Abstract
The bacterial strains resistant to pentachlorophenol (PCP) and hexavalent chromium [Cr(VI)] were isolated from treated tannery effluent of a common effluent treatment plant. Most of the physico-chemical parameters analyzed were above permissible limits. Thirty-eight and four bacterial isolates, respectively were found resistant to >50 μg/ml concentration of [Cr(VI)] and the same level of PCP. Out of the above 42 isolates, only one was found simultaneously tolerant to higher levels of both PCP (500 μg/ml) and Cr(VI) (200 μg/ml), and hence was selected for further studies. To the best of our knowledge, this is the first report in which a native bacterial isolate simultaneously tolerant to such a high concentrations of Cr(VI) and PCP has been reported. The culture growth was best at 0.4% (w/v) glucose as an additional carbon source and 0.2% (w/v) ammonium chloride as a nitrogen source. The growth results with cow urine as a nitrogen source were comparable with the best nitrogen source ammonium chloride. The isolate exhibited resistance to multiple heavy metals (Pb, As, Hg, Zn, Co & Ni) and to antibiotics nalidixic acid and polymixin-B. The efficacy of bacterial isolate for growth, PCP degradation (56.5%) and Cr(VI) bioremediation (74.5%) was best at 48 h incubation. The isolate was identified as Bacillus sp. by morphological and biochemical tests. The 16S rDNA sequence analysis revealed 98% homology with Bacillus cereus. However, further molecular analysis is underway to ascertain its likelyhood of a novel species.
Keywords: Chromium, Heavy metals, Pentachlorophenol, Simultaneous bioremediation
Introduction
In India, leather tanning is one of the well developed industrial sectors. The effluent discharged from tanneries contain organic/inorganic chemicals and toxic metals [1]. The constituents of effluent impart colour, bad odour and eutrophication, consequently affecting the aesthetic quality of water. The heavy metals present in polluted water enter the human body through food chain and may cause adverse health effects [2]. Chromium(VI) is one of the major environmental pollutants which enters the ecosystem by metal finishing, leather tanning, chromate preparation and cooling towers of atomic power plants. In the environment, chromium occurs mainly in trivalent and hexavalent forms. Chromium(VI) is soluble, toxic and carcinogenic [3]. Chromium sulphate [Cr(III)] is used as a tanning agent, resulting in severe ground water contamination around tanneries, which is transformed to Cr(VI), which has been reported to pose health risk in humans, animals and plants [4]. Bioremediation of soluble Cr(VI) can be effectively done by microbes in which it is reduced to less toxic Cr(III) [5]. Several bacteria such as Pseudomonas ambigua [6], Bacillus sp. [7, 8], Serratia marcescens [9] have been reported for Cr(VI) reduction. Serious concern about the toxicity of Cr compounds necessitates recovery and reuse of Cr from tannery effluent as well as other industrial wastes and/or rendering it to a less toxic form [10].
Pentachlorophenol (PCP) is used as a biocide in leather tanning process, and is capable of being biodegradable by only a limited number of bacteria [11, 12]. Apart from Cr(VI), PCP is highly toxic, and a recalcitrant organic compound because of its stable aromatic ring system and high chloride content, thereby persisting in the environment [13]. Pentachlorophenol is toxic to all forms of life since it is an inhibitor of oxidative phosphorylation [14]. The United States Environmental Protection Agency (USEPA) has listed PCP as a priority contaminant because of its proven carcinogenicity and toxicity. Different researchers have reported PCP degrading microorganisms from the natural environment. Several bacterial strains such as Arthrobacter,Pseudomonas,Sphingobium chlorophenolicum, S. marcescens capable of PCP degradation have been reported [15–18].
However, only a limited work has been done toward simultaneous bioremediation of Cr(VI) and phenolics in the tannery effluents [19–21], particularly by native microbes. Most of the researchers have employed either coculture or microbial consortium for simultaneous bioremediation of PCP and Cr(VI). The authors failed to find even a single report in which only one indigenous strain tolerant to high concentration of both PCP and Cr(VI) was employed for simultaneous microbial remediation. If a single potent native strain is available, then its nutritional requirement, growth and maintenance are likely to be more conveniently managed than a coculture or a consortium. In the light of above facts, the present study was aimed at finding a bacterial isolate from the same ecological niche (treated tannery effluent) which can sustain high concentration of Cr(VI) and PCP, and studying its characteristics under various growth conditions so as to ensure that the same isolate could be efficiently employed for simultaneous bioremediation of PCP and Cr(VI) from tannery effluent.
Materials and Methods
Sampling
The treated tannery effluent samples were collected in sterile glass bottles from common effluent treatment plant (CETP) Unnao (India), transported on ice to the laboratory and analyzed within 6 h of collection.
Physico-Chemical Parameters and Metal Analyses
The physico-chemical parameters were determined as per APHA [22], and an average of triplicate for each experiment is being reported. Total chromium {Cr(VI) + Cr(III)} in the samples was determined using Perkin–Elmer 5000 atomic absorption spectrophotometer (AAS) at 357.9 nm, after digestion of samples with a mixture of concentrated nitric (six parts) and perchloric (one part) acids. The digested samples were also analyzed for other heavy metals such as lead, cadmium, zinc, copper, nickel and arsenic using AAS.
Isolation and Screening of PCP and Cr(VI) Resistant Bacteria
Pentachlorophenol resistant bacteria from treated tannery effluent were isolated by standard plate technique [22, 23] on minimal salt medium (MSM) agar plates containing (g/l): KH2PO4, 6.0; Na2HPO4·2H2O, 7.0; MgSO4·7H2O, 0.2; NH4Cl, 2.0 and purified agar, 18 (pH 7.0 ± 0.2) amended with different concentrations (μg/ml) of PCP (50, 100, 150, 200, 250, 300, 350 400, 450, 500, 550) along with glucose 0.4% (w/v) as an additional carbon source [24]. The plates were incubated at 35 ± 1°C for 60 h. The Cr(VI) resistant bacteria were isolated on nutrient agar (NA) amended with different concentrations (μg/ml) of K2Cr2O7 as a source of Cr(VI) (50, 100, 150, 200, 250, 300, 350, 400, 450, 500) and incubated at 35 ± 1°C for 48 h. The bacterial colonies on MSM agar (four isolates) and NA plates (38 isolates) were picked up and purified by repeated streaking on the same medium. The above bacterial isolates (42) were screened on MSM agar plates amended with varying concentrations of PCP (50–500 μg/ml) and 200 μg/ml of Cr(VI), incubated at 35 ± 1°C for 60 h for isolation of most efficient bacterial isolate likely to be employed for further studies on simultaneous bioremediation of Cr(VI) and PCP.
Identification of Selected Bacterial Isolate
The selected bacterial isolate was characterized as per Bergey’s Manual of Determinative Bacteriology [25]. The molecular characterization of isolate was done by 16S rDNA sequence analysis at Institute of Microbial Technology (IMTECH), Chandigarh (India). The bacterial genomic DNA was extracted using Axygen Genomic DNA extraction Kit; using 16S universal primers: 27f (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492r (5′-TACGGYTAACCTTGTTACGACTT-3′), an amplified product of 16S rDNA was obtained which was sequenced using ABI 3130X-Genetic Analyzer (Applied biosystem, USA). The 16S rDNA sequence was deposited to GenBank database to get the accession number, and to identify most similar sequence alignment using www.ncbi.nlm.nih.gov/BLAST [26]. The nucleotide sequences were aligned with Clustal W, and phylogenetic tree was constructed with MEGA 4.1 software using neighbour joining (NJ); the significance of junctions was established using the bootstrap method (1,000 replicates).
Optimization of Culture Medium
Sucrose and glucose were attempted for optimization of carbon and energy source. Different concentrations of sucrose or dextrose as an additional carbon and energy source at 0.2–1.0% (w/v) and ammonium chloride, ammonium nitrate or urea as a nitrogen source at 0.1 and 0.2% (w/v) were supplemented in MSM broth amended with maximum tolerable concentration of PCP (500 μg/ml) and Cr(VI) (200 μg/ml). The cow urine was also attempted as a nitrogen source. In this experiment, MSM broth was prepared in filtered (using 0.2 μm diameter bacterial filter) cow urine instead of distilled water without any other nitrogen source. The broth was inoculated with fresh inoculum of exponential phase culture (at 1% v/v) of 0.86 absorbance having cell density 3.0 × 106 cfu/ml. The flasks were incubated at 35 ± 1°C on a rotatory shaker (New Brunswick Scientific) at 150 rpm for 3 days. The growth was observed at 12 h interval up to 60 h by measuring the absorbance at 600 nm.
Other Heavy Metal Resistance and Antibiotic Sensitivity Test
The promising PCP and Cr(VI) resistant bacterial isolate was studied for other heavy metals resistance and sensitivity to antibiotics. The exponential phase culture of bacterial isolate was inoculated aseptically on NA plates supplemented with other heavy metals such as lead (Pb), arsenic (As), zinc (Zn), cobalt (Co), nickel (Ni) and mercury (Hg) along with Cr at 200 μg/ml. The metal salts used were: nickel chloride, lead acetate, zinc chloride, cobalt nitrate, sodium arsenate and mercuric chloride. The metal concentrations ranged from 25 to 200 μg/ml. The growth was observed after 24 h incubation up to 48 h at 35 ± 1°C. Susceptibility to different antibiotics (viz. methicillin, nalidixic acid, polymixin-B, kanamycin, cephaloridine, co-trimazole, streptomycin and tetracyclin) for selected bacterium was determined by disc diffusion method [27]. The antibiotic impregnated discs (Oxoid) were placed on freshly prepared lawn of bacterial isolate on Mueller Hinton agar plates, and incubated at 35 ± 1°C for 24 h. The bacterial isolate was classified as resistant or susceptible by examining the zone of inhibition on the lawn of bacterial culture.
Growth Study of Selected Bacterium
Optimized MSM broth supplemented with glucose at 0.4% (w/v) and ammonium chloride at 0.2% (w/v) amended with PCP (500 μg/ml) and Cr(VI) (200 μg/ml) individually and in combination were inoculated aseptically with 1% (v/v) culture having 3.0 × 106 cfu/ml (absorbance 0.86). The flasks were incubated at 35 ± 1°C for 60 h on a rotatory shaker at 150 rpm. The growth of bacterial culture was measured at 12 h interval up to 60 h at 600 nm. The growth was also measured in the presence of other heavy metals (μg/ml) such as Pb (175), As (105), Zn (60), Co (80), Ni (105) and Hg (25), in addition to chromium. The resistance concentrations of other heavy metals in this experiment were determined on the basis of previous experiment conducted on the agar plates.
Simultaneous PCP Degradation and Cr(VI) Reduction
The PCP degradation and Cr(VI) reduction under optimized conditions of growth (pH 7.0, 35 ± 1°C) was performed in MSM broth containing PCP (500 μg/ml) and Cr(VI) (200 μg/ml) supplemented with 0.4% (w/v) glucose and 0.2% (w/v) ammonium chloride (as nitrogen source) in 250 ml Erlenmeyer flasks. The uninoculated MSM broth amended with same concentration of PCP and Cr(VI) served as control. The samples were withdrawn at 12 h interval up to 60 h, centrifuged at 10,000 rpm for 10 min at 4°C in cooling centrifuge and decanted the culture supernatant swiftly. The extent of PCP degradation was determined by estimation of chloride ions released in the culture supernatant, which were quantified as per the method of Bergmann and Sanik [28], and were extrapolated against the standard curve of sodium chloride. The method of APHA [22] was followed for the estimation of Cr(VI). The extent of Cr(VI) reduction was determined by extrapolating residual Cr(VI) against K2Cr2O7 standard curve.
Statistical Analysis
All experiments were performed in triplicate. The statistical calculation was done according to the standard method [29]. The results are given as mean ± SD values.
Results and Discussion
Physico-Chemical Parameters and Metal Analyses of Treated Tannery Effluent
Table 1 presents the physico-chemical constituents and heavy metals analyzed in the treated tannery effluent. The results indicate that BOD (104.90 ± 0.25 mg/l), COD (490.93 ± 1.27 mg/l), TDS (2366.43 ± 1.65 mg/l), residual chlorine (5.17 ± 0.18 mg/l), sulphide (9.43 ± 0.20 mg/l), nitrate (15.09 ± 0.05 mg/l), phenol (11.93 ± 0.17 mg/l), oil and grease (19.86 ± 0.67 mg/l) were found much higher than the recommended permissible limits prescribed by the Ministry of Environment and Forest (MOEF) and the USEPA. However, pH (7.3 ± 0.15), temperature (34 ± 0.27°C) and total nitrogen (30.64 ± 0.69 mg/l) were within the recommended permissible limits. The alkalinity is not a pollutant rather a total measure of substances in liquid that has acid-neutralizing ability, and is important for aquatic life as it protects against the pH changes. Although the authors have analyzed total acidity, alkalinity and hardness, the standards are yet to be established in tannery effluents. Total chromium and Cr(VI) were 8.89 ± 0.74 and 1.26 ± 0.05 mg/l, respectively, which were above the permissible limits. The concentrations of other heavy metals detected were lead (0.47 mg/l) and arsenic (0.39 mg/l), which were also above the recommended permissible limits. However, nickel and zinc were found within the recommended permissible limits. Copper and cadmium were detected negligible in the effluent. Nickel mainly affects the digestive tract and central nervous system, and also imparts cytotoxic effect. High doses of zinc for long time may lead to a lower concentration of plasma lipoproteins and decreased copper absorption [30]. The presence of copper ions causes serious toxicological concerns, which is usually known to deposit in brain, skin, liver, pancreas and myocardium [31]. Decreased copper level may also inhibit the transport of iron resulting in anemia [32]. The results suggest that treated tannery effluent released in the environment for various purposes is not satisfactory with regard to its physico-chemical and heavy metal contents. The high values of BOD and COD indicate higher level of organic compounds that are being discharged into the water bodies contributing to their eutrophication.
Table 1.
Physico-chemical and heavy metal analyses of treated tannery effluent
| Physico chemical parameter/heavy metal | Permissible limitb | Obtained value |
|---|---|---|
| pH | 5.5–9.0 | 7.3 ± 0.15ª |
| Temperature | <35°C | 34 ± 0.27 |
| Total solid (mg/l) | – | 3468 ± 1.89 |
| Total suspended solid (mg/l) | 600 | 1102.25 ± 0.22 |
| Total dissolved solid (mg/l) | 2100 | 2366.62 ± 1.65 |
| Total alkalinity (mg/l) | – | 340 ± 3.05 |
| Total acidity (mg/l) | – | 201.33 ± 1.08 |
| Residual chlorine (mg/l) | 1 | 5.17 ± 0.18 |
| Hardness (mg/l) | – | 780.45 ± 1.02 |
| Sulphide (mg/l) | 2.0–5.0 | 9.43 ± 0.20 |
| Oil and grease (mg/l) | 10.0 | 19.86 ± 0.67 |
| B.O.D. (mg/l) | 30.0 | 104.90 ± 0.25 |
| C.O.D. (mg/l) | 250.0 | 490.93 ± 1.27 |
| Total nitrogen (mg/l) | 100.0 | 30.64 ± 0.69 |
| Nitrate (mg/l) | 10.0 | 15.09 ± 0.05 |
| Phenol (mg/l) | 1–5.0 | 11.93 ± 0.17 |
| Cr6+ (mg/l) | 0.1 | 1.26 ± 0.05 |
| Total Cr (mg/l) | 2.0 | 8.89 ± 0.74 |
| Pb2+ (mg/l) | 0.1 | 0.47 |
| Cu2+ (mg/l) | 3.0 | 0.006 |
| As3+ (mg/l) | 0.2 | 0.39 |
| Ni2+ (mg/l) | 3.0 | 0.72 |
| Zn2+ (mg/l) | 5.0 | 0.36 |
| Cd2+ (mg/l) | 2.0 | 0.002 |
aMean value ± SD, b Standards given by Ministry of Environment and Forest (MOEF) and United States of Environment Protection Agency (USEPA)
Isolation and Screening of PCP and Cr(VI) Resistant Bacteria
The microbiological examination of treated tannery effluent revealed the presence of PCP and Cr(VI) tolerant bacteria at 3.0 × 104 and 3.0 × 106 cfu/ml, respectively. A total of 38 bacterial isolates were found resistant to Cr(VI) at >50 μg/ml, while four isolates were resistant to PCP concentration of >50 μg/ml, in the presence of glucose supplemented at 0.4% (w/v) as an additional carbon and energy source. However, the growth was negligible in the absence of glucose indicating the phenomenon of co-metabolism in which microorganisms do not obtain energy from the transformation reaction, rather require another substrate for growth. Dehalogenation and oxidative dehalogenation reactions are important co-metabolism reactions, which may make pesticide molecule accessible for further breakdown [33]. Out of the above bacterial isolates exhibiting tolerance to Cr(VI) and PCP independently, only one was found tolerant to higher levels with simultaneous presence of both PCP (500 μg/ml) and Cr(VI) (200 μg/ml), and hence was selected for further detailed studies. The extended higher levels of Cr(VI) above 200 μg/ml and PCP above 500 μg/ml were found toxic which was evident from no growth on agar plates as well as in broth experiments. Our isolate was found to be more resistant to simultaneous presence of both Cr(VI) and PCP than the strains reported by most of other researchers so far. However, Srivastava et al. [20] have reported that Acinetobacter sp. isolated from pulp and paper mill effluent could tolerate maximum PCP up to 50 μg/ml and Cr(VI) up to 500 μg/ml.
Identification of Selected Bacterial Isolate
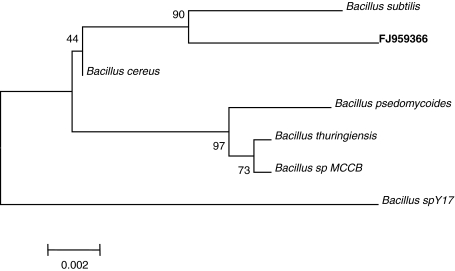
The selected potential bacterial isolate resistant to high simultaneous levels of both PCP (500 μg/ml) and Cr(VI) (200 μg/ml) was subjected to identification by determining its morphological and biochemical characteristics as per Bergey’s Manual of Determinative Bacteriology [25]. The isolate was identified as Bacillus sp. These characteristics observed in our laboratory were also confirmed at the IMTECH, Chandigarh (India). The identification of isolate (MTCC 9777) was further authenticated by 16S rDNA sequence analysis at IMTECH, Chandigarh. Using forward and reverse primers, 1460 bp of 16S rDNA sequence of the isolate was obtained which was deposited to GenBank, and the accession number FJ959366 was obtained. Using BLAST search (www.ncbi.nlm.nih.gov/BLAST) of the obtained sequence, the culture exhibited maximum 98% similarity with Bacillus cereus. The phylogenetic tree constructed with MEGA 4.1 software using NJ is depicted in Fig. 1. The 16S rDNA gene is the most widely accepted gene employed for bacterial classification and identification. Signature nucleotide of 16S rDNA gene allows classification even if a particular sequence has no match in the database, since otherwise unrecognizable isolate can be assigned to phylogenetic branches at the class, family, genus or subgenus levels. However, our isolate needs further molecular characterization to ascertain its identification at species level which may likely turnout to be a novel one.
Fig. 1.
Phylogenetic neighbor-joining tree of isolate created with MEGA 4.1 software
Optimization of Culture Medium
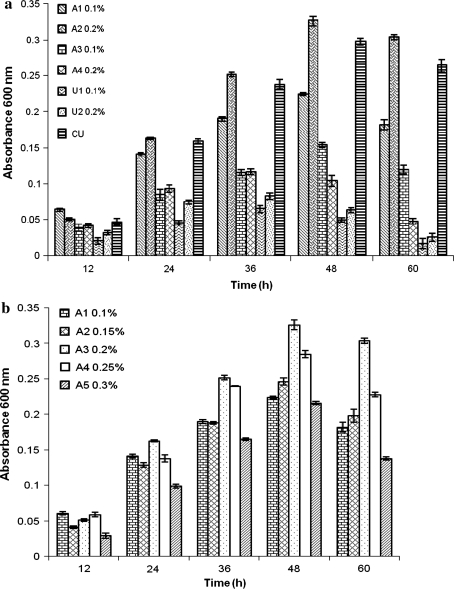
The MSM broth containing 500 μg/ml PCP and 200 μg/ml Cr(VI) was optimized for carbon source (glucose and sucrose) as cosubstrate and for various nitrogen sources including ammonium chloride, ammonium nitrate, urea and cow urine. Different concentrations ranging 0.2–1.0% (w/v) of sucrose or glucose were tried, and observed that maximum growth was evident when glucose was employed as an additional carbon and energy source at 0.4% (w/v) level during the course of 48 h incubation at 35 ± 1°C (not shown). Figure 2a depicts that ammonium chloride was best nitrogen source at 0.2% (w/v) concentration followed by ammonium nitrate and urea. Astonishingly, the growth response in presence of cow urine as a nitrogen source was better than urea, and was almost comparable to ammonium chloride. The better response of cow urine as a nitrogen source could be attributed to the presence of many constituents including potassium, sodium, calcium, magnesium, trace metals, etc. [34]. To the best of authors’ knowledge, cow urine has been attempted for the first time as a source of nitrogen for the sake of bacterial growth.
Fig. 2.
a Effect of nitrogen sources: ammonium chloride (A1 & A2), ammonium nitrate (A3 & A4), urea (U1 & U2) and cattle urine (CU) on growth of the bacterial isolate. (Error bars are standard deviation). b Effect of different concentrations of the best nitrogen source ammonium chloride on the growth of bacterial isolate. (Error bars are standard deviation)
Other Heavy Metal Resistance and Antibiotic Sensitivity
The Cr(VI) and PCP tolerant isolate was also tested for tolerance to other heavy metals based on their presence in tannery effluent. The isolate exhibited pairwise [with Cr(VI) at 200 μg/ml] resistance to heavy metals (μg/ml) such as Pb (175), As (105), Hg (25), Zn (60), Co (80), Ni (105), and also to the multimetal combination of all. Further increase in heavy metal concentration merely by 5 μg/ml individually or in combination rendered the isolate sensitive to all of them (Table 2). The results suggest that our bacterial isolate has an added advantage to withstand the presence of other heavy metals, and perform the desired activity. Such resistance may be possibly due to exclusion of metal species, bioaccumulation, transformation, production of low molecular weight binding proteins, etc. [35]. Since heavy metal resistance is likely to be linked with antibiotic resistance [36], the chromate and other multi heavy metal resistant isolate was tested for its sensitivity to different antibiotics. The isolate was sensitive to antibiotics (mcg/disc) kanamycin (30), methicillin (10), co-trimazole (25), streptomycin (25), tetracyclin (30), cephaloridine (30), while resistant to nalidixic acid (30) and polymixin-B (50) (Table 2). This indicates its broad range environmental adaptation. Such metal tolerant bacterium is very important when it is also antibiotic resistant under metal stress conditions in the environment.
Table 2.
Heavy metal resistance and antibiotic sensitivity test of selected bacterial isolate
| Metals (μg/ml)/antibiotics (mcg/disc) | Sensitive (S)/Resistant (R) |
|---|---|
| Pb (175) | R |
| (180) | S |
| As (105) | R |
| (110) | S |
| Hg (25) | R |
| (30) | S |
| Zn (60) | R |
| (65) | S |
| Co (80) | R |
| (85) | S |
| Ni (105) | R |
| (110) | S |
| Pb (175) + As (105) + Hg (25) + Zn (60) + Co (80) + Ni (105) | R |
| Pb (180) + As (110) + Hg (30) + Zn (65) + Co (85) + Ni (110) | S |
| Kanamycin (30) | S |
| Methicillin (10) | S |
| Nalidixic acid (30) | R |
| Co-trimazole (25) | S |
| Streptomycin (25) | S |
| Polymixin-B (50) | R |
| Tetracyclin (30) | S |
| Cephaloridine (30) | S |
Note Pairwise Cr(VI) 200 μg/ml was present along with individual heavy metal and multi metals
Growth Study of the Isolate
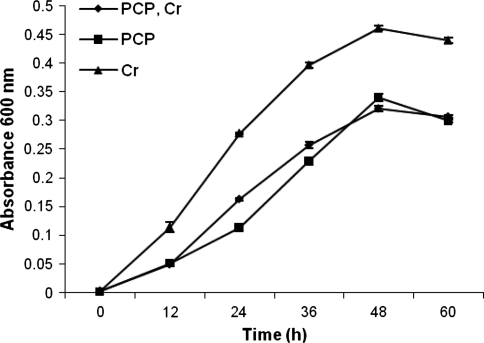
The growth response of bacterial isolate in the presence of Cr(VI) and/or PCP was studied in MSM broth containing 0.4% (w/v) glucose and 0.2% (w/v) ammonium chloride, and the results are presented in Fig. 3. The growth response was maximum at 48 h in the presence of Cr(VI) alone. However, the growth response was less by 26.25% with PCP alone without Cr(VI) and by 30.37% with simultaneous presence of both PCP and Cr(VI). The growth response observed in the presence of PCP alone and PCP + Cr(VI) remained approximately same during the entire course of 60 h growth thereby indicating that the presence of Cr(VI) did not have much inhibitory effect on growth response when xenobiotic PCP was present in the medium. Therefore, our isolate is a potential candidate for simultaneous bioremediation of PCP and Cr(VI).
Fig. 3.
Growth response of bacterial isolate in the presence of PCP and Cr(VI). (Error bars are standard deviation)
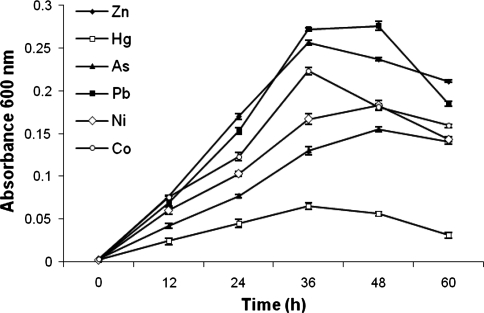
Natural habitats are generally characterized by coexistence of a large number of toxic and nontoxic cations. Therefore, it is necessary to study multiple metal effects on the growth of microorganisms [37]. Figure 4 depicts the resistance of bacterial isolate against other heavy metals in the presence of PCP 500 μg/ml and Cr(VI) 200 μg/ml in MSM broth. The presence of other heavy metals exhibited an inhibitory effect on growth response of the bacterial isolate. The mercury showed maximum and lead minimum inhibitory effect on the growth of isolate. The order of per cent inhibition was: Hg (79.76) > As (51.72) > Ni (43) > Co (27.42) > Zn (20.25) > Pb (14.02). We have analyzed tannery effluent for the presence of heavy metals, and found that total Cr (8.89 mg/l), Pb (0.47 mg/l) and As (0.39 mg/l) exceeded the permissible limits (Table 1). However, our isolate is tolerant to much higher levels of Cr and other heavy metals that are present in the tannery effluent. Multiple heavy metal resistance to Ni, Cr, and Zn has also been reported by Margesin and Schinner [38].
Fig. 4.
Growth response of bacterial isolate in simultaneous presence of PCP and Cr(VI) along with other heavy metals. (Error bars are standard deviation)
The advantage of selecting indigenous bacteria from natural habitats may be for minimization of inhibitory effects from other compounds that may be present along with Cr(VI), since viable organisms must have developed at least some degree of resistance to these compounds. Furthermore, it might be practical to use Cr(VI) reducing microorganisms to reduce other metals simultaneously [39].
Simultaneous PCP Degradation and Cr(VI) Reduction
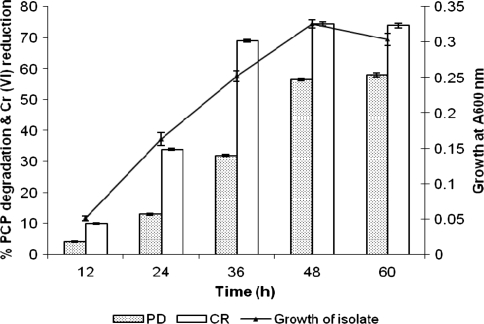
The extent of PCP degradation and Cr(VI) bioremediation was also studied during 60 h growth of Bacillus sp. isolate in the simultaneous presence of PCP and Cr(VI), and the results are depicted in Fig. 5. The results clearly indicate that there is a concomitant increase in bacterial growth during 0–48 h with simultaneous remediation of hexavalent Cr (0–74.5%) and biodegradation of PCP (0–56.5%) up to 48 h incubation. Further increase in time up to 60 h did not have any significant effect on above three determinations, except PCP biodegradation which increased marginally by 1.5% at 60 h, indicating thereby that 48 h is the optimum time for growth and simultaneous bioremediation of Cr(VI) and PCP. The literature available on simultaneous bioremediation of Cr(VI) and PCP by a single indigenous isolate is very scanty. Other researchers have employed either a coculture or a consortium of pure cultures [19, 21] or natural isolates from other than tannery effluents [20]. However, it would be always better to use single strain, if available, as it is convenient to handle and maintain under standard cultural and nutritional conditions.
Fig. 5.
The growth kinetics of Bacillus sp. isolate for establishing its efficacy toward simultaneous bioremediation of pentachlorophenol and Cr(VI). (Error bars are standard deviation)
Conclusion
The Bacillus sp. isolate in this study exhibited very high-level of resistance against Cr and PCP both in broth and on agar medium. Such a high-level of resistance has not been reported in earlier studies undertaken for simultaneous bioremediation of Cr(VI) and PCP by a single indigenous bacterial isolate. The culture requires supplementation of 0.4% (w/v) glucose as an additional carbon and energy source. The cow urine has been attempted, for the first time, as a sole nitrogen source. A higher 56.5% (near maximum) level of PCP biodegradation and maximum simultaneous Cr(VI) bioremediation of 74.5% corresponded with best growth at 48 h incubation. Besides chromium, the isolate has a broad range of multi heavy metal (Pb, Zn, Co, Ni, As, Hg) and antibiotic (nalidixic acid and polymixin-B) resistances which shows a positive sign for application of this strain in the treatment of industrial effluents. Further detailed studies on simultaneous bioremediation of Cr and PCP are underway.
References
- 1.Irshad A, Ali S, Jan MR (1997) Physico chemical studies of industries pollutants. In: Proceedings of NSMTCC’97 on Environ Pollution Feb. 24–26, Islamabad, Pakistan pp 93–99
- 2.Stein JA, Tschudy DP, Coroan PC, Coffins A. Metal pollution. Biol Chem. 1990;245:2213. [Google Scholar]
- 3.Ackerley DF, Gonzalez CF, Park CH, Blake IR, Keyhan M, Martin A. Chromate reducing properties of soluble flavoproteins from Pseudomonas putida and Escherichia coli. Appl Environ Microbiol. 2004;70:873–882. doi: 10.1128/AEM.70.2.873-882.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upreti RK, Srivastava R, Chaturvedi UC. Gut microflora and toxic metals: chromium as a model. Indian J Med Res. 2004;119:49–59. [PubMed] [Google Scholar]
- 5.Chen JM, Hao OJ. Microbial chromium (VI) reduction. Crit Rev Environ Sci Technol. 1998;28:219–225. doi: 10.1080/10643389891254214. [DOI] [Google Scholar]
- 6.Mclean J, Beveridge TJ. Chromate reduction by a Pseudomonad isolated from a site contaminated with chromated copper arsenate. Appl Environ Microbiol. 2001;67:1076–1084. doi: 10.1128/AEM.67.3.1076-1084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camargo FAO, Okele BC, Bento FM, Frankberger WT. Hexavalent chromium reduction by immobilized cells and cell-free extract of Bacillus sp. ES29. Biorem J. 2004;8:23–30. doi: 10.1080/10889860490453140. [DOI] [Google Scholar]
- 8.Rehman A, Zahoor A, Munner A, Hasnain A. Chromium tolerance and reduction potential of a Bacillus sp. env3 isolated from metal contaminated wastewater. Bull Environ Contam Toxicol. 2008;81:25–29. doi: 10.1007/s00128-008-9442-5. [DOI] [PubMed] [Google Scholar]
- 9.Mondaca MA, Campos V, Moraga R, Zaror CA. Chromate reduction in Serratia marcescens isolated from tannery effluent and potential application for bioremediation of chromate pollution. Sci World J. 2002;2:972–977. doi: 10.1100/tsw.2002.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto K, Kato J, Yamo T, Ohtake J. Kinetics and modeling of hexavalent chromium reduction in Enterobacter cloacae. Biotechnol Bioeng. 1993;41:129–133. doi: 10.1002/bit.260410117. [DOI] [PubMed] [Google Scholar]
- 11.Thakur IS, Verma PK, Upadhaya KC. Involvement of plasmid in degradation of pentachlorophenol by Pseudomonas sp. from a chemostat. Biochem Biophys Res Commun. 2001;286:109–113. doi: 10.1006/bbrc.2001.5340. [DOI] [PubMed] [Google Scholar]
- 12.Yang CF, Lee CM, Wang CC. Isolation and physiological characterization of the pentachlorophenol degrading bacterium Sphingomonas chlorophenolica. Chemosphere. 2006;62(5):709–714. doi: 10.1016/j.chemosphere.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Copley SD. Evolution of metabolic pathway for degradation of a toxic xenobiotic: the patchwork approach. Trends Biochem Sci. 2000;25(6):261–265. doi: 10.1016/S0968-0004(00)01562-0. [DOI] [PubMed] [Google Scholar]
- 14.Bock C, Kroppenstedt RM, Schmidt U, Diekmann H. Degradation of prochloraz and 2, 4, 6-trichlorophenol by environmental bacterial strains. Appl Microbiol Biotechnol. 1996;45(1–2):257–262. doi: 10.1007/s002530050680. [DOI] [PubMed] [Google Scholar]
- 15.Edgehill RU. Pentachlorophenol removal from slightly acidic mineral salts, commercial sand, and clay soil by recovered Arthrobacter strain ATCC 33790. Appl Microbiol Biotechnol. 1994;41:142–148. doi: 10.1007/BF00166097. [DOI] [Google Scholar]
- 16.Thakur IS, Verma PK, Upadhaya KC. Molecular cloning and characterization of pentachlorophenol degrading mono oxygenase gene in Pseudomonas sp. from chemostat. Biochem Biophys Res Commun. 2002;290:770–774. doi: 10.1006/bbrc.2001.6239. [DOI] [PubMed] [Google Scholar]
- 17.Dams RI, Paton GI, Killham K. Rhizomediation of pentachlorophenol by Sphingobium chlorophenolicum ATCC 39723. Chemosphere. 2007;68:864–870. doi: 10.1016/j.chemosphere.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Chandra R, Patel DK, Rai V. Isolation and characterization of novel Serratia marcescens (AY927692) for pentachlorophenol degradation from pulp and paper mill waste. World J Microbiol Biotechnol. 2007;23:1747–1754. doi: 10.1007/s11274-007-9424-5. [DOI] [PubMed] [Google Scholar]
- 19.Chirwa FMN, Wang Y-T. Modeling hexavalent chromium reduction and phenol degradation in a coculture biofilm reactor. ASCE J Environ Eng. 2005;131:1495–1506. doi: 10.1061/(ASCE)0733-9372(2005)131:11(1495). [DOI] [Google Scholar]
- 20.Srivastava S, Ahmad AH, Thakur IS. Removal of chromium and pentachlorophenol from tannery effluents. Bioresour Technol. 2007;98:1128–1132. doi: 10.1016/j.biortech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Tziotzios G, Dermou E, Eftychia P, Dorothea V, Dimitris V. Simultaneous phenol removal and biological reduction of hexavalent chromium in a packed-bed reactor. J Chem Tech Biotechnol. 2008;83(7):829–835. [Google Scholar]
- 22.Standard methods for examination of water and wastewater. 20. Washington DC, USA: American Public Health Association, American Water Works Association and Water pollution Control Federation; 1998. [Google Scholar]
- 23.Baldi F, Vaughan AM, Olson GJ. Chromium (VI)-resistant yeast isolated from a sewage treatment plant receiving tannery wastes. Appl Environ Microbiol. 1990;56:913–918. doi: 10.1128/aem.56.4.913-918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfennig N, Lippert KD. Uber das vitamin B12-Bedurfnis phototropher Schwefelbakterien. Arch Mikrobiol. 1966;55:245–256. doi: 10.1007/BF00410246. [DOI] [Google Scholar]
- 25.Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST. Bergey’s manual of determinative bacteriology. 9. Baltimore: Williams & Wilkins; 1994. [Google Scholar]
- 26.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer AW, Kirby WMM, Sherries JC. Truck M antibiotic susceptibility testing by a standardised single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 28.Bergmann JG, Sanik J. Determination of trace amounts of chlorine in naphtha. Anal Chem. 1957;29:241–243. doi: 10.1021/ac60122a018. [DOI] [Google Scholar]
- 29.Steel R, Torrie JH. Principles and procedures of statistics. New York: McGraw Hill Book Co. Inc.; 1992. [Google Scholar]
- 30.Samman S. Trace elements. In: Ann J, Truswell S, editors. Essentials of human nutrition. 2. New York: Oxford University Press; 2002. pp. 1–4. [Google Scholar]
- 31.Davis TA, Volesky B, Vieira RHSF. Sargassum seaweed as biosorbent for heavy metals. Water Res. 2000;34:4270–4278. doi: 10.1016/S0043-1354(00)00177-9. [DOI] [Google Scholar]
- 32.Festa MD, Anderson HL, Dowdy RP, Ellersieck MR. Effects of zinc intake on copper excretion and retention in men. Am J Clin Nutr. 1985;41:285–292. doi: 10.1093/ajcn/41.2.285. [DOI] [PubMed] [Google Scholar]
- 33.Cruger W, Cruger A. Biotechnology: a textbook of industrial microbiology. 2. New Delhi: Panima Publishing Corporation; 1989. pp. 302–303. [Google Scholar]
- 34.Hutton JB, Jury KE, Davies EB. Studies of the nutritive value of New Zealand dairy pastures. NZ J Agric Res. 1965;8:479–496. [Google Scholar]
- 35.Silver S, Misra TK. Plasmid mediated heavy metal resistances. Ann Rev Microbiol. 1988;42:717–743. doi: 10.1146/annurev.mi.42.100188.003441. [DOI] [PubMed] [Google Scholar]
- 36.Basu M, Bhattacharya S, Paul AK. Isolation and characterization of chromium resistant bacteria from tannery effluent. Bull Environ Contam Toxicol. 1997;58:535–542. doi: 10.1007/s001289900368. [DOI] [PubMed] [Google Scholar]
- 37.Verma SK, Singh SP. Multiple chemical resistance in the cyanobacteria, Nostoc muscorum. Bull Environ Contam Toxicol. 1995;54:614–619. doi: 10.1007/BF00192607. [DOI] [PubMed] [Google Scholar]
- 38.Margesin R, Schinner F. Bacterial heavy metal tolerance extreme resistance to nickel in Arthrobacter spp. Strains. Basic Microbiol. 1996;36:269–282. doi: 10.1002/jobm.3620360410. [DOI] [Google Scholar]
- 39.Lovely DR. Bioremediation of organic and metal contaminants with dissimilitory metal reduction. J Ind Microbiol. 1995;14:85–93. doi: 10.1007/BF01569889. [DOI] [PubMed] [Google Scholar]