Abstract
Development of microbial inoculants from rhizobacterial isolates with potential for plant growth promotion and root disease suppression require rigorous screening. Fifty-four (54) fluorescent pseudomonads, out of a large collection of rhizobacteria from broad bean fields of 20 different locations within Imphal valley of Manipur, were initially screened for antifungal activity against Macrophomina phaseolina and Rhizoctonia solani, of diseased roots of broad bean and also three other reference fungal pathogens of plant roots. Fifteen fluorescent pseudomonas isolates produced inhibition zone (8–29 mm) of the fungal growth in dual plate assay and IAA like substances (24.1–66.7 μg/ml) and soluble P (12.7–56.80 μg/ml) in broth culture. Among the isolates, RFP 36 caused a marked increase in seed germination, seedling biomass and control of the root borne pathogens of broad bean. PCR–RAPD analysis of these isolates along with five MTCC reference fluorescent pseudomonas strains indicated that the RFP-36 belonged to a distinct cluster and the PCR of its genomic DNA with antibiotic specific primers Phenazine-1-carboxylic acid and 2, 4-diacetyl phloroglucinol suggested possible occurrence of gene for the potent antibiotics. Overall, the result of the study indicated the potential of the isolate RFP 36 as a microbial inoculant with multiple functions for broad bean.
Keywords: Biocontrol, Rhizobacteria, Vicia faba L., Macrophomina phaseolina, Rhizoctonia solani
Introduction
Broad bean (Vicia faba L.) is an important winter vegetable legume of North-East India and considered as a meat and a skim milk substitute in diet for its high protein and nutritional quality [1]. It responds to application of nitrogenous and phosphatic fertilizer and produces higher yield. However, high cost of fertilizer and related environmental issues are some of the general constraints in increasing its production through application of high dose of fertilizer. Widespread incidence of damping off and root rot diseases is another constraint in successful cultivation of this crop. High relative humidity in atmosphere and heavy texture soils of the region make the plant highly susceptible to Macrophomina phaseolina and Rhizoctonia solani, which are destructive soil-borne plant pathogens associated with damping off and root rot diseases of broad bean. On a global scale, M. phaseolina and R. solani have been reported to be associated with more than 500 plant species [2]. As such, these pathogenic microorganisms are major and chronic threat to food production and ecosystem stability world wide [3]. In North East India, use of pesticide to control these diseases in broad bean is not a common practice but in the season of severe incidence, particularly under wet soil conditions, the damage of crop due to the disease may be total. Under wet soil conditions, chemical control of soil borne pathogen is reported to be less effective. Chemical control is also not desirable due to adverse impacts of pesticides such as development of pathogen resistance, decline in number of non-target beneficial microorganisms in soil and hazards to human health [4]. Under such circumstances, organic inputs such as microbial inoculants hold promise in control of disease and promotion of growth of broad bean in farmers’ field. In general, biological control has been considered as an alternative or a supplementing way of reducing use of chemicals in agriculture [5, 6]. Several rhizobacterial strains have been successfully exploited elsewhere in control of damping off and root diseases of several crops [7, 8] and in management of nutrient under sustainable crop production [9–11]. The mechanisms by which beneficial rhizobacteria promote plant growth may be through production of phytohormone such as IAA [12], antibiotic like substances [13], suppression of deleterious rhizobacteria [14, 15], solubilization of insoluble phosphate [16] and induction of systemic resistance [17]. Therefore, use of rhizobacteria with multiple mechanisms can serve as microbial inoculants in organic method of crop production.
Although, exotic strains of rhizobacteria may be effective in growth promotion and diseases control, molecular technique based evidence suggest that genotypes of beneficial bacteria may be endemic to a biogeographical region [18]. The endemic bacterial pool of a region may contain highly efficient genotypes and is likely to perform better than the exotic strains. By virtue of its being in the Indo-Burma diversity hotspots, Imphal valley is likely to harbour useful microorganisms and to our knowledge there is no previous research done on exploitation of beneficial microorganisms of crop rhizosphere in the valley. In this research, our objective was to isolate and screen rhizobacteria of broad bean grown in the Imphal valley of Manipur for their effectiveness in promotion of growth and control of the damping off and root rot diseases of this crop.
Materials and Methods
Isolation of Fluorescent Pseudomonads and Fungal Pathogens
Twenty broad bean field sites were selected at different locations representing entire Imphal East and Imphal West valley zone of Manipur within Indian region of Indo-Burma diversity hotspot of the world for collection of rhizosphere sample. From each site, 4–5 broad bean roots along with the rhizosphere soils from both healthy and infected plants were randomly selected and uprooted during the winter seasons of 2003–2004. The samples were carried to the laboratory and stored in the freeze (4°C) prior to isolation of rhizosphere bacteria. The measured quantity of roots and the rhizosphere soils were transferred to conical flasks containing 100 ml sterile water and shaken at 120 rpm for 30 min. The suspensions of the samples were serially diluted up to 10−4. One ml was pour plated on King’s B agar medium. Subsequently, the fluorescent colonies were purified by streaking. The purified isolates were maintained in glycerol stock at −20°C. Fungal pathogens were isolated from soil and root samples and identified as M. phaseolina and R. solani based on morphological parameters such as sporangial shape and sexual organs observed under a light microscope [19, 20].
Screening of Fluorescent Pseudomonas Isolates for Inhibition of Fungal Pathogens
The bacterial isolates were screened in vitro for inhibition of M. phaseolina and R. solani obtained from broad bean field along with three other fungi associated with damping off (reference fungal strains, namely Fusarium oxysporum-248, Aspergillus fumigatus-5175 and Sclerotinia sclerotiorum-5575 obtained from IARI, New Delhi), using dual plate assay. Fresh cultures of M. phaseolina and R. solani were grown in PDA medium (Potato infusion-200; Dextrose-20; agar-18 (g l−1). A plug from the growing edges (5 mm diameter) of the M. phaseolina and R. solani colony was placed in the centre of a fresh plate. The bacterial isolates were grown overnight, spotted along the periphery of 90 mm diameter petri disc equidistant from the mycelial plug. These plates (4 replicates) along with the uninoculated control were incubated at 30°C for about 72 h or until the leading edge of the fungus reached the edge of the plate. Antibiosis was assessed by measuring the diameter of the mycelial growth of M. phaseolina and R. solani alone and in presence of the bacterial isolate.
Assessment of Plant Growth Promotion In Vitro and Determination of IAA like Substances and Soluble P in Broth Cultures
Plant growth promotion (PGP) ability of the isolates was assessed in vitro in test tubes by agar slants methods. Surface sterilized broad bean seeds were inoculated with centrifuged pellets of the isolates separately and allowed to grow and root and shoot length of 10 day old plant measured. PGP attributes such as production of IAA like substances and solubilization of P of fifteen promising isolates were determined in broth cultures using standard protocol [21]. Test bacterial cultures were inoculated in 50 ml flasks containing LB broth with tryptophan (2 mg/ml) and incubated at 28 ± 2°C for 3 days. Cultures were centrifuged at 3000 rpm for 30 min. Two mililiter supernatant was mixed with two drops of orthophosphoric acid, 4 ml of Solawaski’s reagent: (50 ml, 35% perchloric acid, 1 ml 0.5 M FeCl2, incubated for 30 min. in dark and optical density (O.D) of the pink color was read at 530 nm using spectrophotometer. The level of IAA in culture filtrates was estimated from a standard graph obtained using graded concentration between 0 and 100 ppm IAA (Sigma). The phosphate solubilizing activity was determined in Pikovskaya’s broth (PK). In PK broth, tricalcium phosphate (TCP) was used as the insoluble phosphate and cultures were grown in an incubator shaker at 30°C and 120 rpm speed. Soluble P-content in cell free extract was determined using Bray’s reagent [22].
PCR–RAPD
Genomic DNA of each of the fifteen promising isolates along with the five reference strains (from MTCC, IMTECH, Chandigarh) were isolated and subjected to RAPD analysis by PCR–RAPD methods [23], using seven oligonucleotide primers (OPA-1, 2, 4, 18, 20, OPD-2 and 5), separately. The reactions were carried out in final volume of 25 μl containing 1× PCR buffer, 1.5 mM MgCl2, 800 μM dNTPs and 1.0–2.0 units Taq DNA polymerase. The reaction mixtures were incubated in a thermocycler at 94°C for 2 min. These were then subjected to 45 cycles consisting of 94°C for 45 s, 37°C for 1 min and 72°C for 2 min.
PCR of Genomic DNA with Gene Specific Primers
To determine whether the genomic DNA of the strain gets amplified with gene specific primers for known antibiotics, Phenazine-1-carboxylic acid and 2,4-diacetyl phloroglucinol produced by the effective biocontrol strains, PCRs were carried out with primer sequences of PHZ1 5′GGC GAC ATG GTC AAC GG 3′, PHZ2 5′CGG CTG GCG GCG TAT AT 3′ and BPR3 5′ GGT GCG ACA TCT TTA ATG GAG TTC 3′, BPR4 5′ CCG CCG GTA TGG AAG ATG AAA AAG TC 3′ [24, 25]. The PCR cycling conditions were as per standard protocol [26]. PCR amplified DNAs were detected by running in 1.5% agarose gel.
Evaluation of Effect of RFP-36 on Broad Bean in Pot
The effect of the isolate RFP-36 on broad bean was evaluated in pot experiment. Inoculum of the culture was produced in 250 ml LB broth under continuous shaking for 24 h on a rotary shaker at 120 rpm and 30°C. Optical density of the broth was adjusted between 0.3 and 0.5 at 600 nm to obtain a cell concentration of 109cfu/ml. The standardized broth culture was then mixed with 1% carboxymethyl cellulose (CMC) for uniform bacterization of the broad bean seeds. Seeds of the susceptible local variety (Mamit amubi) were surface sterilized with 0.1% HgCl2 for 5 min and rinsed repeatedly for 5 times with sterile dist. H2O. Fifty sterilized seeds were placed in the broth culture and 1% CMC mix and incubated for 30 min for uniform adherence of the bacteria on seed surface. Control treatment seeds were treated with the mixture of uninoculated broth and 1% CMC. Both bacterized and control seeds were air dried overnight at room temperature. Inocula of M. phaseolina and R. solani were prepared by incubating mycelial growth in pre-sterilized oat grain for 7 days at 30°C. Fungal inocula were mixed with soil to a level of 105 cfu/g soil. Pot experiments were performed for consecutive two seasons. The treatment combinations of the pot experiment were (i) control (ii) RFP-36 (iii) RFP-36 + M. phaseolina (iv) RFP-36 + R. solani (v) M. phaseolina (vi) R. solani. Each treatment had six replicate pots. Seeds were planted at a depth of approximately 1 cm from the surface of sterilized soil in earthen pots (25 × 25 cm). Germination of seed was recorded on tenth day after seedling. Plants were watered daily. At 60th day after seeding, shoot and root length and biomass of seedling were measured. Percentage of disease incidence (%DI) was calculated at 30th day by using the following formula,
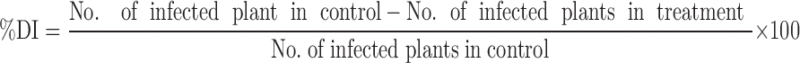 |
Statistical Analysis
The ANOVA approach was used to evaluate the efficiency of rhizobacterial isolates for P-solubilization and IAA production in broth cultures and growth promotion of broad bean. Comparison among means of treatments and appropriate control were made at P = 0.05. Pool data of the green house experiments of two consecutive seasons were subjected to ANOVA. Standard deviations of replicate values of zone of inhibition in dual plate assay were calculated only for the antagonized fungus.
Results and Discussion
Antagonistic plant-associated bacteria (APB) are an important functional group of beneficial bacteria responsible for the control of soil-borne fungal pathogen [27]. Occurrence of PGP attributes in APB may reinforce their effectiveness as microbial inoculants. In this study, a large number of morphologically different colonies were obtained from broad bean rhizosphere on King’s B agar, out of which 54 fluorescent Pseudomonas isolates (FPIs) were screened for antagonism against M. phaseolina and R. solani and PGP ability on broad bean. Fifteen FPI were able to inhibit M. phaseolina and R. solani in dual plate assay, as evident from the inhibition zone of 8–29 mm in case of M. phaseolina and 8–28 mm in case of R. solani (Table 1 and Fig. 1). In earlier dual plate assay, inhibition zone of 5 and 10 mm were recorded in case of M. phaseolina and R. solani by Pseudomonas auruginosa RsB29 [27]. The indigenous FPIs were not as effective in inhibition of the reference fungal pathogens used in this study. Interestingly, the reference fluorescent Pseudomonas strains (FPSs) were not effective in suppression of either the fungal pathogens of broad bean roots or the other reference plant root pathogen.
Table 1.
Inhibition zone of fungal pathogens of broad bean in dual plate assay due to inoculation with rhizobacterial pseudomonads and the reference strains
| Pseudomonads isolates | Inhibition zone of fungal pathogen (mm) | ||||
|---|---|---|---|---|---|
| Rhizoctonia solani | Macrophomina phaseolina | Fusarium oxysporum | Aspergillus fumigatus | Sclerotinia slerotiorum | |
| Control | 0 | 0 | 0 | 0 | 0 |
| RFP-36 | 28.0 ± 0.3 | 29.0 ± 0.4 | 8.0 ± 0.5 | 8.5 ± 0.5 | 10.0 ± 1.0 |
| RFP-59 | 9.0 ± 1.5 | 8.5 ± 0.9 | 0 | 8.0 ± 1.0 | 0 |
| RFP-18 | 8.0 ± 0.5 | 8.0 ± 0.5 | 8.0 ± 0.5 | 0 | 0 |
| RFP-LKBI | 12.5 ± 1.0 | 12.0 ± 1.2 | 8.0 ± 0.3 | 0 | 0 |
| RFP-LABI | 14.0 ± 1.2 | 12.0 ± 1.0 | 0 | 0 | 0 |
| RFP-56 | 8.5 ± 0.9 | 9.5 ± 0.9 | 0 | 0 | 0 |
| RFP-11 | 8.5 ± 1.0 | 0 | 0 | 8.5 ± 0.9 | 0 |
| RFP-44 | 18.0 ± 1.2 | 20.0 ± 1.2 | 8.0 ± 0.9 | 0 | 8.0 ± 1.5 |
| RFP-50 | 8.0 ± 1.0 | 8.0 ± 0.9 | 0 | 0 | 8.0 ± 0.5 |
| RFP-38 | 8.5 ± 1.5 | 9.0 ± 1.5 | 8.5 ± 0.6 | 0 | 0 |
| RFP-35 | 0 | 9.0 ± 1.0 | 0 | 0 | 0 |
| RFP-LAL | 14.0 ± 1.0 | 14.0 ± 1.2 | 8.5 ± 0.5 | 0 | 0 |
| RFP-PROG | 11.0 ± 1.5 | 10.0 ± 0.5 | 8.0 ± 1.5 | 0 | 0 |
| RFP-TOR | 10.0 ± 1.2 | 10.0 ± 1.5 | 0 | 0 | 0 |
| RFP-YM | 9.5 ± 1.0 | 9.0 ± 1.0 | 8.0 ± 1.0 | 0 | 0 |
| MTCC-438 | 8.0 ± 0.9 | 0 | 0 | 0 | 0 |
| MTCC-2759 | 8.0 ± 0.5 | 8.0 ± 0.9 | 0 | 0 | 0 |
| MTCC-2581 | 0 | 8.0 ± 1.0 | 0 | 7.0 ± 1.0 | 0 |
| MTCC-102 | 7.0 ± 1.0 | 8.0 ± 1.0 | 0 | 0 | 0 |
| MTCC-103 | 7.0 ± 1.0 | 8.0 ± 1.5 | 0 | 8.0 ± 1.5 | 0 |
Values are mean of four replicates ± standard deviation
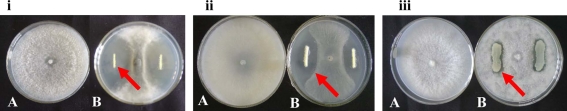
Fig. 1.
Antagonistic effect of fluorescent Pseudomonas isolate RFP-36 on iM. phaseolina and iiR. solaniiii MTCC-103 (Pseudonomas fluorescens) strain against M. phaseolina on PDA medium (A = control and B = Test plates)
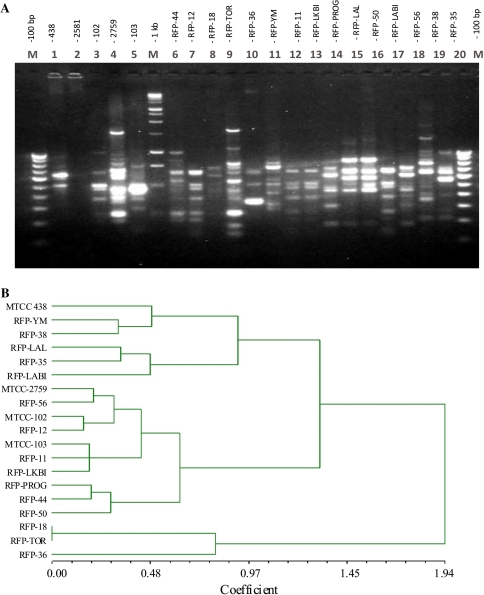
Inocula of the 15 indigenous FPIs and the reference FPSs stimulated growth of root and shoot of broad bean grown in test tubes compared to the uninoculated control, although the reference FPSs were not as effective as the superior indigenous FPIs. Highest level of growth stimulation was observed due to inoculation with the isolate RFP-36 followed by RFP-44 (Table 2). These two isolates also produced significant level of IAA like substances and soluble P in culture filtrate of PKA broth media supplemented with tryptophan and TCP. Level of IAA like substance ranged from of 24.1–66.7 μg/ml culture filtrate (Table 2). Earlier studies reported level of IAA in vitro to vary from 3.6 to 11.8 ppm in case of fluorescent pseudomonas of pea rhizosphere [28], and 2.0–21.6 ppm in rice rhizospheres [10]. Similarly, in previous research, range of soluble P was found to vary from 1.38 to 15.7 ppm in culture filtrates due to inoculation with fluorescent pseudomonas from different crop rhizosphere [29]. However, in none of these studies a clear correlation between these attributes and plant growth stimulation has been observed. In our study also, there was no significant correlation between these attributes and plant growth stimulation. Plant growth is stimulated by many other factors besides PGP parameters of microbial inoculants. Nevertheless, based on the PGP attributes, antagonisms of root fungal pathogen in vitro and broad bean growth in agar slants, clear evidence was obtained that the endemic pool of rhizobacteria of broad bean fields of Imphal valley contain FPIs which were superior than exotic reference FPSs. Earlier, Cho et al. [18] provided molecular technique based evidence on occurrence of certain degree of endemicity among FPSs in soils of biogeographical regions of the world. Our results suggest that endemic strains may also vary in functional properties for potential utility of superior isolates in local inoculum development programme. PCR-RAPD data also indicated distinctness of the indigenous FPIs and the reference FPSs of this study. Out of the four RAPD markers used in PCRs, only the primers OPA-4 generated discernable multiple band in 1.5% agarose (Fig. 2a) and the resultant dendogram (Fig. 2b) showed majority of the indigenous FPIs in one group of clusters and the remaining in another group along with the reference FPSs. Out of the 15 isolates, RFP-36 was found to be the most effective inhibitor of M. phaseolina and R. solani in vitro (Table 1) and it also separated into a distinct cluster in the dendogram (Fig. 2b). In some recent publication, it has been suggested that specificity of individual biocontrol bacteria in control of specific soil pathogen is linked to unique genetic machinery mediated functional properties [30]. Therefore, molecular mechanism of effective antagonism of RFP 36 against the two notorious soil borne pathogen need to be investigated in future research.
Table 2.
Level of soluble P and IAA like substances in culture filtrate and stimulation of growth of broad bean due to inoculation with fluorescent pseudomonas and reference strains grown in vitro for 5 and 10 days
| Treatment | Root length (cm) | Shoot length (cm) | Extent of P-solubilization | IAA like substances | ||
|---|---|---|---|---|---|---|
| 5 days | 10 days | 5 days | 10 days | (μg/ml) | (μg/ml) | |
| Control | 6.12 | 7.38 | 6.36 | 8.05 | 6.36 | 5.23 |
| RFP-36 | 8.37 | 10.52 | 39.56 | 11.90 | 39.56 | 49.7 |
| RFP-59 | 7.9 | 8.4 | 38.20 | 8.95 | 38.20 | 32.4 |
| RFP-18 | 6.5 | 7.8 | 12.70 | 8.8 | 12.70 | 32.4 |
| RFP-LKBI | 7.8 | 9.4 | 32.80 | 9.2 | 32.80 | 35.3 |
| RFP-LABI | 7.2 | 8.8 | 17.30 | 9.4 | 17.3 | 42.5 |
| RFP-56 | 7.8 | 9.0 | 40.56 | 10.0 | 40.56 | 28.4 |
| RFP-11 | 6.4 | 7.4 | 14.36 | 8.85 | 14.36 | 37.9 |
| RFP-44 | 7.9 | 9.6 | 56.80 | 10.8 | 56.80 | 39.5 |
| RFP-50 | 6.8 | 8.9 | 13.20 | 9.6 | 13.20 | 28.4 |
| RFP-38 | 8.1 | 10.0 | 44.90 | 10.8 | 44.90 | 66.7 |
| RFP-35 | 7.1 | 8.80 | 15.40 | 10.2 | 15.4 | 29.1 |
| RFP-LAL | 7.2 | 8.6 | 27.96 | 9.0 | 27.96 | 38.3 |
| RFP-PROG | 6.3 | 7.4 | 16.96 | 8.5 | 16.96 | 24.3 |
| RFP-TOR | 7.2 | 8.4 | 39.33 | 8.95 | 39.33 | 24.1 |
| RFP-YM | 6.8 | 8.9 | 12.80 | 8.8 | 12.80 | 33.5 |
| MTCC-438 | 6.76 | 7.9 | 19.93 | 8.4 | 19.93 | 29.3 |
| MTCC-2759 | 6.6 | 8.1 | 32.13 | 8.6 | 32.13 | 33.5 |
| MTCC-2581 | 6.8 | 7.8 | 36.86 | 8.2 | 36.86 | 30.9 |
| MTCC-102 | 6.8 | 8.2 | 15.80 | 9.4 | 15.80 | 53.1 |
| MTCC-103 | 7.1 | 8.0 | 38.72 | 9.2 | 38.72 | 29.8 |
| LSD (P = 0.05) | 0.2840 | 0.3896 | 8.6101 | 0.4389 | 6.2052 | 4.88 |
Fig. 2.
Electrophoretic pattern of polymerase chain reaction products (PCR) products (primers OPA-4) of the 20 IBSD Pseudomonas isolates and three reference strains from IMTECH, Chandigarh. a Dendogram showing genetic relatedness among the 20 Pseudomonas isolates of IBSD collection and three reference strains obtained from IMTECH, Chandigarh based on RAPD analysis. b The tree was constructed by using UPGMA, Disc coefficient method
RFP-36 was found to be very effective on broad bean in in vitro experiment. The two pathogens adversely affected seed germination, reduced shoot and root length and subsequently plant biomass also reduced significantly, as compared to the plants in the uninoculated control treatment. As expected, RFP-36 increased seed germination and seedling biomass of broad bean significantly. This isolate could also reduce the adverse effect of M. phaseolina and R. solani on plates of dual inoculated pots (Table 3). Seedlings (unbacterized) in M. phaseolina and R. solani infested soil developed distinct symptoms of charcoal rot and root rot diseases. In the pathogen inoculated pot, % disease incidence was 52 and 58% for M. phaseolina and R. solani, respectively and % disease incidence decreased to 27 and 25% due to bacterization of seeds with RFP-36. These pooled data of two consecutive experiments clearly indicated that the RFP-36 could exhibit PGP and biocontrol potential against M. phaseolina and R. solani in soil where the indigenous microflora was eliminated by autoclaving. It will be interesting to observe whether the isolates exhibit similar effect in unsterilized soil in presence of indigenous microflora.
Table 3.
Effect of seed bacterization with RFP-36 strain on percentage seed germination, disease incidence and growth parameters of broad bean seedling in M. phaseolina and R. solani inoculated in green house
| Treatment | Seed | Disease | Shoot length (cm) | Root length (cm) | Seedling |
|---|---|---|---|---|---|
| Germination | incidence | Biomass | |||
| (%) | (%) | (g/plant) | |||
| 60 days | |||||
| Control | 78 | 0.0 | 34.0 ± 2.0 | 14.0 ± 1.5 | 16.62 |
| RFP-36 | 88 | 0.0 | 40.0 ± 3.0 | 21.0 ± 1.0 | 27.86 |
| RFP-36 + M. phaseolina | 85 | 27.0 ± 1.5 | 36.0 ± 2.2 | 12.5 ± 1.2 | 20.21 |
| RFP-36 + R. solani | 84 | 25.0 ± 2.0 | 38.0 ± 2.5 | 14.5 ± 2.0 | 21.28 |
| M. phaseolina | 58 | 52.0 ± 2.4 | 22.0 ± 2.6 | 7.2 ± 1.5 | 6.91 |
| R. solani | 52 | 58.0 ± 2.0 | 21.0 ± 1.5 | 5.2 ± 2.0 | 7.72 |
| LSD (P = 0.05) | 1.47 | 2.4 | 2.1 | 1.2 | 3.74 |
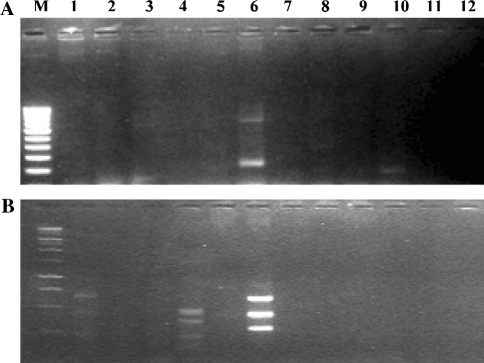
There are volume of literature on plant growth promotion by beneficial bacteria [31] and quite a few on biocontrol potential of fluorescent pseudomonas. Research work on screening fluorescent bacteria with multiple functions is relatively less [32]. Limited data on molecular characterization of our strain in this study suggest that RFP-36 could be a unique isolate. We obtained distinct products mostly for RFP-36 and RFP-44 when PCRs of genomic DNA of the isolates with specific primer sequence for specific antibiotic were carried out. The genomic DNA of isolate RFP-36 showed PCR products with primer sequence for 2,4-diacetyl phloroglucenol and for phenazine-1-carboxylic acid and similarly RFP-44, another efficient biocontrol isolate of our collection, also showed bands from PCR with specific primer for phenazine-1-carboxylic acid. Reference strain MTCC-2759 also showed bands although faintly with specific primers for phenazine-1-carboxylic acid (Fig. 3). However, antifungal activity of MTCC -2759 was observed to be much less when compared with the two local strains. This result suggests that the two local isolates might possess genes related to the two antibiotics. Earlier, Zhang [33] reported positive results from PCR of genomic DNA of few pseudomonas isolates with PHZ1/PHZ2 primers. However, we could not use a control strain positive for PCR with PHZ1/PHZ2 primers, in our study, to draw final conclusion on related gene for the bioactive principle in the strain. More research on performance in field situation and characterization of the superior isolates and the bioactive principles against the pathogens is currently under progress.
Fig. 3.
Polymerase chain reaction products (PCR) of gene specific primer for 2,4-diacetyl phloroglucenol (a) and for phenazine-1-carboxylic acid (b), Lane M 100 bp DNA marker, 1 MTCC-2759, 2 MTCC-103, 3 MTCC-102, 4 RFP-44, 5 RFP-12, 6 RFP-36, 7 RFP-18, 8 RFP-LKBI, 9 RFP-PROG, 10 RFP-LAL, 11 RFP-50, 12 RFP-56
References
- 1.Chavan JK, Kute LS, Kadam SS. Broad bean––nutritional chemistry, processing and utilization. In: Salunkhe DD, Kadam SS, editors. CRC hand book of world legumes. Boca Raton: CRC Press; 1989. pp. 223–245. [Google Scholar]
- 2.Wyllie DG. Charcoal rot of soyabean-current status. In: Wyllie TD, Scott DH, editors. Soyabean diseases of the north central region. St Paul: APS Press; 1988. [Google Scholar]
- 3.Weger LA, Bij AJ, Dekkers LC, Simons M, Wijffelman CA, Lugtenberg BJJ. Colonization of the rhizosphere of crop plants by plant-beneficial pseudomonads. FEMS Microbiol Ecol. 1995;17:221–228. [Google Scholar]
- 4.Gerhardson B. Biological substitutes for pesticides. Trends Biotechnol. 2002;20:338–343. doi: 10.1016/S0167-7799(02)02021-8. [DOI] [PubMed] [Google Scholar]
- 5.Postma J, Montanari M, Boogert PHJF. Microbiol enrichment to enhance the disease suppressive activity of compost. Eur J Soil Biol. 2003;39:157–163. doi: 10.1016/S1164-5563(03)00031-1. [DOI] [Google Scholar]
- 6.Welbaum G, Sturz AV, Dong J, Nowak J. Fertilizing soil microorganisms to improve productivity of agro ecosystems. Crit Rev Plant Sci. 2004;23:175–193. doi: 10.1080/07352680490433295. [DOI] [Google Scholar]
- 7.Whipps JM, et al. Ecological consideration involved in commercial development of biological control agents for soil-borne diseases. In: Ilsas, et al., editors. Modern soil microbiology. Wellington EMS: Marcel Dekker; 1997. pp. 215–243. [Google Scholar]
- 8.Hass D, Defago G. Biological control of soil-borne pathogens by fluorescent pseudmonads. Nat Rev Microbiol. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- 9.Chanway CP. Inoculation of tree roots with plant growth promoting soil bacteria: an emerging technology for reforestation. For Sci. 1997;43:99–112. [Google Scholar]
- 10.Thakuria D, Talukdar NC, Goswami C, Hazarika S, Boro RC, Khan MR. Characterization and screening of bacteria from rhizosphere of rice grown in acidic soils of Assam. Curr Sci. 2004;86:978–985. [Google Scholar]
- 11.Thakuria D, Talukdar NC, Goswami C, Hazarika S, Kalita MC, Bending GD. Evaluation of rice-legume-rice cropping system on grain yield, nutrient uptake, nitrogen fixation and chemical, physical and biological properties of soil. Biol Fertil Soils. 2009;47:237–251. doi: 10.1007/s00374-008-0320-4. [DOI] [Google Scholar]
- 12.Cattelen AJ, Hartel PG, Fuhrmann JJ. Screening for plant growth promoting rhizobacteria to promote early soyabean growth. Soil Sci Soc Am J. 1999;63:1670–1680. doi: 10.2136/sssaj1999.6361670x. [DOI] [Google Scholar]
- 13.Alexander DB, Zuberer DA. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils. 1991;12:39–45. doi: 10.1007/BF00369386. [DOI] [Google Scholar]
- 14.Kloepper JW, Leong J, Teintze M, Scrhorth MN. Enhanced plant growth by siderophores produced by plant growth promoting rhizobacteria. Nature. 1980;286:885–886. doi: 10.1038/286885a0. [DOI] [Google Scholar]
- 15.Dileep Kumar BS, Bezbaruah B. Plant growth promotion and fungal pest control through an antibiotic and siderophore producing fluorescent Pseudomonas strain from tea (Camellia sinensis (L.) O.Kuntze) plantations. Indian J Exp Biol. 1997;35:289–392. [Google Scholar]
- 16.Richardson AE (2003) Making microorganisms mobilize soil phosphorus. In: First virtual international meeting on microbial phosphate solubilization. http://webcd.usal.es/web/psm/abstracts/Richardson2.htm. pp 1–5
- 17.Loon LC, Bakker P, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Ann Rev Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- 18.Cho Jae-Chang, Tiedji JM. Biogeography and degree of endemicity of Fluorescent pseudomonas strains in soil. Appl Environ Microbiol. 2000;66:5446–5448. doi: 10.1128/aem.66.12.5448-5456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plaats-Niterink Van Der AJ (1981) Monograph of fungal studies. In: Mycology. Central Bureau Voor Schimmel Culture, Baarn
- 20.Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. New York: Macmillan; 1987. [Google Scholar]
- 21.Gordon DA, Webber RP. Colorimetric estimation of indole-3-acetic acid. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bray RH, Kurtz LT. Determination of total, organic and available form of phosphorus in soils. Soil Sci. 1945;59:39–45. doi: 10.1097/00010694-194501000-00006. [DOI] [Google Scholar]
- 23.Souza JT, Raaijimakkers JM. Polymorphisms within the prnD and Phc genes from pyrrolnitrin and pyoluteorin-producing Pseudomonas and Bulkholderia spp. FEMS Microbiol Ecol. 2003;43:21–34. doi: 10.1111/j.1574-6941.2003.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 25.Spadden Mc, Gardener BB, Mavrodi DV, Thomashow LS, Weller DM. A rapid polymerase chain reaction-based assay characterizing rhizosphere populations of 2, 4-diacetyl phloroglucinol-producing bacteria. Phytopathology. 2001;91:44–5423. doi: 10.1094/PHYTO.2001.91.1.44. [DOI] [PubMed] [Google Scholar]
- 26.Raajmaker JM, Weller DM, Thomashow LS. Frequency of antibiotic-producing Pseudomonas spps. In natural environments. Appl Environ Microbiol. 1997;63:881–887. doi: 10.1128/aem.63.3.881-887.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saikia Ratul, Singh Kiran, Arora DilipK. Suppression of Fusarium-Wilt and charcoal rot of chickpea by Pseudomonas aeruginosa RsB29. Indian J of Microbiol. 2006;44(3):181–184. [Google Scholar]
- 28.Pal KK, Dey R, Bhatt DM, Chaullan SM. Enhancement of groundnut growth and yield by plant growth promoting rhizobacteria. Int Arachis Newsl. 1999;19:51–53. [Google Scholar]
- 29.Chen Y, Mei R, Lu S, Liu L, Kloepper JW (1994) The use of yield increasing bacteria as plant growth promoting rhizobacteria in Chinese agriculture. In: Gupta VK, Utkhede R (eds) Management of soil born diseases, M/S Narosa Pub. House, New Delhi, pp 1–13
- 30.Bloemberg GV, Lugtenberg BJJ. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol. 2001;4(4):343–350. doi: 10.1016/S1369-5266(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 31.Biswas JC, Ladha JK, Dazzo FB, Yanni YG, Rolfe BG. Rhizobial inoculation influences seedling vigor and yield of rice. Agron J. 2000;92:880–886. doi: 10.2134/agronj2000.925880x. [DOI] [Google Scholar]
- 32.Hilali A, Przrost D, Broughton WJ, Antoun A. Effects of inoculation with strains of Rhizobium leguminoarum biovar trifolii on wheat growth in two soils of Moroco. Can J Microbiol. 2001;47:590–593. doi: 10.1139/cjm-47-6-590. [DOI] [PubMed] [Google Scholar]
- 33.Y Zhang (2004) Biocontrol of sclerotinia stem rot of Canola by bacterial antagonists and study of biocontrol mechanisms involved. Ph.D thesis, University of Manitoba, Canada