lee KY. Photosynthetic microorganisms in environmental biotechnology. Hong Kong: Springer-Verlag; 2001. [Google Scholar]
- 31.Sandau E, Sandau P, Pulz O. Heavy metal sorption by microalgae. Acta Biotechnol. 1996;16:227–235. doi: 10.1002/abio.370160402. [DOI] [Google Scholar]