Abstract
Two plant growth promoting rhizobacteria––Sinorhizobium meliloti RMP1 and Pseudomonas aeruginosa GRC2 were studied for integrated nutrient management to obtain improved yield of Brassica juncea. Low concentrations of urea and diammonium phosphate (DAP) stimulated the growth of both S. meliloti RMP1 and P. aeruginosa GRC2. 1 M of urea and 0.35 M of DAP was found lethal for RMP1, while 1.3 M and 0.37 M concentrations of urea and DAP proved to be toxic for GRC2. Lc50 was observed as 0.49 M of urea and 0.15 M of DAP for RMP1, and 0.66 M urea and 0.18 M of DAP for GRC2. Urea and DAP adaptive variants of RMP1 and GRC2 was isolated. Adaptive bacterial variants had better growth rates at sub-lethal (Lc50) concentrations of urea and DAP as compared to non-adaptive variants. They also retained plant growth promoting attributes similar to non adaptive variants. GRC2 and RMP1 did not affect the growth of each other and were chemotactically active for DAP, urea as well as root exudates of B. juncea. Both the isolates colonized well in the rhizosphere of B. juncea, as their populations were recorded ≈5 log10 cfu g−1 after 120 days. Interestingly, the colonization ability was found even better when both strains were co-inoculated, as their population was recorded in the range of ≈6 log10 cfu g−1 after 120 days. In field trials, application of RMP1 and GRC2 resulted in significant increase in biomass and yield of B. juncea as compared to control. However, yield was better with application of half dose and full dose of recommended fertilizers. Interestingly, the biomass as well as yield improved further when both isolates were applied together along with half dose of recommended fertilizers.
Keywords: Sinorhizobium meliloti, Pseudomonas aeruginosa, Urea, Diammonium phosphate
Introduction
The modern cultivation practices exploit millions of ton of chemicals in the form of inorganic fertilizers and pesticides along with various adjuvants, solvents, carriers and diluents, which are applied to the soil or sprayed on the plants. Excessive use of these chemicals exerts deleterious effects on soil microorganism, and also affects the fertility status of soil and pollutes environment [1]. Chemicals used to control pathogens disturb environment, subvert ecology, degrade soil productivity and mismanage water resources [2]. Moreover, because of induction of chemical resistance by fungicides in fungal plant pathogens and non-target side effects on other plant pathogens, alternatives are required to substitute chemicals with bacterial fertilizers and bio-pesticides [3]. A significant alternative is the use of microorganisms in biological control as non-hazardous strategy [4]. Plant growth-promotion and biological control of soil borne pathogens with plant growth-promoting rhizobacteria (PGPR) has been intensively investigated [5].
The Brassica oil seed crops including Brassica juncea, B. napus, B. rapa and B. campestris are the world’s third most important source of oil seeds and edible oil [6]. Presently, chemical fertilizers such as urea (N-46%) and diammonium phosphate (DAP; N-18% and P-46%) are applied as source of nitrogen and phosphorus, however, their utilization efficiency remains low in the farmer’s field due to loss by volatilization, denitrification, leaching, and conversion into unavailable forms. In the last decade, integrated use of chemical and biofertilizers for improving crop productivity and improvement the soil fertility for sustainable crop production has gained significant importance [7]. To attain this, an approach of blending chemical fertilizers with chemical adaptive bacterial strains derives the synergistic benefits [8].
Pertaining to these facts, in present study, two rhizospheric isolates Sinorhizobium meliloti RMP1 and Pseudomonas aeruginosa GRC2 were studied for their potential for integrated nutrient management studies to improve growth and yield of B. juncea and reduce use of chemicals. These isolates are well known for their plant growth-promoting (PGP) activities [5, 9]. RMP1 is known to produce siderophore, indole acetic acid (IAA), solubilzed insoluble phosphate and inhibited growth of charcoal rot disease causing Macrophomina phaseolina, while GRC2 is known to have hydrocyanic acid (HCN) and chitinase production ability. GRC2 is also antagonistic to Macrophomina phaseolina, Fusarium oxysporum and Sclerotia sclerotiorum [5]. Further, to substantiate applicability of Sinorhizobium meliloti RMP1 and Pseudomonas aeruginosa GRC2 in rhizosphere, their chemotactic behaviour towards two chemical fertilizers—urea and diammonium phosphate (DAP) was evaluated. Also, they were applied in field along with reduced dose of fertilizers, as individual trials or in co-inoculated conditions. The competence of RMP1 and GRC2 in B. juncea rhizosphere was also experimented.
Materials and Methods
Microorganisms
The rhizobacterial strains viz., S. meliloti RMP1 and P. aeruginosa GRC2 were obtained from departmental culture collection, Department of Botany and Microbiology, Gurukul Kangri University, Hardwar (India). S. meliloti RMP1 and P. aeruginosa GRC2 were maintained on yeast extract mannitol (YEM) agar medium and Kings’ B agar medium. Both strains were stored at −20°C in 20% glycerol until mentioned otherwise.
Isolation of Urea and DAP Adaptive Variants of S. meliloti RMP1 and P. aeruginosa GRC2
The lethal concentrations of urea and DAP on bacterial strains were determined by growing them in medium having different concentrations of urea (0.01–1.5 M) and DAP (0.01–1.00 M). For this, YEM broth was used for S. meliloti and King’s B broth for P. aeruginosa GRC2. The log phase cultures (108 cells ml−1) were transferred under aseptic conditions to the respective supplemented medium and incubated at 28°C 150 rpm for 48 h. Optical density was measured at 610 nm after every 6 h interval. Growth stimulatory, sub-lethal (Lc50) and lethal (Lc100) concentrations of urea and DAP were determined by calculating specific growth rate and, growth rate of control/specific growth rate of treatment (Vo/V) as described earlier by Maheshwari and Saraf [10]. The adaptive variants of both RMP1 and GRC-2 were raised against the sub-lethal concentrations (Lc50) of urea and DAP by transferring the surviving colonies on growth medium and medium supplemented with sub-lethal (Lc50) concentrations of urea and DAP, respectively [11]. The plant growth-promoting activities of chemical fertilizer adaptive variants of strains RMP1 and GRC2 were examined as described earlier [5, 9].
Interaction Study Between Strains RMP1 and GRC2, and Preparation of Root Exudates
Effect of interaction between the strains RMP1 and GRC2 on their growth rates was determined according to Sindhu et al. [12]. Supernatants of log phase cultures (108cells ml−1) of RMP1 and GRC2 were prepared by centrifuging the late-log phase cultures at 12,000×g for 20 min. The supernatants were passed through membrane filter (0.45 μM). The Whatman filter paper discs (1 cm) soaked in supernatant of GRC2 were placed on YEM plates pre-inoculated with RMP1 and disc soaked with supernatant of RMP1 was placed on King’s B solidified plates pre-inoculated with GRC2. The inhibition of growth around the filter disc was assessed and recorded after every 6 h of incubation at 28°C, in form of zone of inhibition (if any). For preparation of root exudates, the seeds were surface sterilized with 0.5% sodium hypochlorite (NaOCl) for 5 min and soaked in 0.75% H2O2 for 3 min. Germinated seeds with root length of about 5–6 mm were transferred to sterilized glass tubes (170 mm length, 30 mm diameter) containing 20 ml of sterile half strength Knop’s solution [13]. Contamination free root exudates solutions were stored at −20°C for further use.
Chemotactic Behavior of Strains for DAP, Urea and Root Exudates
The chemotactic behavior of the strains RMP1 and GRC2 towards root exudates of B. juncea, urea and DAP was determined by capillary assay method [14]. The appropriate concentrations (equal to Lc50) of urea and DAP were added to the buffer and filled into the capillaries, inserted into the cell suspensions of both adaptive variants (106–107 cells ml−1 of buffer) on a glass slide. Root exudates were used in their original concentrations for capillary assay. The cells in the capillaries were plated on to the YEM agar and King’s B agar media for RMP1 and GRC2, respectively after every 30 min of incubation at 28°C, and incubated for 24–48 h at 28°C, after which the total numbers of colonies were scored. Aspartic acid (200 μM) was experimented as positive control while capillary tubes containing buffer alone served as negative control. The chemotaxis index (C.I.) was determined according to Lopez-de-Victoria and Lowell [15], as the ratio of number of bacterial cells accumulated in the test capillaries containing either urea, DAP or root exudate with respect to control.
Seed Bacterization
Method of Weller and Cook [16] was used for seed bacterization. Bacterial strains RMP1 and GRC2 were grown in YEM broth and King’s B broth, respectively for 48 h at 28°C in a bioreactor (BIOFRRM-L Scigenics India Pvt. Ltd.). Both the cultures were centrifuged at 7100×g for 15 min at 4°C. The culture supernatants were discarded and pellets were washed and resuspended in sterile distilled water (SDW) to get final bacterial population density of approximately 108cells ml−1. The cell suspensions of strains RMP1 and GRC2 (1:1) was mixed with 1% carboxymethylcellulose (CMC) solution to form slurry coated on the surface of seeds, as described earlier [5]. Seeds of B. juncea coated with 1% CMC slurry without bacterial strains served as control.
Field Trials
Field trials were carried out in district Haridwar, India (29°66′ 40″N lat., 78°13′E long.) in sandy loam soil (77.3% sand, 13.6% silt, 11.7 clay, total organic C 0.098%, pH 6.4 having 36% water holding capacity). Trials were carried out in 100 m2 field plots during October 2004 to February 2005. The recommended dose of chemical fertilizers for the crop of B. juncea was 100 kg h−1 nitrogen, in two split doses in the form of urea and 40 kg h−1 phosphate in form of DAP, in two split doses viz. N50+50, P20+20 as suggested [17]. Bacterized and non-bacterized seeds were sown on randomized field design in five sets of treatments with three replication of each treatment. (I) seeds bacterized with RMP1, (II) seeds bacterized with GRC2 (III) non-bacterized seeds + low dose of chemical fertilizers (N25+25 P20) (IV) non-bacterized seeds + recommended dose of chemical fertilizers (N50+50, P40) (V) seeds bacterized with adaptive variants of strains RMP1 and GRC2 (VI) seeds bacterized with adaptive variants of strains RMP1 and GRC2 + low dose of chemical fertilizers (N25+25 P20) and (VII) control (non-bacterized seeds and without chemical fertilizers). The crop was irrigated at different intervals as and when required. Seed germination (%) was recorded on the tenth day after sowing (DAS). Growth and yield parameters were recorded after 120 DAS.
Root Colonization
In our previous studies it was observed that S. meliloti RMP1 resistant to 100 μg ml−1 ampicillin [9] while P. aeruginosa GRC2 was resistant to 100 μg ml−1 streptomycin [5]. B. juncea plants, bacterized with strains were sampled after 30, 60, 90 and 120 DAS and bacterial population on the roots was measured. The roots were cut into 1 cm long segments and 1 g of root segments was dipped in 5 ml of SDW and vortex for 5 min. Suitable dilution of the suspension was poured into Petri plates containing YEM agar (ampicillin 100 μg ml−1) and King’s B agar (streptomycin 100 μg ml−1) to estimate population of RMP1 and GRC2, respectively. Cfu per gram of root segment was enumerated after 24 h of incubation at 28°C. Population dynamics of RMP1 and GRC2 along with other aerobic bacteria were recorded after 30, 60, 90 and 120 DAS.
Statistical Analysis
For each treatment, samples were obtained from three replicate plots per treatment in the completely randomized block design. Bacterial counts (cfu g−1) in broth cultures and soil were analyzed after logarithmic transformation. The data were analyzed and considered to be significantly different at P ≤ 0.05 and P ≤ 0.01 [18].
Results
Isolation of Chemical Fertilizers (Urea and DAP) Adaptive Variants of S. meliloti RMP1 and P. aeruginosa GRC2
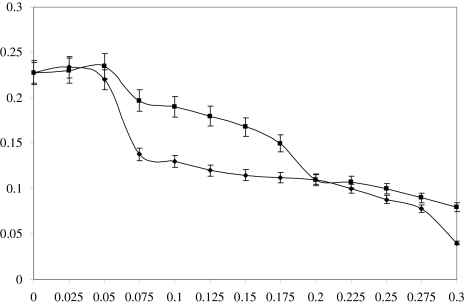
Decrease in growth rate of both RMP1 and GRC2 strains was observed at higher concentrations of urea and DAP, respectively. The growth of RMP1 was completely inhibited at 1 M of urea and 0.35 M of DAP, while in case of GRC2, 1.3 and 0.37 M concentrations of urea and DAP proved to be toxic as evidenced by absence of growth on respective medium, hence considered as lethal concentrations. However, the growth rate of RMP1 and GRC2 were found to increase at low concentrations of urea (0.04 and 0.3 M) and DAP (0.037 M), respectively (Figs. 1, 2). Lc50 was observed as 0.49 M of urea and 0.15 M of DAP for RMP1, and 0.66 M urea and 0.18 M of DAP for GRC2, as 50% decrease in growth rates of RMP1 and GRC2 was recorded at these concentrations as compared to control (data not shown). Interestingly, adaptive bacterial variants showed increase in growth rates at sub-lethal (Lc50) concentrations of urea and DAP. These tolerant variants showed growth pattern identical to non adaptive variants, and also gave PGP activities similar to non adaptive variants (data not shown).
Fig. 1.
Effect of urea concentration on specific growth rate of RMP1 (diamond) and GRC2 (square). Error bars indicate standard error of the mean, where error bars are not visible; they are smaller than the marker
Fig. 2.
Effect of DAP concentration on specific growth rate of RMP1 (diamond) and GRC2 (square). Error bars indicate standard error of the mean, where error bars are not visible; they are smaller than the marker
Interaction Between RMP1 and GRC2, and Chemotaxis Study
P. aeruginosa GRC2 did not inhibit the growth of S. meliloti RMP1 under co-culture conditions in disc test as no inhibition zone was observed around the culture discs of GRC2 on the lawn of RMP1. Similarly, RMP1 did not suppress the growth of GRC2.
RMP1 and GRC2 and their adaptive variants showed good chemotaxis index (C.I.) towards root exudates of crop plant, urea and DAP. The C.I. of adaptive variants for urea and DAP was found invariably higher than respective non adaptive variants, as evidenced by their better chemotactic movement towards crystal of urea and DAP in comparison to wild strains of RMP1 and GRC2. The C.I. of RMP1 and GRC2 and their adaptive variants was almost same for root exudates (Table 1).
Table 1.
Capillary assay of chemotaxis of Sinorhizobium meliloti RMP1 and Pseudomonas aeruginosa GRC2 towards root exudates of Brassica juncea and chemical fertilizers (urea and diammonium phosphate)
| Treatment | RMP1 | RMP1 Urea-DAP adaptive variant | GRC2 | GRC2 Urea-DAP adaptive variant |
|---|---|---|---|---|
| C.I. | C.I. | C.I. | C.I. | |
| Root exudates | 15.67 ± 0.83 | 17.5 ± 0.89 | 15.17 ± 0.44 | 15.88 ± 0.59 |
| Urea | 11.27 ± 0.51 | 13.35 ± 0.88 | 11.53 ± 0.54 | 12.96 ± 0.98 |
| DAP | 25.16 ± 0.77 | 28.49 ± 0.67 | 23.97 ± 0.73 | 25.39 ± 0.79 |
| Aspartic acid (Positive control) | 53.22 ± 4.25 | 53.42 ± 4.02 | 52.39 ± 4.77 | 61.62 ± 3.2 |
| Chemotaxis Buffer (Negative control) | 1.0 ± 0.2 | 1.0 ± 0.31 | 1.0 ± 0.12 | 1.0 ± 0.25 |
C.I. Chemotaxis index (the ratio bacterial number accumulated in the test capillaries containing urea, DAP and root exudates to that of control), DAP diammonium phosphate. *data are an average of three replicates
Root Colonization
Both RMP1 and GRC2 showed significant increase in rhizospheric population when inoculated individually. The population of RMP1 increased from log10 4.20 cfu g−1 root (after 30 DAS) to log10 5.83 cfu g−1 root (120 DAS), while population of GRC2 increased from log10 4.98 cfu g−1 root to log10 5.99 cfu g−1 root, in the same duration, in individual trials (Table 2). However, population of both isolates increased considerably when co-inoculated as compared to individual trials. When co-inoculated with GRC2 the population of RMP1 increased from log10 4.29 cfu g−1 root (30 DAS) to log10 6.02 cfu g−1 root (120 DAS). Similarly, in presence of RMP1, the population of GRC2 increased from log10 4.58 cfu g−1 root to log10 6.12 cfu g−1 root in same duration (Table 2).
Table 2.
Bacterial population in the rhizosphere of Brassica juncea var Pusa Jaikisan
| Bacteria | Log10 value cfu g−1 root | |||
|---|---|---|---|---|
| 30 DAS | 60 DAS | 90 DAS | 120 DAS | |
| RMP1 | 4.20 ± 0.14 | 5.32 ± 0.22 | 5.72 ± 0.15 | 5.83 ± 0.14 |
| GRC2 | 4.98 ± 0.14 | 5.74 ± 0.12 | 5.94 ± 0.22 | 5.99 ± 0.19 |
| RMP1 (when co-inoculated with GRC2) | 4.29 ± 0.12 | 5.35 ± 0.21 | 5.55 ± 0.18 | 6.02 ± 0.22 |
| GRC2 (when co-inoculated with RMP1) | 4.58 ± 0.11 | 5.71 ± 0.19 | 5.82 ± 0.19 | 6.12 ± 0.24 |
DAS Days after of sowing. Values are mean of 10 randomly selected plants
Regression coefficient (r) value: RMP1 = 0.969; GRC2 = 0.963
Field Trial
Dry root and shoot weight, root and shoot length were increased significantly in all the treatments as compared to control 82.97 and 89.36% increase in fresh shoot weight of B. juncea plants, and seed yield per hectare was recorded 631 and 639 kg by treatment of RMP1 or GRC2, respectively. The synergistic effect of RMP1 and GRC2 was apparent in co-inoculated trial, for growth enhancement of B. juncea. 34.24 and 114.89% increase in shoot length and fresh shoot weight was recorded in co-inoculated trial as compared to control. Further, plant growth and yield was significantly improved with application of half dose and full dose of fertilizers. Interestingly, application of urea and DAP along with adaptive bacterial inoculants RMP1 and GRC2 resulted in maximum increase in number of pods per plant. It was interesting to note that the total grain yield (kg ha−1) was almost similar to that obtained after application of recommended dosages of urea and DAP. Approximately 35% increase in grain yield was obtained with application of either of bacterial strain in individual trials; while co-inoculation of both strains resulted in 109.04% increase in seed yield as compared to control. However, application of both isolates along with reduced dose of fertilizers resulted in 111.77% increase in seed yield with respect to control, which was almost similar to treatment, where full dose of fertilizers were applied (110.06%). Growth and yield parameters obtained from different treatments are summarized in Table 3.
Table 3.
Effect of integrated use of chemical fertilizers and co-inoculants (RMP1 + GRC2) on growth and yield of Brassica juncea after 120 DAS
| Treatments | Seed germination (%) | Root | Shoot | Yield | |||||
|---|---|---|---|---|---|---|---|---|---|
| Length (cm) | Fresh wt. (g) | Dry wt. (g) | Length (cm) | Fresh wt. (g) | Dry wt. (g) | No of siliquae plant−1 | Seed yield Hect−1 (kg) | ||
| RMP1 | 85 | 15.2 | 13.7 | 8.4 | 180 | 86 | 41 | 246 | 631 |
| GRC2 | 87 | 15.9 | 14.1 | 8.9 | 184 | 89 | 43 | 249 | 639 |
| N25+25 P20 | 89 | 16.8 | 15.3 | 9.2 | 190 | 96 | 52 | 291 | 781 |
| N50+50 P20+20 | 88 | 19.8 | 20.1 | 11.3 | 201 | 106 | 62 | 337 | 981 |
| RMP1 + GRC2 | 90 | 19.5 | 20.0 | 10.1 | 196 | 101 | 60 | 315 | 978 |
| RMP1 + GRC2 + N25+25 + P20 | 95 | 20.2 | 21.8 | 12.4 | 204 | 108 | 69 | 341 | 989 |
| Control | 79 | 11.2 | 10.7 | 5.9 | 146 | 47 | 28 | 146 | 467 |
| SEM | 1.46 | 0.86 | 0.75 | 0.37 | 0.51 | 0.29 | 0.43 | 0.79 | 0.83 |
| CD @ 1% | 6.53 | 3.85 | 3.36 | 1.65 | 2.31 | 1.33 | 1.94 | 3.56 | 3.71 |
| CD @ 5% | 4.6 | 2.71 | 2.36 | 1.16 | 1.62 | 0.93 | 1.36 | 2.50 | 2.61 |
Values are mean of 10 randomly selected plants from each set
N50+50 P20+20 full doses of chemical fertilizers, N25+25 P20 half dose of chemical fertilizers
Discussion
In present study, the growth of P. aeruginosa GRC2 and S. meliloti RMP1 was subjected to increased concentrations of urea and DAP. The low doses were found to stimulate the bacterial growth, but increasing the concentration of urea and DAP resulted in decreased the growth rate, and also lead to cell lysis and death, which was in accordance to earlier observations [19]. Recently, Bhattacharya and Roy [20] found that higher concentrations of urea are inhibitory to rhizobial growth, because of alteration in cell membrane permeability and/or effect on cellular DNA synthesis, but extremely low doses urea proved stimulatory for growth rate of rhizobia, which is similar to our findings. Urea and DAP tolerant variants of both strains were obtained and it was observed that their PGP characteristics were restored in vitro. Both RMP1 and GRC2 were good colonizers of B. juncea rhizosphere. The population of both isolates increased ten times after 120 DAS, as compared to population of 30 DAS. Their colonization ability was further substantiated by the finding that both RMP1 and GRC2 were attracted towards B. juncea root exudates. Also, root exudates of B. juncea had not any adverse affect on bacterial survival. All the desired characteristics were restored even after acquisition of tolerance to urea and DAP in both isolates. Both strains had urease activity.
DAP when applied to the field release ammonia and provide HPO4−, a soluble and available form of inorganic phosphate but soon after it become unavailable due to low solubility and high sorption capacity in soil. Acidification was responsible for inorganic phosphate solubilization by RMP1 which had been reported to form acids [9], resulting drop in pH and cause phosphate soubilization [21]. Whereas, H+ excreting ATPase seems to be improbable with GRC2 for phosphate solubilization mechanisms. Ability of phosphate solubilization was restored in RMP1 and GRC2 after acquisition of resistance to urea and DAP. Variant strains also restored other PGP activities like siderophore production and suppression of the phytopathogenic fungus i.e. Macrophomina phaseolina in spite of the presence of urea or DAP, which encourage the utilization of these strains further in rhizosphere. RMP1 and GRC2 both colonized well in the rhizosphere of B. juncea as proved by the regression coefficient (r) values, 0.969 and 0.963 for RMP1 and GRC2, respectively (Table 2). Earlier it was reported that S. meliloti [22] and P. aeruginosa [23] secreted acyl homoserine lactones (AHLs) molecules that directly play a role in the root colonization and their behavior in rhizosphere through quorum sensing (intercellular communication) [24]. Since RMP1 and GRC2 are PGPR strains, hence role played by AHLs is an added advantage that can not be ruled out as these are common among plant-associated species in comparison to that of soil borne species [25].
Distinct microbial populations in rhizosphere frequently interact with each other. Therefore mixed inoculants (combination of microorganisms) that interact synergistically are currently being devised, which yield better and quick results [26]. The secondary metabolites produced by RMP1 and GRC2 were non-reactive against each other; hence both were able to co-exist. Berggren et al. [27] also observed neutral behavior of P. putida towards R. leguminosarum under controlled conditions. It has been suggested that bacteria that attain colony-forming units of about ≈103 per gram or higher on root mass can be considered as good colonizers [28], and we obtained population of RMP1 and GRC2 of ≈105 per gram in individual trials. The rhizospheric competence of both strains increased in presence of each other as their population was recorded in the range of ≈106 per gram. During present study, enhanced plant growth and yield parameters revealed the significance of integrated use of co-inoculant of variants with reduced dose of urea and DAP. The co-inoculation of RMP1 and GRC2 proved very effective for growth promotion of B. juncea, when applied with half dose of fertilizers. 103.73% increase in fresh root weight and 117.8% increase in yield was recorded with the co-inoculation of both strains with half dose of fertilizers. This was in accordance to the findings of root colonization studies. Earlier, Gupta et al. [5] obtained enhanced nodule weight in pea when inoculated with Pseudomonas GRC2. Recently, Pandey and Maheshwari [29] reported commensalism between Sinorhizobium and Burkholderia sp. resulting in growth enhancement of pigeon pea when applied together. The increase in grain yield with co-inoculation of both strains and half dose of fertilizers was almost similar to that obtained after application of recommended doses of urea and DAP. The results clearly suggest possibility of reduction in chemical use by application of RMP1 and GRC2. Saraf and Sood [11] suggested possible exploitation of pesticide resistant mutant rhizobial strains for integrated use with chemical fertilizers. Tripathi et al. [30] observed 48% increase in gram yield by using Rhizobium with half dose of chemical fertilizers. Similarly, Okon [31] reported 5–30% enhancement in growth and yield of cereals by inoculation with Azospirillum sp. with reduced dose of fertilizers. Mohiuddin et al. [32] suggested integrated use of biofertilizers with reduced dose of chemical fertilizers in wheat. However, here we report reduction of chemical fertilizer and growth enhancement of B. juncea by integrated use of two PGPR, chemotactically active for these fertilizers, with immense potential for these chemical fertilizer adaptive variants for commercial and environmental benefits.
Acknowledgments
Financial support for this research from TMOP & M-CSIR, Government of India is gratefully acknowledged.
Contributor Information
Dinesh K. Maheshwari, Phone: +91-1334-246-767, FAX: +91-1334-246-767, Email: maheshwaridk@gmail.com
Piyush Pandey, Email: piyushgkp@rediffmail.com.
References
- 1.Sharma R. Use of biofertilizers in rainfed agriculture. Agrobios Newsl. 2004;2:16–17. [Google Scholar]
- 2.Ayala S, Rao EVSP. Perspective of soil fertility management with a focus on fertilizer use for crop productivity. Curr Sci. 2002;82:797–807. [Google Scholar]
- 3.Dekker J. Acquired resistance to fungicides. Ann Rev Phytopathol. 1976;14:405–428. doi: 10.1146/annurev.py.14.090176.002201. [DOI] [Google Scholar]
- 4.Cook RJ, Thomoshow LS, Weller DM, Fujimoto D, Mazzola M, Bangera G, Kim D. Molecular mechanism of defense by rhizobacteria against root diseases. Proc Nat Acad Sci. 1995;92:4197–4201. doi: 10.1073/pnas.92.10.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta CP, Dubey RC, Maheshwari DK. Plant growth enhancement and suppression of Macrophominaphaseolina causing charcoal rot of peanut by fluorescent Pseudomonas. Biol Fertil Soils. 2002;35:399–405. doi: 10.1007/s00374-002-0486-0. [DOI] [Google Scholar]
- 6.Raj L, Singh VP, Kaushik CD. Stability analysis in crop seed and mustard for resistance to Alternaria leaf blight and white rust. Ind Phytopathol. 1997;50:513–519. [Google Scholar]
- 7.Yasari E, Esmaeili Azadgoleh MA, Mozafari S, Alashti MR. Enhancement of growth and nutrient uptake of Rapeseed (Brassica napus L.) by applying mineral nutrients and biofertilizers. Pak J Biol Sci. 2009;12:127–133. doi: 10.3923/pjbs.2009.127.133. [DOI] [PubMed] [Google Scholar]
- 8.Vargas MAT, Mandes IC, Hungaria M. Response of field-grown bean (Phaseolus vulgaris L.) to Rhizobium inoculation and nitrogen fertilization in two Carrados soils. Biol Fertil Soils. 2000;33:228–233. doi: 10.1007/s003740000240. [DOI] [Google Scholar]
- 9.Arora NK, Kang SC, Maheshwari DK. Isolation of siderophore producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr Sci. 2001;81:673–677. [Google Scholar]
- 10.Maheshwari DK, Saraf M. Effect of carbaryl and 2, 4-D to nitrogenase and uptake hydrogenase in agar cultures and root nodules formed by Rhizobium leguminosarum. J Gen Appl Microbiol. 1994;40:569–574. doi: 10.2323/jgam.40.569. [DOI] [Google Scholar]
- 11.Saraf M, Sood N. Influence of monocrotophos on growth oxygen uptake and exopolysaccharide production of Rhizobium NCIM 2771 on chickpea. J Indian Bot Soc. 2002;81:154–157. [Google Scholar]
- 12.Sindhu SS, Gupta SK, Dadarwal KR. Antagonistic effect of Pseudomonas spp on pathogenic fungi and enhancement of growth of green gram (Vigna radiata) Biol Fertil Soils. 1999;29:62–68. doi: 10.1007/s003740050525. [DOI] [Google Scholar]
- 13.Hewitt EJ. Sand and water culture methods used in the study of plant nutrition. Farnham Royal Bucks: Commonwealth Agricultural Bureaux; 1952. p. 84. [Google Scholar]
- 14.Alder JA. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichiacoli. J Gen Microbiol. 1973;74:77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-de-victoria G, Lowell CR. Chemotaxis of Azospirillum species to aromatic compounds. Appl Environ Microbiol. 1993;59:2951–2955. doi: 10.1128/aem.59.9.2951-2955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grim AC, Harwood CS. Nay-H, a catabolic plasmid-encoded receptor is required for chemotaxis of Pseudomonas putida to the aromatic hydrocarbon naphthalene. J Bacteriol. 1999;181:3310–3316. doi: 10.1128/jb.181.10.3310-3316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad A, Khan I, Abdin MZ. Interactive effect of nitrogen and sulphur on nitrogen harvest of rapeseed-mustard. Ind J Plant Physiol. 2001;6:46–52. [Google Scholar]
- 18.Gomez AK, Gomez AA. Statistical procedure for agricultural research. New York: John Wiley and Sons; 1984. [Google Scholar]
- 19.Strzeleowa A. The effect of urea on spheroplast formation in Rhizobium. Acta Microbiol Pol. 1970;2:23–24. [PubMed] [Google Scholar]
- 20.Maheshwari DK, Gupta M. Diverse effect of two organocarbamets nematicides on nitrogen assimilation of Rhizobium japonicum 2002 in free-living culture. Biochem Physiol Pflanzen. 1991;187:316–322. [Google Scholar]
- 21.Balasubramaniun A, Palaniappan SP. Effects of two pesticides on oxidative metabolism of cowpea bacteroids. Curr Sci. 1983;52:674–675. [Google Scholar]
- 22.Bhattacharya R, Roy TK. Microbiological and electron microscopic studies of urea treated Rhizobium sp. Cells Acta Microbiol Pol. 2000;49:201–206. [PubMed] [Google Scholar]
- 23.Pearson JP, Delden C, Iglewski BH. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol. 1999;181:1203–1210. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fray RG. Altering plant-microbe interaction through artificially manipulating bacterial quorum sensing. Ann Bot. 2002;89:245–253. doi: 10.1093/aob/mcf039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elasri M, Delorme S, Lemanceau P, Stewart G, Laue B, Glickmann E, Oger PM, Dessaux Y. Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soilborne Pseudomonas spp. App Environ Microbiol. 2001;67:1198–1209. doi: 10.1128/AEM.67.3.1198-1209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bashan Y. Inoculants of plant growth promoting bacteria for use in agriculture. Biotechnol Adv. 1998;16:729–770. doi: 10.1016/S0734-9750(98)00003-2. [DOI] [Google Scholar]
- 27.Berggren I, Vuurde JWL, Martensson AM. Factor influencing the effect of deleterious Pseudomonas putida rhizobacteria on initial infection of pea roots by Rhizobium leguminasorum bv viceae. Appl Soil Ecol. 2001;17:97–105. doi: 10.1016/S0929-1393(01)00130-5. [DOI] [Google Scholar]
- 28.Scher FM, Kloepper JW, Singleton C, Zaleska I, Laliberte M. Colonization of soybean roots by Pseudomonas and Serratia spp. relationship to bacterial motility, chemotaxis and generation time. Phytopathol. 1988;78:1055–1059. doi: 10.1094/Phyto-78-1055. [DOI] [Google Scholar]
- 29.Pandey P, Maheshwari DK. Commensalism between Sinorhizobium and Burkholderia in binary-culture consortium for plant growth promotion. Curr Sci. 2007;92:1137–1142. [Google Scholar]
- 30.Tripathi RS, Dubey CS, Khan AW, Agarwal KB. Effect of application of Rhizobium inoculum on yield of gram varieties in chambal command area of Rajasthan. Sci Cult. 1975;41:266–269. [Google Scholar]
- 31.Okon Y. Response of cereal and forage grasses to inoculation with N2- fixing bacteria. In: Veerger C, Newton WE, editors. Advances in nitrogen fixation research. The Haugue/Wageningen : Martinus Nifhoff & Junk Publishers; 1984. pp. 303–309. [Google Scholar]
- 32.Mohiuddin MD, Das AK, Ghose DC. Growth and productivity of wheat as influenced by integrated use of chemical fertilizer, biofertilizer and growth regulator. Ind J Plant Physiol. 2000;5:334–338. [Google Scholar]