Abstract
Two chromium-resistant bacteria (IFR-2 and IFR-3) capable of reducing/transforming Cr(VI) to Cr(III) were isolated from tannery effluents. Isolates IFR-2 and IFR-3 were identified as Staphylococcus aureus and Pediococcus pentosaceus respectively by 16S rRNA gene sequence analyses. Both isolates can grow well on 2,000 mg/l Cr(VI) (as K2Cr2O7) in Luria-Bertani (LB) medium. Reduction of Cr(VI) was found to be growth-associated in both isolates and IFR-2 and IFR-3 reduced 20 mg/l Cr(VI) completely in 6 and 24 h respectively. The Cr(VI) reduction due to chromate reductase activity was detected in the culture supernatant and cell lysate but not at all in the cell extract supernatant of both isolates. Whole cells of IFR-2 and IFR-3 converted 24 and 30% of the initial Cr(VI) concentration (1 mg/l) in 45 min respectively at 37°C. NiCl2 stimulated the growth of IFR-2 whereas HgCl2 and CdCl2 significantly inhibited the growth of both isolates. Optimum temperature and pH for growth of and Cr(VI) reduction by both isolates were found to be between 35 and 40°C and pH 7.0 to 8.0. The two bacterial isolates can be good candidates for detoxification of Cr(VI) in industrial effluents.
Keywords: Bioremediation, Chromium, Tannery effluents
Introduction
Chromium is used in different industrial processes and released into the environments. Leather processing industries (LPI) use chromium material (chrome liqueur or chrome powder) for tanning of leather. Residual chromium thus is discharged in solid or liquid effluents. Soluble hexavalent chromium [Cr(VI)] is extremely toxic and shows mutagenic and carcinogenic effects on biological systems due to its strong oxidizing nature [1]. Cr(III) is less toxic and bioavailable than Cr(VI), as it readily forms insoluble oxides and hydroxides above pH 5 [2].
In Bangladesh, about 250 leather processing industries at Hazaribagh, South Western part of the Dhaka city, discharge both liquid and solid wastes into canals and rivers with residual chromium. This causes chromium contamination not only of water but also aquatic lives, land, vegetables farming and crops thus posing a serious health hazard. The most direct and severe effect comes out with the leather-cut wastes, which are conventionally being processed into feed ingredient (as a protein source). This chromium (tetravalent or hexavalent form) content produce thus might be a good source of chromium migration into the food chain.
A wide variety of bacteria have been reported for reducing/transforming Cr(VI) to Cr(III), under aerobic and anaerobic condition, e.g. Intrasporangium Sp. Q5-1, Bacillus sp. ES29, Escherichia coli, Enterobacter cloacae, Pseudomonas fluorescens LB300 [3–7]. Application of Cr-resistant bacteria for detoxification of Cr(VI) has been considered as an economical, effective and safe procedure over conventional physical and chemical methods [8].
We have isolated two chromium resistant bacteria from tannery effluents collected from drain water nearby tannery industries at Hazaribagh area. These bacteria are capable of reducing Cr(VI) to Cr(III). We are working to develop a bioremediation process for treatment of chromium-polluted tannery effluents as well as leather-cut wastes for conversion of the latter into a safe poultry feed ingredient.
Materials and Methods
Sample Collection and Processing
Tannery effluent/drain water was collected from Hazaribagh Tannery area of Dhaka city in sterile bottles and immediately transferred to the laboratory. Samples were diluted in normal saline and inoculated (0.1 ml) on Luria-Bertani (LB) agar plate containing 500 mg/l of potassium dichromate (K2Cr2O7) as Cr(VI) by spread plate method. Plates were incubated at 37°C for 4 days. Useful single colonies were selected and sub-cultured onto fresh LB agar medium and preserved at 4°C or in 10% glycerol stock for further studies.
Identification by 16S rRNA Gene Sequence
Genomic DNA of bacterial isolates were isolated according to the method described by Sambrook et al. [9]. Gene fragments specific for the highly variable V3 region of the bacterial 16S rRNA gene was amplified by PCR as described by Ovreås et al. [10] using universal bacterial primers L340GCF and K517R (Sigma, USA) in a thermal cycler (MJ Research Inc., Watertown, USA). The sequence of the L340GCF primer, which included a 40 base pair GC-clamp attached to the 5′-end, was 5′-CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGGACTCCTACGGGAGGCAGCAG-3′ and the sequence of the K517R primer was 5′-ATTACCGCGGCTGCTGG-3′. The PCR products were subjected to 2% agarose gel electrophoresis, stained with ethidium bromide and visualized on a UV transilluminator for the presence of about 200 bp PCR products. Amplified 16S rRNA gene PCR products were purified using StrataPrep® PCR purification kit (Stratagene, USA) according to the manufacturers protocol. Sequencing reactions were carried out using ABI-Prism Big dye terminator cycle sequencing ready reaction kit and the PCR products were purified by a standard protocol. The purified cycle sequenced products were analyzed with an ABI-Prism 310 genetic analyzer. The chromatogram sequencing files were edited using Chromas 2.32. The homology of the 16S rRNA gene sequences was checked with the 16S rRNA gene sequences of other organisms that had already been submitted to GenBank database using the BLASTN [11] (http://www.ncbi.nih.gov/BLAST/) algorithm.
Effect of Chromium(VI) Concentration on Growth
The effect of varying concentrations (0–2,000 mg/l) of Cr(VI) (as K2Cr2O7) on the tolerance of the microbial growth was examined in duplicate in LB broth (5 ml) at 37°C under 150 rpm in a water bath (N-Biotek, Inc. Korea). The growth was monitored measuring OD at 600 nm.
Chromium(VI) Assay
Hexavalent chromium was determined colorimetrically with a spectrophotometer (Genesys 5, USA) using the S-diphenylcarbazide (DPC) (Nacalai Tesque, Inc., Japan) method [12]. The DPC reagent was prepared by adding 24 ml of 85% H3PO4 to 56 ml distilled water. This solution was mixed with 0.076 g DPC previously dissolved in 20 ml of 95% ethanol. The reagent was stored in dark at 4°C. Cr(VI) in the sample was assayed by adding 125 μl of the DPC reagent to 1 ml of chromium samples, mixed gently and kept at room temperature for 20 min. The absorbance of the color produced was measured at 540 nm using a spectrophotometer. Cr(VI) concentration in the sample was calculated from a standard curve using K2Cr2O7 as standard.
Determination of Chromate Reductase Activity
The reaction mixture for the chromate reductase activity contained 2 mg/l Cr(VI) as K2Cr2O7 in 0.5 ml of 100 mM phosphate buffer, pH 7.0 held at 37°C in a water bath. The reaction was initiated by the addition of 0.5 ml of the enzyme (as culture supernatant, cell extract supernatant or cell lysate) and residual Cr(VI) concentration was measured after 1 h following the method described above [12]. One unit of enzyme activity was defined as 1 μmol of Cr(VI) reduced/min/ml at 37°C.
Time Course of Growth and Cr(VI) Reduction
The time course of growth and Cr(VI) reduction/transformation was evaluated with 20 mg/l K2Cr2O7 in shake flask culture (N-Biotek, Inc. Korea) containing 100 ml LB broth at 37°C, 150 rpm. Samples (1.5 ml) were withdrawn at 0.5 or 2 h intervals. The growth was monitored measuring OD at 600 nm. Samples were centrifuged (Eppendorf, Germany) at 10,000 rpm for 5 min and the supernatants were assayed for residual Cr(VI) concentration.
Cell Fractionation for Localization of Cr(VI) Reduction and Chromate Reductase Activity
Bacteria were grown overnight in 100 ml of LB medium with 20 mg/l K2Cr2O7 at 37°C with orbital shaking (150 rpm). Thereafter, cells of each isolate were harvested by centrifugation (Biofuge Primo, Heraeus, Germany) of 30 ml culture at 5,000 rpm for 10 min. Culture supernatant was collected and the cell pellet was resuspended in 30 ml phosphate buffer (100 mM, pH 7.0). Cells in an ice bath were disrupted with an ultrasonic probe (550 Sonic Dismembranator, Fisher Scientific, Pittsburgh). Power was applied ten times in 30 s pulses with 30 s intervals. The sonicate was centrifuged at 16,000 rpm at 4°C for 20 min. Cell extract supernatant was transferred in a fresh tube and was kept in ice. Cell lysate was also resuspended in 30 ml phosphate buffer. Total chromium and hexavalent chromium in three fractions (culture supernatant, cell extract supernatant and cell lysate) were determined by Graphite Furnace Atomic Absorption (GFAA) spectrometer (AAnalyst 800, Perkin Elmer, USA) and diphenylcarbazide method [12], respectively.
Chromium Reduction by Whole Cell in Buffer
Bacterial cells were grown in 50 ml LB broth in a 250 ml Erlenmayer flask. After 20 h of incubation at 37°C and 150 rpm agitation, cells were harvested from 1.5 ml culture by centrifugation at 10,000 rpm for 5 min. The cell pellet in an eppendorf tube was washed twice with phosphate buffer and resuspended in 0.5 ml phosphate buffer (100 mM, pH 7.0). Cr(VI) reduction was initiated by adding 0.5 ml of K2Cr2O7 (2 mg/l) in phosphate buffer and incubated at 37°C in a water bath for 45 min. The reaction mixture was centrifuged at 10,000 rpm for 5 min and the supernatant was analysed for residual Cr(VI) determination by the method as described above. A reaction blank was prepared by adding 0.5 ml phosphate buffer to 0.5 ml of K2Cr2O7 (2 mg/l) and considered as 100% Cr(VI). Phosphate buffer was used to set the spectrophotometer to zero.
Effect of Different Metals on the Growth of Bacterial Isolates
The effect of different salts on the growth of bacterial isolates was examined in LB broth (5 ml) supplemented with 200 mg/l of CuCl2, ZnCl2, NiCl2, CdSO4, and HgCl2. The tubes were inoculated with 25 µl of the inoculum and incubated at 37°C and 150 strokes/min in a water bath for 20 h. The growth of the bacterial isolates was measured spectrophotometrically at 600 nm.
Effect of Temperature and pH on the Growth and Cr(VI) Reduction
The effect of temperature and pH on the growth and Cr(VI) reduction by bacterial isolates were investigated using LB medium (5 ml) containing 10 mg/l Cr(VI) as K2Cr2O7. Media were inoculated with 25 µl of overnight cultures of the bacterial isolates (OD600 1.2–1.7). Chromium reduction and growth was studied at various incubation temperatures (25–45°C). For the effect of pH, sterilized culture medium was adjusted to pH 5.0, 6.0, 7.0, 8.0, 9.0 and 10.0 with predetermined amounts of sterilized 0.1 N HCl or 1 N NaOH and the tubes were incubated at 37°C in a water bath. Growth was measured after 24 h of incubation by taking absorbance at 600 nm. The culture was centrifuged (10,000 rpm, 5 min) and the supernatant was used to determine the residual Cr(VI) concentration.
Results and Discussion
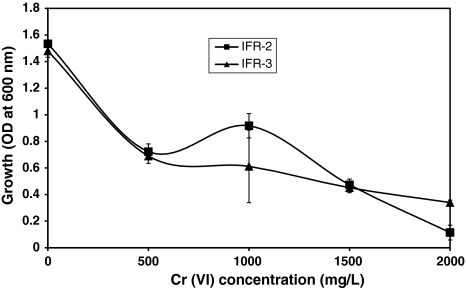
Eight unique colonies were selected from Cr(VI) containing LB plates, inoculated with effluent/drain water collected from Hazaribagh Tannery area, Dhaka. These isolates were further inoculated in LB broth supplemented with different concentrations of Cr(VI) to determine their Cr(VI) tolerance between 500 and 2,000 mg/l. Isolates IFR-2 and IFR-3 showed highest level of tolerance, growing well in presence of 2,000 mg/l Cr(VI) (Fig. 1) whereas other isolates did not grow well above 1,000 mg/l Cr(VI). Therefore isolates IFR-2 and IFR-3 were selected for further studies. Camargo et al. [4] isolated some chromium resistant bacteria that can tolerate or reduce Cr(VI) at concentrations of 1,500–2,500 mg/l.
Fig. 1.
Effect of Cr(VI) as K2Cr2O7 concentrations on the growth of bacterial isolates (IFR-2, IFR-3)
The isolates IFR-2 and IFR-3 were identified by both morphological and the 16S rRNA gene sequence analyses technique (Table 1). Isolate IFR-2 showed 98% identity with Staphylococcus aureus subsp. aureus and isolate IFR-3 was 100% identical to Pediococcus pentosaceus ATCC 25745. According to the scientific literatures, these species of bacteria have never been reported as chromium-resistant bacteria.
Table 1.
Identification of chromium(VI)-reducing bacteria by 16S rRNA gene sequence analyses
| Isolate | Organism | Accession no. | E value | Identity (%) |
|---|---|---|---|---|
| IFR-2 | S. aureus subsp. aureus | NC_009641.1 | 1e−66 | 98 |
| IFR-3 | P. pentosaceus ATCC 25745 | NC_008525.1 | 2e−69 | 100 |
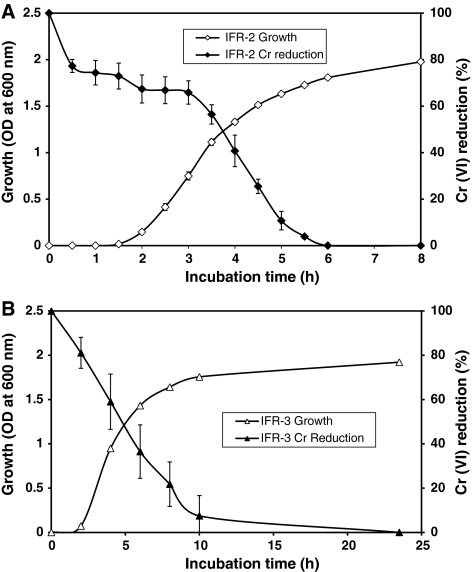
Time courses of growth and Cr(VI) reduction or detoxification by chromate reductase of isolates IFR-2 and IFR-3 were investigated in presence of an initial Cr(VI) concentration of 20 mg/l in shake flask culture. Cr(VI) reduction by both isolates were found to be growth-associated (Fig. 2). Isolate IFR-2 reduced 90% of Cr(VI) in 5 h (Fig. 2A) while IFR-3 reduced 90% of Cr(VI) in 10 h of incubation (Fig. 2B). Cr(VI) was completely reduced after 6 and 24 h respectively by isolates IFR-2 and IFR-3. It has been reported that Bacillus Sp. ES29 reduced 90% of Cr(VI) added (2 mg/l) to the medium in less than 6 h [13]. Thacker and Madamwar [14] have shown that the bacterial isolate DM1 reduces 50 ppm of chromium to 0 ppm in 54 h. They added a second aliquot of chromium which was reduced to 0 ppm in 99 h, and the third aliquot was reduced to 21.8 ppm in 126 h.
Fig. 2.
Time course of growth and Cr(VI) (20 mg/l) reduction by bacterial isolates IFR-2 (A) and IFR-3 (B)
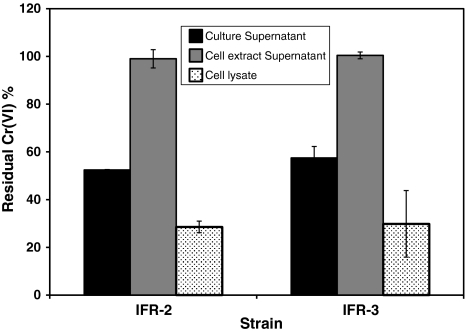
In the culture supernatants of final samples of the above experiment, Cr(VI) added in the culture (20 mg/l) medium was not detected at all (Table 2) while AAS results revealed a total Cr of 19.81 (±0.37) and 19.27 (±0.33) mg/l respectively in the culture supernatants of isolates IFR-2 and IFR-3. These results suggest that Cr(VI) was almost completely reduced by both isolates in the extracellular environment as very negligible amount of Cr(VI) and total Cr were detected in the cell extract supernatants of isolates IFR-2 and IFR-3 (Table 2). To investigate the location of enzymatic reduction, the chromate reductase activity was measured in the culture supernatant, cell lysate and cell extract supernatant. Chromate reductase activity was detected in the culture supernatant and cell lysate but not at all in the cell extract supernatant of both isolates. The residual Cr(VI) concentration in the cell lysate and culture supernatants of IFR-2 and IFR-3 ranged between 30 and 60% (Fig. 3) while the cell extract supernatant retained 100% of Cr(VI) (1 mg/l). The specific activity of chromate reductase was calculated from this data and ranged between 0.033 to 0.053 µmol Cr(VI) reduced/min/mg protein in the cell lysate and culture supernatants respectively (Table 3). We therefore presume that chromate reductase is a membrane-bound enzyme in both isolates and is also secreted in the extracellular environment. Camargo et al. [13] observed chromate reductase activity in the cell-free extract and soluble fraction but very low activity in the membrane fraction of Bacillus sp. ES29. Wang et al. [15] reported that chromate reductase activity is preferentially associated in the membrane fraction of E. cloacae HO1. Membrane bound chromium reduction activity has also been reported in P. fluorescens LB300 [7].
Table 2.
Determination of Cr(VI) and total Cr as K2Cr2O7 concentrations in the culture supernatants and cell extract supernatants of bacterial isolates by DPC method and Atomic Absorption Spectrometer, respectively
| Fraction | Cr(VI) concentration (mg/l) | Total Cr concentration (mg/l) | ||
|---|---|---|---|---|
| IFR-2 | IFR-3 | IFR-2 | IFR-3 | |
| Culture supernatant | 0.0 | 0.0 | 19.81 (±0.37) | 19.27 (±0.33) |
| Cell extract supernatant | 0.64 (±0.01) | 0.53 (±0.04) | 0.021 (±0.01) | 0.032 (±0.01) |
Fig. 3.
Cr(VI) reduction by different fractions of bacterial isolates
Table 3.
Determination of protein concentration and chromate reductase specific activity in the culture supernatant and cell lysate of bacterial isolates
| Fraction | Protein concentration (mg/ml) | Chromate reductase specific activity (µmol Cr(VI) reduced/min/mg protein) | ||
|---|---|---|---|---|
| IFR-2 | IFR-3 | IFR-2 | IFR-3 | |
| Culture supernatant | 0.067 | 0.061 | 0.051 (±0.01) | 0.053 (±0.02) |
| Cell lysate | 0.13 (±0.01) | 0.153 (±0.04) | 0.04 (±0.01) | 0.033 (±0.01) |
Chromate reduction activities of whole cells of bacterial isolates were determined at 37°C. Whole cells of IFR-2 and IFR-3 reduced 24 and 30% of the initial Cr(VI) concentration (1 mg/l) in 45 min respectively. Initial cell density has been shown to have a dramatic effect on Cr(VI) reduction [16]. A number of factors may be contributing to the observed trends, including Cr(VI) toxicity acclimation time needed to readjust cells to low nutrient loads before initiation of reduction.
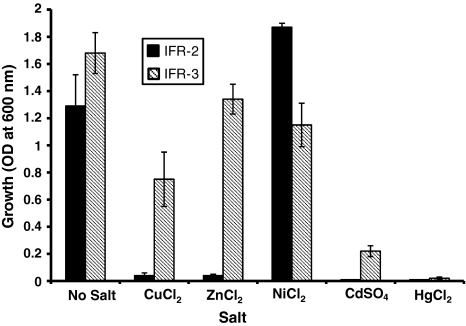
The effects of different salts on the growth of bacterial isolates were also investigated. The growth of isolate IFR-2 was stimulated in presence of NiCl2 and very mild growth was observed in presence of CuCl2 and ZnCl2 whereas growth was almost completely inhibited in presence of CdCl2 and HgCl2 (Fig. 4). Isolate IFR-3 showed good growth in presence of ZnCl2, NiCl2 and CuCl2, mild growth in presence of CdCl2 and almost no growth in presence of HgCl2 (Fig. 4). Basu et al. [17] have reported that Hg2+ and Pb2+ are toxic to bacterial isolates and inhibited their growth.
Fig. 4.
Effect of different metals (200 mg/l) on the growth of bacterial isolates
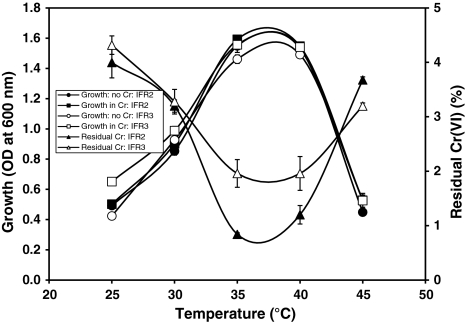
The optimum temperatures for growth and Cr(VI) reduction was found between 35 and 40°C for both isolates IFR-2 and IFR-3 (Fig. 5). The growth of both isolates increased with the increase of temperature up to 35°C and decreased at 45°C. Cr(VI) reduction by both isolates also increased with the increase of temperature up to 35°C and decreased above 40°C (Fig. 5). However, more than 95% of Cr(VI) was reduced by both isolates between temperatures 25–45°C. No significant difference was observed in the presence or absence of Cr(VI) on the growth of both isolates at different temperatures. Thacker and Madamwar [14] reported a maximum reduction of Cr(VI) at 35°C by the bacterial isolate DM1. An NAD(P)H dependent hexavalent chromium reductase was purified from Pseudomonas ambigua which showed activity over a wide range of temperature from 40 to 70°C [18].
Fig. 5.
Effect of temperatures on the growth and Cr(VI) (10 mg/l) reduction by bacterial isolates
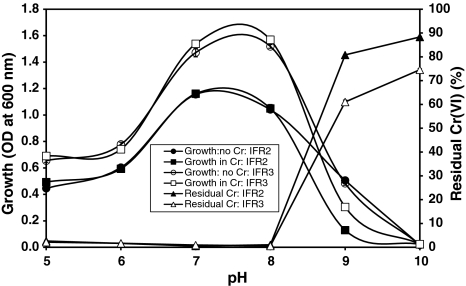
The bacterial isolates IFR-2 and IFR-3 can grow and reduce more than 95% Cr(VI) in a wide range of pH between 5.0 to 8.0 as seen in Fig. 6. The optimum pH for growth and Cr(VI) reduction by both isolates was found between pH 7.0 and 8.0. Growth and Cr(VI) reduction by both isolates was significantly reduced at pH above 8.0 (Fig. 6). Optimal Cr(VI) reduction was shown to be directly related to the optimum pH (8.0) for the growth of Bacillus isolates [4]. McLean et al. [16] reported a wide range of pH between 6.0 to 9.0 for Cr(VI) reduction by P. synxantha.
Fig. 6.
Effect of pH on the growth and Cr(VI) (10 mg/l) reduction by bacterial isolates
Chromate tolerance mechanisms in bacteria have been reported to include reduction, methylation, precipitation at the cell surface, blocking cellular uptake by altering the uptake pathway and removal from the cytoplasm by efflux pumps [19–21]. In the present study the mechanism of chromate tolerance was not investigated. However, our results in this study provide a basis for assessing the potential of using indigenous Cr(VI)-reducing novel bacteria for bioremediation application. Development of economic and efficient bioremediation technique based on immobilized whole cell system for the reduction and removal of toxic Cr from tannery effluents and solid wastes may help to cope with the extensive use of Cr in leather tanning.
Acknowledgment
This work was supported with grant in aid by the Ministry of Science and Information and Communication Technology, Government of the People’s Republic of Bangladesh.
References
- 1.McLean J, Beveridge TJ. Chromate reduction in Pseudomonad isolated from a site contaminated with chromate copper arsenate. Appl Environ Microbiol. 2001;67:1076–1084. doi: 10.1128/AEM.67.3.1076-1084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai D, Sass BM, Moore DA. Chromium(III) hydrolysis constants and solubility of chromium(III) hydroxide. Inorg Chem. 1987;26:345–349. doi: 10.1021/ic00250a002. [DOI] [Google Scholar]
- 3.Yang J, Minyan H, Wang G. Removal of toxic chromate using free and immobilized Cr(VI)-reducing bacterial cells of Intrasporangium sp. Q5-1. World J Microbiol Biotechnol. 2009;25:1579–1587. doi: 10.1007/s11274-009-0047-x. [DOI] [Google Scholar]
- 4.Camargo FAO, Bento FM, Okeke BC, Frankenberger WT. Chromate reduction by chromium-resistant bacteria isolated from soils contaminated with dichromate. J Environ Qual. 2003;32:1228–1233. doi: 10.2134/jeq2003.1228. [DOI] [PubMed] [Google Scholar]
- 5.Shen H, Yi-Tin W. Characterization of enzymatic reduction of hexavalent chromium by Escherichia coli ATCC 33456. Appl Environ Microbiol. 1993;59(11):3771–3777. doi: 10.1128/aem.59.11.3771-3777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang PC, Mori T, Komori K, Sasatsu M, Toda K, Ohtake H. Isolation and characterization of Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Appl Environ Microbiol. 1989;55:1665–1669. doi: 10.1128/aem.55.7.1665-1669.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bopp LH, Chakrabarty AM, Ehrlich HL. Chromate resistance plasmid in Pseudominas fluorescens. J Bacteriol. 1983;155:1105–1109. doi: 10.1128/jb.155.3.1105-1109.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganguli A, Tripathi AK. Bioremediation of toxic chromium from electroplating effluent by chromate reducing Pseudomonas aeruginosa A2Chr in two bioreactors. Appl Microbiol Biotechnol. 2002;58:416–420. doi: 10.1007/s00253-001-0871-x. [DOI] [PubMed] [Google Scholar]
- 9.Sambrook J, Fritsch EF, Miniatis T. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 10.Ovreås L, Forney L, Daae FL, Torsvik V. Distribution of bacterioplankton in meromictic lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR amplified gene fragments coding for 16S rRNA. Appl Environ Microbiol. 1997;63:3367–3373. doi: 10.1128/aem.63.9.3367-3373.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett RJ, James BR. Chromium. In: Sparks DL, editor. Methods of soil analysis, Part 3, SSSA Book Series 5. Madison: SSSA; 1996. pp. 683–701. [Google Scholar]
- 13.Camargo FAO, Okeke BC, Bento FM, Frankenberger WT. In vitro reduction of hexavalent chromium by a cell-free extract of Bacillus sp. ES29 stimulated by Cu2+ Appl Microbiol Biotechnol. 2003;62:569–573. doi: 10.1007/s00253-003-1291-x. [DOI] [PubMed] [Google Scholar]
- 14.Thacker U, Madamwar D. Reduction of toxic chromium and partial localization of chromium reductase activity in bacterial isolate DM1. World J Microbiol Biotechnol. 2005;21:891–899. doi: 10.1007/s11274-004-6557-7. [DOI] [Google Scholar]
- 15.Wang PC, Mori T, Toda K, Ohtake H. Membrane associated chromate reductase activity from Enterobacter cloacae. J Bacteriol. 1990;172:1670–1672. doi: 10.1128/jb.172.3.1670-1672.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLean SJ, Beveridge TJ, Phipps D. Isolation and characterization of a chromium-reducing bacterium from a chromated copper arsenate-contaminated site. Environ Microbiol. 2000;2(6):611–619. doi: 10.1046/j.1462-2920.2000.00143.x. [DOI] [PubMed] [Google Scholar]
- 17.Basu M, Bhattacharya S, Paul AK. Isolation and characterization of chromium-resistant bacteria from tannery effluents. Bull Environ Contam Toxicol. 1997;58:535–542. doi: 10.1007/s001289900368. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Miyata N, Horitsu H, Kawai K, Takamizawa K, Yutaka Tai Y, Okazaki M. NAD(P)H-dependent chromium(VI) reductase of Pseudomonas ambiga G-1.: a Cr(V) intermediate formed during the reduction of Cr(VI) to Cr(III) J Bacteriol. 1992;174:5340–5345. doi: 10.1128/jb.174.16.5340-5345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shuttleworth KL, Richard FU. Sorption of heavy metals to the filamentous bacterium Thiothrix strain Al. Appl Environ Microbiol. 1993;59:1274–1282. doi: 10.1128/aem.59.5.1274-1282.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovely DR, Philips EJP. Reduction of chromate by Desulfovibrio vulgaris and its C3 cytochrome. Appl Environ Microbiol. 1994;60:726–728. doi: 10.1128/aem.60.2.726-728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramírez-Díaz MI, Díaz-Pérez C, Vargas E, Riveros-Rosas H, Campos-García J, Cervantes C. Mechanisms of bacterial resistance to chromium compounds. Biometals. 2008;21:321–332. doi: 10.1007/s10534-007-9121-8. [DOI] [PubMed] [Google Scholar]