Abstract
Bacterial infections of the central nervous system, especially acute infections such as bacterial meningitis require immediate, invariably empiric antibiotic therapy due to the widespread emergence of resistance among bacterial species. Nosocomial infections by Pseudomonas aeruginosa have been described with an increasing trend towards multidrug resistance. P. aeruginosa isolates n = 53 (66%) isolated from the cerebrospinal fluid (CSF) were used for this study. Antibiotic resistance in 53 P. aeruginosa clinical isolates from 80 CSF samples were evaluated. Of these, n = 42 (80%) of the isolates showed multidrug resistance to more than eight antibiotics and n = 17 (32%) isolates were found to be imipenem resistant P. aeruginosa (IMPR-Pa). Genotypical examination by ERIC based PCR revealed minor genetic variations. Polymicrobial infections are common in the CSF samples. However, high prevalence of P. aeruginosa as an opportunistic pathogen has been developing with increased resistance to antimicrobial agents and thus becoming a significant threat.
Keywords: Pseudomonas aeruginosa, Prevalence, CSF, Nosocomial infections, MIC, Antibiotics
Introduction
Cerebrospinal fluid (CSF) is a clear body fluid that occupies the subarachnoid space and the ventricular system around and inside the brain. CSF is considered sterile. Detection of microbes in CSF, even in low numbers, provides valuable information about possible infection. CSF is susceptible to infection by a number of opportunistic bacterial pathogens, including Pseudomonas aeruginosa, Klebsiella pneumoniae and Enterobacter cloacae [1].
Pseudomonas aeruginosa is an invasive, gram-negative opportunistic pathogen that causes a wide range of severe infections that include bacteremia, pneumonia, meningitis, urinary tract and wound infections [2]. It is the one of the common nosocomial pathogens causing iatrogenic meningitis infection in CSF. P. aeruginosa has now become a major cause of nosocomial infections due to its remarkable propensity to rapidly acquire resistance determinants to a wide range of antibacterial agents. Of note P. aeruginosa has a greater ability to develop resistance to virtually any antibiotic to which it is exposed, because of multiple resistance mechanisms that can be present within the pathogen [3].
The most common resistance mechanism is production of beta-lactamases, including penicillinases, cephalosporinases, and carbapenemases [4]. In addition, various efflux pump systems are capable of actively removing every antibiotic from the intracellular milieu [5]. Increasing rates of antimicrobial resistance among P. aeruginosa are a problem worldwide. Data from the United States collected in 2002 reflect an increase of 37% resistance to fluoroquinolones, 32% resistance to imipenem, and 22% resistance to ceftazidime in P. aeruginosa isolates when compared to similar data collected from 1997 to 2001 [2].
The Infectious Diseases Society of America (IDSA) identified P. aeruginosa among the top seven pathogens threatening our healthcare-delivery system and as a crucial example of unmet medical need [2]. Analysis clearly demonstrated that P. aeruginosa is resistant to all clinically significant antibiotics including carbapenems, which are considered last-line antibiotics for therapy. However, since the first isolation of plasmid mediated metallo beta lactamase from P. aeruginosa in 1991 [6] increasing rates of imipenem resistant metallo beta lactamase (MBL) P. aeruginosa producing strains have become a serious problem. Such strains are resistant to multiple antibiotics as they hydrolyze all beta-lactams and are resistant to clinically available inhibitors like clavulanic acid and EDTA [7]. In spite of increasing susceptibility of patients to bacterial infections being well corroborated, the long lasting illness associated with these infections remains poorly understood.
Strain typing is an epidemiologically important tool for recognizing outbreaks of infection, detecting cross-transmission of nosocomial pathogens and determining the source of the infection. Phenotypic methods face problems of low reproducibility due to varied expressions of phenotypic characters such as sporadic expression of virulence genes or antigens. Many of the currently used molecular techniques offer a good discriminatory power in evaluating the utility of the particular typing method [8].
Genotypic methods used for typing and characterization of clinical strains include plasmid analysis, restriction endonuclease analysis, pulsed-field gel electrophoresis (PFGE), DNA sequencing, ribotyping, restriction fragment length polymorphism studies (RFLP), randomly amplified polymorphic DNA (RAPD), amplified fragment length polymorphism (AFLP), and repetitive sequence-based PCR (rep-PCR). The rep-PCR method uses primers that target noncoding repetitive sequences interspersed throughout the bacterial genome and is an established approach for subspecies classification and strain delineation of bacteria [9]. Two such groups of repetitive elements are the enterobacterial repetitive intergenic consensus (ERIC) sequences common to Gram-negative enteric bacteria, and the BOX elements, originally detected in Streptococcus pneumoniae [10].
The present study is an attempt (1) to characterize clinical isolates collected from CSF by antimicrobial susceptibility testing for clinically relevant antimicrobials, and (2) to determine the genetic diversity of these P. aeruginosa strains for our population.
Materials and Methods
Bacterial Strains and Growth Conditions
In this study, 53 strains of P. aeruginosa were isolated from 80 CSF samples collected during March to October 2008 from three hospital and two diagnostic centers. Bacteria were identified by biochemical tests [11], and stored at −20°C. Standard strain P. aeruginosa ATCC 27853 was used as control.
Susceptibility Testing
Antimicrobial Susceptibility
Antibiotics were tested by the Kirby–Bauer method [12] using nine antibiotics discs including: gentamicin, amikacin, tobramycin, ceftazidime, cefepime, imipenem, ampicillin, carbenicillin, ciprofloxacin and norfloxicn (Hi-media, Mumbai). The plates were incubated at 37°C for 24 h before the zones of inhibition were measured. Bacterial strains that demonstrated resistance to three or more categories of antibiotics were defined as multidrug resistant. The P. aeruginosa ATCC 27853 strain was adopted as the standard for quality control.
MIC
Minimum inhibition concentrations (MIC) were determined on plates of Muller-Hinton agar containing serial two-fold dilutions of each antibiotic [13]. Bacterial suspensions of 104 colony-forming units (CFU)/ml were inoculated onto the surface of the plates, and results were recorded after overnight incubation at 37°C. The MIC was defined as the lowest antibiotic concentration with no visible growth.
Detection of MBLs
Metallo beta lactamase producing P. aeruginosa was suspected when the isolate was resistant to ceftazidime and imipenem. Various methods have been recommended for screening MBL. These include the modified Hodge test, double disc synergy test using imipenem and EDTA discs or ceftazidime and EDTA discs or EDTA impregnated imipenem discs and EDTA impregnated meropenem discs.
We used zone enhancement with EDTA impregnated imipenem discs [14] for phenotypic determination of MBLs. Test organisms were inoculated on to plates with Mueller–Hinton agar. A 0.5 M EDTA solution was prepared by dissolving 186.1 g of disodium EDTA·2H2O in 1,000 ml of distilled water and adjusting it to pH 8.0 using NaOH. The mixture was sterilized by autoclaving. EDTA solution was added to ceftazidime discs to obtain a desired concentration of 750 μg. The EDTA impregnated antibiotic discs were dried immediately in an incubator and stored at −20°C in airtight vials. Then, 10 μg imipenem discs (with and without EDTA) were placed on the surface of an inoculated agar plate. The inhibition zones of imipenem and imipenem EDTA discs were compared after 16–18 h of incubation in air at 35°C. Strains with enhancement zone in imipenem EDTA discs were recognized as MBL producing P. aeruginosa.
Preparation of Chromosomal DNA
Cells from an overnight culture in BHI broth collected by centrifugation were suspended in lysis buffer (phosphate-buffered [PBS] containing 1% sodium dodecyl sulfate [SDS] and 100 μg/ml proteinase K). The cell suspension was incubated at 37°C for 1 h and equal volume of phenol:chloroform (1:1) mixture was added to cell suspension and vortexed. The samples were centrifuged and the aqueous phase was transferred to a fresh tube. The DNA was precipitated by adding 100 μl of 3 M sodium acetate and 3 vol. of cold absolute alcohol then air-dried and suspended in 50 μl of TE buffer (10 mM Tris–HCl [pH 8.0], 1 mM EDTA).
Genotyping
Clonal distributions of the strains were studied by enterobacterial repetitive intergenic consensus (ERIC)–PCR genotyping. ERIC2–PCR amplification was carried with the conserved primers [9], a total volume of 20 μl amplification buffer (10 mM Tris–HCl, pH 9.0, 1.5 mM MgCl2, 50 mM KCl and 0.01% Gelatin), was mixed with 0.25 mM of each dNTP, 20 pmol of primer, 25 ng of template DNA and 1 U/reaction of Taq DNA polymerase. The thermal cycler was programmed for 35 cycles of 1 min at 94°C, 1 min at 53°C and 2 min at 72°C with 8 min final extension period. A sample of 10 μl from each reaction was analyzed by gel electrophoresis in a 2% agarose gel with ethidium bromide, using 1 kb ladder as a molecular-weight marker. Minor differences in band intensity, as well as weak bands, were not considered to define the types.
Results
Antibiotic Profile and Resistance Rate Among the P. aeruginosa Isolates
In this study from the 80 CSF samples, 53 (66%) P. aeruginosa were isolated. About 42/53 (80%) were found to be multidrug resistant. Among these, 17 (32%) were resistant to imipenem. The imipenem resistant isolates 17/53 were also resistant to tobramycin, gentamicin, ampicillin, ceftazidime, cefepime, ciprofloxacin and norfloxacin. Overall, 11/53 (20%) were found resistant only to carbenicillin and 14/53 (25%) isolates were resistant to amikacin (Table 1).
Table 1.
Incidence of resistance in P. aeruginosa to three different groups of antibiotics
| Antibiotics | Incidence of resistance | |
|---|---|---|
| No. of resistant isolates | Percentage | |
| Aminoglycosides | 70.00 | |
| Tobramycin (Tb) | 24 | 44.00 |
| Gentamicin | 51 | 96.00 |
| Amikacin (Ak) | 14 | 25.00 |
| β-Lactams | 74.66 | |
| Imipenem (I) | 17 | 32.00 |
| Ampicillin (Ap) | 53 | 100.00 |
| Carbencillin (Cb) | 11 | 20.00 |
| Ceftazidime (Ca) | 53 | 100.00 |
| Cefepime (Cp) | 53 | 100.00 |
| Fluoroquinolones | 100.00 | |
| Ciprofloxacin (Cf) | 53 | 100.00 |
| Norfloxacin (Nx) | 53 | 100.00 |
Determination of MIC’s in P. aeruginosa Isolates
The 15 multidrug resistant clinical isolates of P. aeruginosa had imipenem, gentamicin, ciprofloxacin and ceftazidime MIC’s ranging from 4–64, 16–2,048, 2–32 and 16–1,024 μg/ml, respectively (Table 2).
Table 2.
MIC’s of imipenem, gentamicin, ciprofloxacin and ceftazidime in P. aeruginosa isolates determined by agar dilution method
| P. aeruginosa isolate | MIC of imipenem, μg/ml (mean ± SD) |
MIC of gentamicin, μg/ml (mean ± SD) |
MIC of ciprofloxacin, μg/ml (mean ± SD) |
MIC of ceftazidime, μg/ml (mean ± SD) |
|---|---|---|---|---|
| PA1 | 4 ± 1.15 | 512 ± 4.1 | 4 ± 0.87 | 16 ± 0.76 |
| IMP-PA2 | 32 ± 4.0 | 32 ± 4 | 2 ± 0.83 | 32 ± 1.25 |
| PA3 | 4 ± 1.5 | 128 ± 2.51 | 2 ± 0.76 | 64 ± 1.32 |
| PA4 | 8 ± 2.0 | 64 ± 4.5 | 8 ± 1.25 | 16 ± 1.04 |
| IMP-PA5 | 16 ± 1.5 | 1,024 ± 5.5 | 16 ± 0.57 | 128 ± 2.5 |
| PA6 | 16 ± 0.57 | 64 ± 3.51 | 8 ± 1.0 | 64 ± 0.5 |
| PA7 | 8 ± 1.0 | 128 ± 2.08 | 32 ± 2.0 | 128 ± 2.08 |
| IMP-PA8 | 64 ± 2.7 | 512 ± 0.5 | 8 ± 2.0 | 1,024 ± 2.51 |
| IMP-PA9 | 64 ± 0.57 | 64 ± 1.73 | 8 ± 3.05 | 256 ± 4.04 |
| PA10 | 8 ± 3.05 | 192 ± 2.08 | 4 ± 0.8 | 128 ± 2.25 |
| PA11 | 8 ± 1.52 | 256 ± 3.05 | 8 ± 2.5 | 640 ± 1.73 |
| IMP-PA12 | 32 ± 3.51 | 256 ± 2.0 | 16 ± 0.57 | 256 ± 5.03 |
| IMP-PA13 | 16 ± 1.52 | 512 ± 3.05 | 8 ± 2.51 | 512 ± 0.56 |
| PA14 | 8 ± 2.51 | 2,048 ± 4.05 | 32 ± 1.25 | 128 ± 0.76 |
| PA15 | 16 ± 2.51 | 512 ± 1.0 | 4 ± 1.0 | 256 ± 2.0 |
Detection of MBL’s
Of the 53 isolates of P. aeruginosa, 17 isolates (32%) were found resistant to carbapenems (imipenem) and 50 (20.8%) were found to be MBL producers confirmed by disc potentiation method. The P. aeruginosa ATCC 27853 did not exhibit any zone size enhancement with EDTA impregnated imipenem discs.
Genetic Relationship of Multidrug Resistant P. aeruginosa Isolates Based on ERIC–PCR
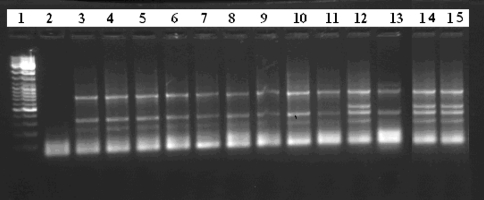
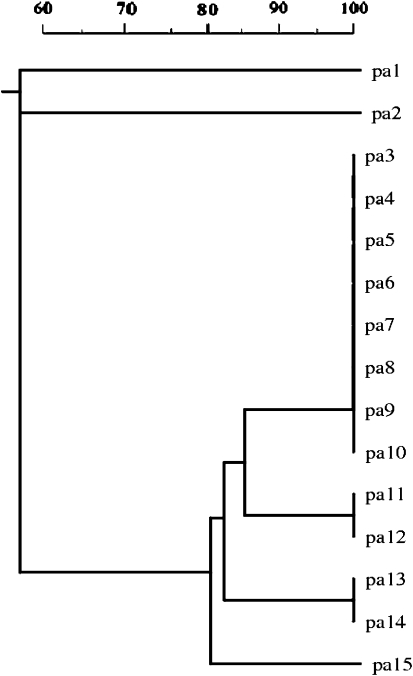
From the 15 multidrug resistant P. aeruginosa isolates, two visually different banding patterns were generated. The amplicon size were in the range of 200–2,650 bp. Amongst the 15 multidrug resistant P. aeruginosa isolates, 12 isolates exhibited similar banding pattern and were monomorphic, while only 3 isolates exhibited polymorphism associated with a different banding pattern than the former (Fig. 1). The monomorphic isolates were resistant isolates were resistant to six antibiotics, while the latter three were resistant to more than eight antibiotics. Of the two groups obtained, most of the monomorphic isolates were grouped into one genotype and the polymorphic isolates were grouped into second genotype (Fig. 2).
Fig. 1.
ERIC–PCR-generated DNA fingerprints of P. aeruginosa isolates. Lanes: 1 1 kb ladder marker, 2 negative control; 3 ATCC control, 4–15 local isolated strains
Fig. 2.
Dendrogram of genetic relationship between 15 isolates of multidrug-resistant P. aeruginosa obtained with ERIC2 primer. The scale above indicates the similarity index
Discussion
The rapid appearance of multidrug resistant organisms has led to a concurrent increase in central nervous system (CNS) infections caused by gram negative bacteria. Furthermore, the clinical value of antibiotics that remain active against such bacteria in the CNS is limited by their decreased penetration of the blood–brain barrier. P. aeruginosa is the most frequently isolated bacterium in hospitals and they show high intrinsic resistance to many structurally diverse antibiotics [15].
In the present study, incidence of P. aeruginosa was found to be 66%. Similar observations were made by Jones et al. [16]. The antimicrobial susceptibility results of P. aeruginosa against three groups of antibiotics indicated the high degree of resistance to beta-lactams (74%) followed by aminoglycosides (70%) and flouroquinolones (100%). Bijayini et al. [17], found 64 (70%) were resistant to ceftazidime, 68 (75%) to piperacillin, 54 (59%) to piperacilin/tazobactam, 81 (89%) to ticarcillin/clavulinic acid, 75 (82%) to cefoperazone, 67 (74%) to amikacin, 74 (81%) to cefepime, 65 (71%) to levofloxacin, 72 (79%) to ciprofloxacin and 63 (69%) to aztreonam by the Kirby–Bauer method. Similar levels of antibiotic resistance were demonstrated in the present study with ceftazidime, cefepime and ciprofloxacin. In recent years, emerging imipenem resistance has been reported in India [18].
Comparatively the incidence of resistance observed in the present study was higher than the earlier reports. However, the available data indicates the prevalence of resistance among the P. aeruginosa isolates varies between countries and difference can be attributed to the variation of resistance to antimicrobials based on extent of exposure to various antibiotics and their differences in prescription patterns and/or quality of infection control practices. Therefore, our results are moderately correlating with the earlier reported results [16, 17].
The MIC of four antibiotics was determined for 15 clinical isolates of P. aeruginosa. The MIC of imipenem for the 15 multidrug resistance stains ranged from 4 to 64 μg/ml, gentamicin (16–2,048 μg/ml), ciprofloxacin (2–32 μg/ml) and ceftazidime (16–1,024 μg/ml).
Recent reports of P. aeruginosa outbreaks were due to multidrug resistant genotypes, which complicate the treatment of infections. In a similar study, Speijer et al. [19] concluded that the small number of identified patient-to-patient transmissions (5 among 49 patients with P. aeruginosa) and the large number of genotypes found indicated that most P. aeruginosa strains originated from the patients themselves. Hsueh et al. [20] who traced the spread of a single strain of P. aeruginosa and increased concern about multidrug resistant strains in the report over a period of several years [20].
Carbapenems are the drugs of choice for multidrug resistant P. aeruginosa and ESBL producing organisms. However, resistance to carbapenems due to reduced uptake of drug leads to imipenem/meropenem resistant isolates [21]. Varying resistance (4–60%) towards imipenem and meropenem been reported worldwide [22–24]. In this study, we observed a resistance of 32% to imipenem among the P. aeruginosa isolates, while 20.8% of screened bacteria were MBL-positive. The results of the present study demonstrate that the MBL-producing strains have significantly higher rates of resistance to β-lactam antibiotics.
The ERIC–PCR analysis grouped P. aeruginosa isolates into two major profiles as shown in the Fig. 1. The DNA relatedness was determined based on unweighted pair group using arithmetic averages of Dice coefficient as shown in Fig. 2. Thus, the study revealed the minor genetic variations occurred during the isolation and collection period.
In conclusion, despite the increased frequency of multidrug resistance in P. aeruginosa, there exists a relative paucity of information regarding antimicrobial resistance. Our study beneficially help assists in identification of the causative agents of the infection. The experience with the isolates suggested that the surveillance for multidrug resistant P. aeruginosa should be maintained and careful infection control measures and cautious use of antibiotics must be promoted.
References
- 1.Yamamoto Y. PCR in diagnosis of infection: detection of bacteria in cerebrospinal fluids. Clin Diagn Lab Immunol. 2002;9:508–514. doi: 10.1128/CDLI.9.3.508-514.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George HT, John B, John EE, David G, Michael S, John G. Bad bugs need drugs: an update on the development pipeline from the availability task force of the infectious diseases society of America. Clin Infect Dis. 2006;42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 3.Livermore DM. Pseudomonas proteins pumps and carbapeanems. J Antimicrob Chemother. 2001;47:247–250. doi: 10.1093/jac/47.3.247. [DOI] [PubMed] [Google Scholar]
- 4.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 5.Poole K. Aminoglycoside resistance in P. aeruginosa. J Antimicrob Agents Chemother. 2008;49:479–487. doi: 10.1128/AAC.49.2.479-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe M, Shizuko I, Matsuhisa I, Susumu M. Transferable imipenem resistance in P. aeruginosa. J Antimicrob Agents Chemother. 1991;35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush K. Metallo b-lactamase: a class apart. Clin Infect Dis. 1998;27:S48–S53. doi: 10.1086/514922. [DOI] [PubMed] [Google Scholar]
- 8.Olive M, Jain P. Principles and applications of methods for DNA-based typing of microbial organisms. J Clin Microbiol. 1999;37:1661–1669. doi: 10.1128/jcm.37.6.1661-1669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hulton CS, Higgins CF, Sharp PM. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol. 1991;5:825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 11.Gilardi GL. Identification of Pseudomonas and related bacteria. Glucose nonfermenting gram-negative bacteria in clinical microbiology. Boca Raton: CRC Press; 1978. pp. 15–44. [Google Scholar]
- 12.Performance standards for antimicrobial disk susceptibility tests; approved standard. 10. Wayne: Clinical and Laboratory Standards Institute; 2009. [Google Scholar]
- 13.Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 8. Wayne: Clinical and Laboratory Standards Institute; 2009. [Google Scholar]
- 14.Yong D, Lee K, Yum JH, Shin HB, Rossolini GM, Chong Y. Imipenem-EDTA disk method for differentiation of metallo-beta-lactamase producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002;40:3798–3801. doi: 10.1128/JCM.40.10.3798-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmeli Y, Nicolas T, George M, Matthew H. Emergence of antibiotic-resistant P. aeruginosa. Comparison of risks associated with different antipseudomonal age. Antimicrob Agents Chemother. 1999;43:1379–1382. doi: 10.1128/aac.43.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones RN, Marshall SA, Pfaller MA. Nosocomial enterococcal blood stream infections in the SCOPE program: antimicrobial resistance, species occurrence, molecular testing results, and laboratory testing accuracy. Diagn Microbiol Infect Dis. 1997;29:95–102. doi: 10.1016/S0732-8893(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 17.Bijayini B, Anupam D, Purva M, Kapil A. High prevalence of carbapenem resistant P. aeruginosa at a tertiary care centre of north India. Are we under-reporting? Indian J Med Res. 2008;128:324–325. [PubMed] [Google Scholar]
- 18.Hemalatha V, Sekar U, Kamat V. Detection of metallo betalactamase producing P. aeruginosa in hospitalized patients. Indian J Med Res. 2005;122:148–152. [PubMed] [Google Scholar]
- 19.Speijer H, Savelkoul PH, Bonten MJ, Stobberingh EE, Tjhie JH. Application of different genotyping methods for P. aeruginosa in a setting of endemicity in an intensive care unit. J Clin Microbiol. 1999;37:3654–3661. doi: 10.1128/jcm.37.11.3654-3661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsueh PR, Teng LJ, Yang PC, Chen YC, Ho SW, Luh KT. Persistence of a multidrug-resistant P. aeruginosa clone in an intensive care burn unit. J Clin Microbiol. 1998;36:1347–1351. doi: 10.1128/jcm.36.5.1347-1351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rie N, Yuka A, Hideaki I, Koji Y, Terutaka H, Hajme M. Carbapenem derivatives as potential inhibitors of various β-lactamases, including class B metallo-β-lactamases. Antimicrob Agents Chemother. 1999;43:2497–2503. doi: 10.1128/aac.43.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendiratta DK, Deotale V, Narang P. Metallo beta lactamase producing P. aeruginosa in a hospital from rural area. Indian J Med Res. 2005;121:701–703. [PubMed] [Google Scholar]
- 23.Alan PG, Chanwit T, Richard A, Thomas M, Louie J, Wally K, David M, Livermore P, Neil W. Nosocomial outbreak of carbapenem-resistant P. aeruginosa with a new blaIMP allele, blaIMP-7. J Antimicrob Agents Chemother. 2002;46:252–258. doi: 10.1128/AAC.46.1.255-258.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magalhães V, Lins AK, Magalhães M. Metallo-β-lactamase producing P. aeruginosa strains isolated in hospitals in Recife, Pe, Brazil. Braz J Microbiol. 2005;36:123–125. [Google Scholar]