Abstract
Himalayan soils undergo dramatic temporal changes in their microclimatic properties. The soil habitats in the high altitude cold habitats of Himalayas are little explored with respect to bacterial diversity and metabolic potentials of the bacterial species. Soil habitat in Western Himalayas is dominated by the genera of Pseudomonas, Arthrobacter, Bacillus, and Flavobacterium. Strains were found to be diverse in their metabolic potentials to utilize different carbon sources by growing them on media containing 114 different sole carbon sources. Bacillus sp. STL9 was supported by the lowest number (12.3%) of the carbon sources while growth was observed in 73.7% of the carbon sources tested for the Pseudomonas sp. SPS2. Carbohydrates appeared to be preferred carbon sources for these Himalayan isolates followed by amino acids and proteins. These microbes also produced various extra-cellular hydrolytic enzymes having biotechnological potentials, lipase being the one secreted by most strains (85.7%) followed by β-galactosidase (42.8%). Antibiotic resistance profiling for 85 different antibiotics has also been described.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-011-0092-7) contains supplementary material, which is available to authorized users.
Keywords: Bacteria, Microbial diversity, Western Himalayas, Antibiotic resistance
Introduction
The great mountain system of Himalayas forms a barrier between the Tibetan Plateau to the north and the alluvial plains of the Indian subcontinent to the south. The Himalayas include the highest mountains in the world, with more than 110 peaks rising to elevations of 24,000 feet (7,300 m) or more above sea level. Most part of the mountains rise above the line of perpetual snow. The soil types in Himalayas are diverse and include; a high humus content soil, Podzolic soils (infertile, acidic forest soils) and even saline soils in the dry high plains.
The soil habitats in the high altitude cold habitats of Himalayas are little explored with respect to bacterial diversity. These hilly terrains differ from their polar counterparts in showing extreme seasonal variations in temperature and are surrounded by land masses where average temperature is higher and support mesophilic bacterial communities. The Himalayan soil environments are distinct from those in polar-regions and are characterized by dramatic seasonal shifts in physical and biochemical properties. These changes are further confounded by altitude gradient of climatic changes. Winter is characterized by intermittent snow cover and fluctuating subfreezing temperatures; summer has intense, desiccating sunshine punctuated by infrequent rains [1].
Bacteria are renowned for their rapid evolution in response to novel selection pressure. Any environment subject to varying selection, either spatially or temporally may harbor suites of bacteria that are capable of rapid change. The emergence and spread of antibiotic resistance [2] are perhaps the best known examples. Many more examples of rapid evolution of metabolic characters have been described for a diverse range of bacterial species, and most cases involve the emergence of novel genes and their spread in environments that are subject to marked human impact [3, 4]. Although important information about the metabolic versatility of bacteria in human-impacted environments can be gleaned from the literature [5, 6], whether such versatility is a general property of natural microbial communities is less well known.
We have previously carried out phylogenetic diversity study in the soil samples from these Himalayan habitats using partial 16S rDNA sequence data. The cultured bacterial strains belonged to Protobacteria (largely gamma-Proteobacteria), Firmicutes, Actinobacteria, and Bacteroidetes [7]. Most of the strains belonged to the genus Bacillus (30%), followed by Pseudomonas (24%), Arthrobacter (12%), and Flavobacterium (6%). In this study, we report characterization of representative strains of Bacillus, Pseudomonas, Arthrobacter, and Flavobacterium with respect to their metabolic capabilities. Potential of these microbes to produce various extracellular hydrolytic enzymes, and antibiotic resistance profile for 85 different antibiotics have also been described. This is the first report describing the metabolic characteristics of dominant, cultivated, aerobic bacterial strains from the permanently cold habitats of Western Himalayas. This habitat is little explored with respect to microbial composition and physiological characteristics of the residing strains.
Materials and Methods
Isolation of Bacteria and Growth Conditions
The surface soil samples were collected from different altitudes of Western Himalayan range situated at 34.10 N and 77.40 E. The temperature of the soil ranged from −20 to +10°C at the time of sampling. The samples were brought to the laboratory under ice and stored at −20°C. Soil samples were used for measurement of volatile solid content (drying at 450°C for 5 h) as per the standard protocols [8].
Ten fold serial dilutions of samples in sterilized normal saline were surface spread on ABM agar plates containing peptone, 5 g; yeast extract, 3 g; agar, 15 g and distilled water, 1,000 ml. Sets of triplicate plates for each dilution were incubated at 10°C. Colonies were picked up after 7 days of incubation at 10°C, purified and maintained in 15% (w/v) glycerol at −70°C.
Qualitative Assay for Production of Extracellular Enzymes
Qualitative assay for extracellular protease, amylase, β-galactosidase, lipase, cellulase, and alkaline phosphatase was carried out as described earlier [9]. Hemolytic activity was tested on blood agar medium.
Carbon Source Utilization
The ability of the culture to utilize a carbon source was checked by taking carbon compound in minimal medium without citrate (1.05% K2HPO4, 0.45% KH2PO4, 0.1% (NH4)2SO4, 1.5% agar). Carbohydrates, alcohols, organic acids, and phenolics were used at a concentration of 20 mM and amino acids at 10 mM as described by Meichichi et al. [10]. Tests were scored positive when growth of strains was observed when compared with strains grown in minimal medium without carbon source. Chemicals were obtained from Sigma, USA and Hi-Media, India and were of reagent grade.
Antibiotic Sensitivity and Isolation of Plasmid DNA
Sensitivity of the cultures to various antibiotics was tested by using antibiotic discs supplied by Hi-media Pvt. Ltd., India. ABM agar medium in 90 mm diameter sterile petri-dishes to a depth of 4 mm was used for surface spreading freshly grown cultures. Plates were incubated at 20°C aerobically for 48 h and zone of growth inhibition was analyzed by zone reader. The antibiotics used and their respective concentrations are listed in supplementary Table S2.
Plasmid DNA was extracted and purified by the method of Sambrook et al. [11] and also using the commercial kit (Qiagen, GmbH) as per manufacturer’s instructions.
Results and Discussion
Aerobic, heterotrophic strains SMT9 (AM689953), SPS2 (AM689946), SCP7 (AM690003), SMT7 (AM690032), SKT4a (AM690004), STL9 (AM690035), and RSS4 (AM690029) exhibited 98, 96, 97, 98, 98, 98, and 97% identity at 16S rRNA gene sequence level with Pseudomonas fluorescens, Pseudomonas veronii, Pseudomonas frederiksbergensis, Arthrobacter agilis, Arthrobacter methylotrophus, Bacillus soli, and Flavobacterium limicola, respectively. The strains were selected from the set of 173 cultured and uncultured bacteria obtained from various environmental samples from Western Himalayas on the basis of their novelties both at 16S rRNA gene sequence and phenotypic level [7].
Production of Extracellular Hydrolytic Enzymes
Ability of microbes to secrete six different extra-cellular hydrolytic enzymes was tested in addition to the hemolytic activity (Table 1). Isolates produced one or more (up to 4) hydrolytic enzymes, lipase being the one secreted by most strains (85.7%) followed by β-galactosidase (42.8%). Cellulase and hemolytic activity were not observed among the selected strains. Protease (28.5%), alkaline phosphatase (28.5%), and amylase (14.2%) were moderate in prevalence. Strain SPS2, SMT7, SKT4a, and STL9 produced more than one extra-cellular hydrolytic enzyme, while strain RSS4 did not produce any of extra-cellular hydrolytic enzymes tested. The production of extracellular enzymes by the isolates, like amylase, lipase, alkaline phosphatase etc. could be playing a key role in organic matter cycling in the Himalayan soil habitats. Enzymatic activities of aminopeptidase and β-glucosidase in Antarctic Ross Sea sediments have been investigated by Fabiano and Danovare [12]. These enzymes were shown to play an important role in organic matter diagenesis.
Table 1.
Morphological characteristics and extracellular hydrolytic enzymes produced by bacterial strains from Western Himalayas
| Strains | Source | Cell morphologyb | Amylasea | Proteasea | Lipasea | Cellulasea | β-galactosidasea | Alkaline phosphatasea | Hemolytic activity |
|---|---|---|---|---|---|---|---|---|---|
| SMT9 | Moist, sandy, dark grey soil from magnetic hill, Siachen. Altitude—11,300 ft | Gram −, rods, length 1–2 μm | − | − | + | − | − | − | − |
| SPS2 | Soil sample from Partapur, Siachen. Altitude—10,400 ft | Gram −, rods, length 1–2 μm | − | +++ | ++++ | − | − | + | − |
| SCP7 | Soil sample from Central Post, Siachen. Altitude—12,000 ft | Gram −, rods, length 1–2 μm | − | − | + | − | − | − | − |
| SMT7 | Moist, sandy, dark grey soil from magnetic hill, Siachen. Altitude—11,300 ft | Gram +, cocci, length 1 μm | − | + | + | − | + | − | − |
| SKT4a | Soil sample Khardungla top, Siachen. Altitude—18,400 ft | Gram +, rods, length 0.5 μm | − | − | + | − | ++ | − | − |
| STL9 | Humic soil from Leh. Altitude—11,400 ft | Gram +, rods, length 2–4 μm | ++ | − | + | − | ++ | ++ | − |
| RSS4 | Soil from a river bank, Siachen. Altitude—12,200 ft | Gram −, rods, length 2–4 μm | − | − | − | − | − | − | − |
aZone of hydrolysis (diameter) after 4 days at 20°C: + = 5–10 mm, ++ = 10–20 mm, +++ = >20 mm
bGram − = Gram negative, Gram + = Gram positive
Culturable bacteria, capable of producing cold adapted hydrolytic enzymes from sea-ice of high latitude ocean of Arctic, have been reported by Yu et al. [13]. Majority of the psychrophilic strains were able to secrete various psychrophilic enzymes into the medium at 4°C. Among these, lipases were most frequently observed (62.6%), followed by glutinases (51.4%) and amylases (40.5%). The present study also shows prevalence of extracellular hydrolytic enzymes in a similar fashion, except the alkaline phosphatase activity which was probably not studied by the authors.
Utilization of Different Carbon Sources by Strains Showing Novel Characteristics
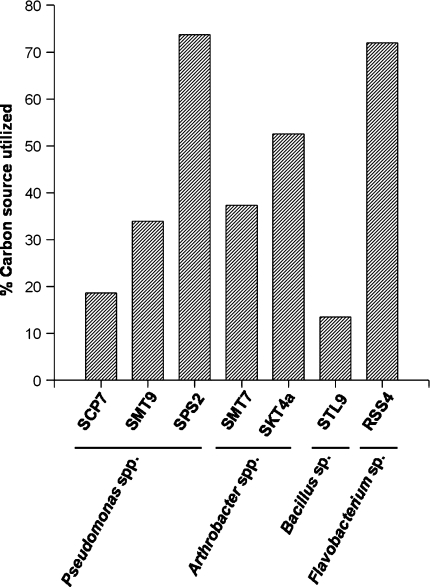
The bacterial strains showed remarkable diversity in their range of metabolic capabilities. Bacillus sp. STL9 (supplementary Table S1; Fig. 1), was supported by the lowest number (12.3%) of the carbon sources; growth was observed in 73.7% of the carbon sources tested for the Pseudomonas sp. SPS2. There were marked differences in the metabolic idiosyncrasies of the strains even within the highly versatile group of pseudomonads. Pseudomonas strain SCP7 utilized merely 17.5% of the total carbon sources tested, while the other strains SMT9 and SPS2 were more versatile and could grow on 32.4 and 73.7% of the carbon sources, respectively. Pseudomonas, an enormously diverse genus of the γ-Proteobacteria, is an important member of soil microbial communities [5]. Members of the genus have been isolated from essentially all environments studied [6], including alpine soil, where it was identified as the most prevalent culturable genus [14]. The genus exhibits remarkable metabolic variation [15]. The great metabolic flexibility of Pseudomonas species perhaps allows them to inhabit variable environments such as those reported in the present study. One strategy might be the evolution of strains that are capable of utilizing a large number of different carbon sources for growth. Alternatively, because the alpine environment is highly heterogeneous with pockets of specific carbon compounds, a large number of different strains that have recently gained or lost the ability to grow on particular sources of carbon may exist.
Fig. 1.
Metabolic potentials of cold active bacterial strains from Western Himalayas. The ability of the culture to utilize a carbon source was checked by taking carbon compound in minimal medium without citrate. Carbohydrates, alcohols, organic acids, and phenolics were used at a concentration of 20 mM and amino acids at 10 mM
Arthrobacter sp. SKT4a (53.5%) and Flavobacterium sp. RSS4 (71.9%) were the other two bacterial species with remarkable metabolic potentials. High alpine soil environments are characterized by dramatic seasonal shifts in physical and biochemical properties. It has been suggested that changes in many organic compounds lead to shifts in selection pressures over time [16, 17]. Moreover, as soils change from wet to dry, the spatial distribution of sources of carbon for growth becomes more heterogeneous. Bacterial species that are most responsive to these severe and shifting selection gradients are likely to evolve more rapidly in response to these changes. The remarkable metabolic versatility of Flavobacterium strain RSS4 in the present investigation is of special interest. Members of this group have been reported from ice samples from non-polar glacier [18], though their metabolic potentials have not been explored. The present study suggests that species of Pseudomonas and Flavobacterium could be playing a significant role in biogeochemical cycles in the soil habitats of Western Himalayas.
Carbohydrates appeared to be preferred carbon sources for these Himalayan isolates followed by amino acids and proteins (supplementary Table S1). Among amino acids and proteins the affinity of isolates greatly varied, some of the amino acids were either not utilized [dl-β-phenylalanine, d-serine, and 3-(3,4-dihydroxyphenyl) dl-alanine] or utilized by a single bacterial strain (l-hydroxyproline, l-tryptophan, and l-threonine). On the other hand a few amino acids were extensively utilized as sole carbon source. For example l-cystine and d-alanine supported growth of all the seven selected Himalayan strains while, l-aspartic acid was utilized by 5 strains and l-proline, and glycine by six out of seven strains tested. Similarly, utilization profile of sugars had a broad spectrum with aesculin and α-cyclodextrin not utilized by any of the isolates on one hand, as against d-trehalose, d-mannose, d-fructose, d-mandelic acid, β-cyclodextrin, and Ca-d-saccharate being utilized by most of the strains.
Alcohols, organic acids and phenolic compounds are not preferred carbon sources for the Himalayan isolates and a tendency to use them was only observed in strain RSS4 (58.6% of alcohols, organic acids and phenolics), SPS2 (68.9%) and SKT4a (34.4%). Among alcohols, organic acids and phenolics; succinic acid, dl-2-Amino-n-butyric, acid and benzoic acid are the most commonly used carbon sources.
The carbon source utilization profile for the selected Himalayan isolates was consistent with the results obtained with 17 cold tolerant strains of Pseudomonas isolated from alpine soil [19], in that, glycine, benzoate, starch, maltose, trehalose, sucrose, and cellobiose were utilized by equivalent proportion of strains from the two habitats. However, these two groups exhibited marked difference in their preference for citrate, acetate, and casein. With respect to the metabolic potential of Pseudomonas species, the Himalayan isolates appear to be more versatile towards utilization of various carbon sources.
Antibiotic Resistance and Prevalence of Plasmids Among Selected Strains
All the seven selected strains were tested for resistance to 85 different antibiotics (Fig. 2; supplementary Table S2). Maximum resistance was observed in the Flavobacterium sp. RSS4 (56.4%), followed by Pseudomonas sp. SPS2 (47%), and Pseudomonas sp. SMT9 (42.3%). Occurrence of antibiotic resistance were extremely low for the Bacillus sp. STL9 (12.3%) and Arthrobacter sp. SKT4a (10.5%).
Fig. 2.
A comparison of antibiotic sensitivity profile among bacterial strains from Western Himalayas. Sensitivity of the cultures to various antibiotics was tested by using antibiotic discs on ABM agar medium. Freshly grown cultures were surface spread, plates were incubated at 20°C aerobically for 48 h, and zone of growth inhibition was analyzed by zone reader. Resistant = no zone of inhibition; weakly resistant <3 mm of inhibition zone; sensitive ≥3 mm of inhibition zone
All the strains were highly sensitive to antibiotics, sparfloxacin, spectinomycin, streptomycin, nalidixic acid, pefloxacin, oxacillin, lomefloxacin, minocycline, tetracycline, moxifloxacin, levofloxacin, oxytetracycline, and norfloxacin at the concentrations tested. Resistance to cefpodoxime, cephalexin, cephradine, cephaloridine, sulphadiazine, oleandomycin, metronidazole, lincomycin, and methicillin was frequently observed and more than 50% of the strains could grow on these antibiotics at the concentration tested. Curiously, we did not observe presence of any plasmid in the strains SMT9, SKT4a, and STL9. Attempts to extract plasmid were made using various isolation protocols but we could not isolate intact plasmids in these Himalayan strains. Strains SPS2, SCP7, SMT7, and RSS4 harbored (>10 kb) plasmids, though the preparations were of poor quality showing extensive degradation of the plasmid DNA.
Prevalence of antibiotic resistance among bacteria from the pristine environment of Himalayas is of concern in the context of the fact that snow and ice-covers are used by the human population in Himalayas, as drinking water sources. The high frequency of antibiotic resistance observed among selected Himalayan isolates is in marked discordance with the generally accepted hypothesis that bacteria found in pristine habitats display low level of antibiotic resistance [20].
Antibiotic resistance profiles have been largely studied in bacteria from clinical sources and such information is scanty with respect to environmental bacteria. Kelch and Lee [21] studied antibiotic resistance patterns of Gram-negative bacteria isolated from mesophilic environmental sources including rivers and Bay of Tillamook, Oregon. For a total of 2,445 Gram-negative bacteria studied, a correlation of antibiotic resistance pattern from different sources was observed indicating their common origin. It was hypothesized that bacteria which share a common environment also share a common mode for the development of antibiotic resistance. This hypothesis does not seem to hold good in present investigation as there is extreme heterogeneity of antibiotic resistance profile among isolates from similar environmental niches.
Antibiotic resistance genes have an environmental origin, sometimes as an antibiotic protective mechanism and sometimes with a different function [22].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgment
We thank Dr R Vijayaraghwan, Director, DRDE Gwalior for providing all facilities and support required for this study.
References
- 1.Greenland D, Losleben M. Climate. In: Bowman WD, Seastedt TR, editors. Structure and function of an alpine ecosystem. New York: Niwot Ridge; 2001. pp. 15–31. [Google Scholar]
- 2.Alberti S, Ortali V, Salas EJ. On the transduction of certain metabolic characters in staphylococci. Ann Ist Super Sanita. 1965;1:61–66. [PubMed] [Google Scholar]
- 3.Hall RM, Collis CM. Antibiotic resistance in gram-negative bacteria: the role of gene cassettes and integrons. Drug Resist Updates. 1998;1:109–119. doi: 10.1016/S1368-7646(98)80026-5. [DOI] [PubMed] [Google Scholar]
- 4.Nemergut DR, Martin AP, Schmidt SK. Integron diversity in heavy metal contaminated mine tailings and inferences about integron origin and evolution. Appl Environ Microbiol. 2004;70(2):1160–1168. doi: 10.1128/AEM.70.2.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palleroni NJ. Genus I Pseudomonas Migula 1894. In: Krieg NR, Holt JG, editors. Bergey’s manual of systematic bacteriology. Baltimore: Williams and Wilkins; 1984. pp. 141–199. [Google Scholar]
- 6.Palleroni NJ. Introduction to the family Pseudomonadaceae. In: Balows A, Truper H, Dworkin M, Harder W, Schleifer KH, editors. The prokaryotes. 2. New York: Springer-Verlag; 1992. pp. 3071–3079. [Google Scholar]
- 7.Gangwar P, Alam SI, Bansod S, Singh L. Bacterial diversity of soil samples from Western Himalayas, India. Can J Microbiol. 2009;55:564–577. doi: 10.1139/W09-011. [DOI] [PubMed] [Google Scholar]
- 8.Rand MC, Greenberg AE, Taras MJ, Franson MA (1975) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, 1193 p
- 9.Alam SI, Singh L. Proteolytic heterotrophic bacteria of cyanobacterial assemblage from Schirmacher oasis, Antarctica, capable of growing under extreme conditions. Curr Sci. 2002;83:1000–1004. [Google Scholar]
- 10.Meichichi T, Labat M, Patel BKC, Woo THS, Thomas P, Garcia JL. Clostridium methoxybenzovorans sp. nov., a new aromatic 0-demethylating homoacetogen from an olive mill waste water treatment digester. Int J Syst Bacteriol. 1999;49:1201–1209. doi: 10.1099/00207713-49-3-1201. [DOI] [PubMed] [Google Scholar]
- 11.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Cold Springer Harbor: Cold Springer Harbor Laboratory Press; 1990. [Google Scholar]
- 12.Fabiano M, Danovare R. Enzymatic activity, bacterial distribution, and organic matter composition in sediments of the Ross Sea (Antarctica) Appl Environ Microbiol. 1998;64(10):3838–3845. doi: 10.1128/aem.64.10.3838-3845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y, Li HR, Chen B, Zeng YX, He JF. Phylogenetic diversity and cold-adaptive hydrolytic enzymes of culturables psychrophilic bacteria associated with sea-ice from high latitude ocean, Arctic. Wei Sheng Wu Xue Bao. 2006;46(2):184–190. [PubMed] [Google Scholar]
- 14.Mancinelli RL. Population-dynamics of alpine tundra soil bacteria, Niwot Ridge, Colorado Front Range, USA. Arct Alp Res. 1984;16:185–192. doi: 10.2307/1551070. [DOI] [Google Scholar]
- 15.Palleroni NJ, Doudoroff M. Some properties and taxonomic subdivisions of genus Pseudomonas. Annu Rev Phytopathol. 1972;10:73–100. doi: 10.1146/annurev.py.10.090172.000445. [DOI] [Google Scholar]
- 16.Lipson DA, Schmidt SK, Monson RK. Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biol Biochem. 2000;32:441–448. doi: 10.1016/S0038-0717(99)00068-1. [DOI] [Google Scholar]
- 17.Schmidt SK, Lipson DA, Ley RE, Fisk MC, West AE. Impacts of chronic nitrogen additions vary seasonally and by microbial functional group in tundra soils. Biogeochemistry. 2004;69:1–17. doi: 10.1023/B:BIOG.0000031028.53116.9b. [DOI] [Google Scholar]
- 18.Xiang S, Yao T, An L, Xu B, Wang J. 16S rRNA sequences and differences in bacteria isolated from the Muztag Ata glacier at increasing depths. Appl Environ Microbiol. 2005;71(8):4619–4627. doi: 10.1128/AEM.71.8.4619-4627.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer AF, Lipson DA, Martin AP, Schadt CW, Schmidt SK. Molecular and metabolic characterization of cold-tolerant alpine soil Pseudomonas sensu stricto. Appl Environ Microbiol. 2004;70(1):483–489. doi: 10.1128/AEM.70.1.483-489.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leff LG, Dana JR, McArthur JV, Shimkets LG. Detection of Tn5-like sequences in kanamycin-resistant stream bacteria and environmental DNA. Appl Environ Microbiol. 1993;59:417–421. doi: 10.1128/aem.59.2.417-421.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelch WJ, Lee JS. Antibiotic resistance patterns of gram-negative bacteria isolated from environmental sources. Appl Environ Microbiol. 1978;3:450–456. doi: 10.1128/aem.36.3.450-456.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alonso A, Rojo F, Martinez JL. Environmental and clinical isolates of Pseudomonas aeruginosa show pathogenic and biodegradative properties irrespective of their origin. Environ Microbiol. 1999;1:421–430. doi: 10.1046/j.1462-2920.1999.00052.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.