Abstract
The relationship of Foot-and-Mouth Disease virus antigen payload and number of dose of vaccine conferring protection against virus challenge in goats was studied. Goats vaccinated with oil adjuvant Foot-and-Mouth Disease vaccines containing different antigen payloads with or without booster resisted virulent challenge at 21 days post-vaccination or 7 days after booster respectively. However, localized sub-clinical infection was observed in two vaccinated goats on 35 days post-challenge. RNA could be detected from 31.8% of vaccinated goats (102.69–104.99 viral RNA copies per cotton swab of nasal secretions) on day 35 post-challenge. Since no live virus could be isolated after 5 days post-challenge, the risk of these animals transmitting the disease was probably very low. The finding showed that oil adjuvant Foot-and-Mouth Disease vaccines containing antigen payload of 1.88 μg may prevent or reduce the local virus replication at the oropharynx and shedding of virus from nasal secretions and thereby reduce the amount of virus released into the environment subsequent to exposure to live virus. This study also showed that goats with poor sero conversion to vaccination can be infected without overt clinical signs and became carriers like sheep.
Keywords: Foot-and-mouth disease, Goats, Oil adjuvant vaccine, Vaccination, Immunity, Carrier status
Introduction
Foot-and-Mouth Disease (FMD) is endemic in India and outbreaks are recorded throughout the year. FMD virus (FMDV) serotypes O, A and Asia 1 are prevalent. All the ruminants are affected and the disease has been studied extensively in cattle, less so in buffaloes and sheep but poorly in goats. Vaccination of goats results in protective immune response and development of colostral antibodies [1]. Small ruminants are vaccinated only in the face of FMD outbreak and vaccination programs do not include small ruminants. There is paucity of information on the role of goats in FMD epidemiology and transmission. Also the role of vaccine in the control of FMD in goats is poorly studied. The dose of the aqueous vaccine per animal varies from 2 to 3 ml and small ruminants usually receive one-third of cattle dose. However, in the case of oil based vaccines for a typical dose volume of 2 ml for large ruminants, the small ruminants usually receive one half of a cattle dose [2]. In the present study we made an attempt to study the effect of vaccination and the payload of antigen in the vaccine in prevention of FMD against virulent challenge and subsequent effects such as persistence of virus and immune response in goats.
Materials and Methods
Vaccination, Challenge and Sampling
Thirty Osmanabadi goats aged between 6 and 12 months were used in this study. The details of antigen payloads in experimental oil adjuvant Foot-and-Mouth vaccine formulations, vaccination and challenge schedule is given in Table 1. Animals were challenged with 1,000 cattle tongue ID 50 (CTID50) virulent O1 Manisa virus intradermally into the coronary band [3].
Table 1.
Details of vaccination and challenge of goats
| Groups | No of goats | Antigen payload (μg/2 ml) | Vaccination | Challenge | |
|---|---|---|---|---|---|
| Primary | Booster | ||||
| 1 | 6 | 15 (4×) | 0 day | 21st day | 28th day |
| 2 | 6 | 3.75 (1×) | 0 day | 21st day | 28th day |
| 3 | 6 | 15 (4×) | 0 day | Nil | 21st day |
| 4 | 6 | 3.75 (1×) | 0 day | Nil | 21st day |
| 5 | 6 | Unvaccinated controls | Nil | Nil | With the other groups |
Vaccinations were staggered to allow simultaneous challenge of all goats in each group. Group 3 and 4 animals received only one vaccine while Group 1 and 2 received one vaccine and a booster dose on day 21 post-vaccination. The groups 3 and 4 received the vaccine 7 days after the groups 1 and 2. As a result all the groups were challenged on the same day i.e. day 21 post-vaccination for groups 3 and 4 and day 28 post-vaccination for groups 1 and 2
Clotted blood for serology and detection of antibodies against FMDV non-structural protein (NSP) was collected on 0, 5, 14, 21 and 28 days post-vaccination (dpv) and 0, 5, 10, 15, 21, 28 and 35 days post-challenge (dpc). Heparinised blood and nasal secretions were collected daily up to 10 days post-challenge and thereafter at weekly intervals up to 35 days post-challenge. These materials were used for virus isolation (VI) and quantitative real-time RT-PCR (qRT-PCR). Probang samples were collected from the upper oesophagus and pharynx on 0, 3, 5, 10, 15, 21, 28 and 35 days post-challenge for VI and qRT-PCR. The goats were monitored for clinical signs of disease and temperatures were recorded daily. A subjective scoring system with slight modification [4] was used to evaluate the progression of disease in these animals. Individual clinical signs were scored as follows: lesions in the inoculated foot—1; Mouth lesions—2; lesions on one foot other than the inoculated foot—2; lesions on two feet other than the inoculate foot—3; lesions on three feet other than the inoculate foot—4; The scores were then added. The lesion score from the inoculated feet was not considered as generalization of the disease. A goat could therefore score a maximum of six points.
Virus Isolation
Nasal secretions and probang samples were examined for the presence of live virus by bovine primary thyroid (BTY) cell culture inoculation [5] and confirmatory antigen ELISA [6]. BTY cell culture supernatants from samples showing no sign of CPE after 72 h were pooled and re-passaged once and the absence of FMDV was confirmed by antigen ELISA as mentioned above.
Real-Time Quantitative RT-PCR Assay for Detection of Viral RNA
The amount of viral RNA in blood, nasal secretions and probang samples were quantified by real-time RT-PCR (qRT-PCR) as described previously [7]. For the generation of standard curves, a FMDV RNA standard was synthesized in vitro from a plasmid containing a 79 base pair insert of the internal ribosomal entry site (IRES) of a type O FMDV (kindly provided by Dr. Donald P. King, Institute for Animal Health, UK) using a MEGAScripTM T7 kit (Ambion, USA) as described previously [4]. The CT values were assigned to each reaction and samples with a CT value of 35 or less were considered positive [8].
Serology
Antibodies to FMDV NSP 3ABC were tested using Cedi test® FMDV-NS (Cedi-Diagnostics, The Netherlands) and an in-house indirect ELISA [9]. Serum antibody titres were measured [10] and expressed as the reciprocal of the final dilution of serum in the serum/virus mixture which neutralized an estimated 100 TCID50 of virus at the 50% end-point estimated according to the method of Kärber [11].
Statistical Analysis
Statistical analysis of the serum antibody titres was carried out by methods described by Snedecor and Cochran [12]. The 95% confidence interval was calculated for each serum titre (Mean ± 1.96 × Standard Error). Student’s t test was used to compare the amount of virus excreted or virus genome in the blood, nasal secretions and probang samples.
Results
Development of Clinical FMD
Five out of six control goats exhibited pyrexia (≥40°C) and clinical signs of FMD, recording a highest clinical score of 6 on days 2–5 post-challenge. All vaccinated goats were protected against challenge. However, three goats each in group 3 and 4 showed pyrexia (≥40°C) from 2 to 7 days post-challenge and thereafter body temperature dropped to normal range.
Detection of Virus/Virus Nucleic Acid in Blood, Nasal secretion and Probang Samples
Blood
Five out of six unvaccinated goats showed the presence of viral RNA in blood one day after challenge (Mean = 102.38 copies per ml) and persisted up to 9 days post-challenge. Virus could be isolated up to 5 days post-challenge in unvaccinated goats. Neither virus nor viral RNA was detected throughout the study period in the vaccinated goats.
Nasal Secretions
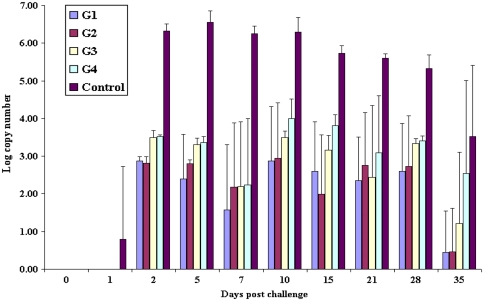
Viral RNA could be detected in nasal secretions from both vaccinated and unvaccinated goats up to 35 days post-challenge. Live virus could be isolated from nasal secretions of both vaccinated and unvaccinated goats up to 5 days post-challenge. The unvaccinated control group excreted a significantly higher (P < 0.001) higher mean viral RNA copy number than the vaccinated group (Fig. 1) from days 2–35 post-challenge. The group 1 goats (4× with two doses) excreted significantly low (P < 0.001) low levels of mean viral RNA copy numbers than group 3 goats (4× with single dose) at 2 days post-challenge. Group 2 goats (1× with two dose) excreted significantly low (P < 0.001) low levels of mean viral RNA copy numbers than group 4 goats (1× with single dose) at 2, 5 and 15 days post-challenge. Group 3 goats (4× with single dose) excreted significantly low (P < 0.05) low levels of mean viral RNA copy numbers than group 4 goats (1× with single dose) at 10 and 15 days post-challenge.
Fig. 1.
Mean FMDV viral RNA copy numbers detected over time by qRT-PCR from cotton bud samples collected from nose of vaccinated and unvaccinated goats. Between the vaccinated groups there was significant difference in the excretion of virus RNA (P < 0.05) and between the vaccinated and control groups there was a highly significant difference in the excretion of virus RNA (P < 0.001) on all days post-challenge
Probang Samples
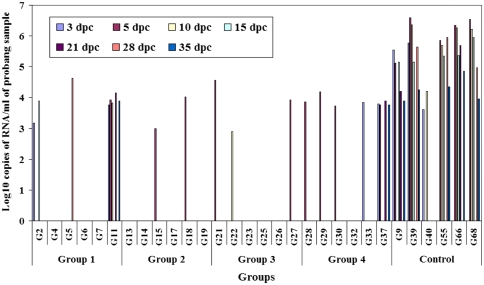
All of the unvaccinated control goats excreted virus in the probang samples up to 35 days post-challenge (Fig. 2). No correlation could be established between virus isolation and qRT-PCR. Two goats in group 1 did not shed the virus throughout the study period. Two other goats scored positive on 3, 5 and 28 days post-challenge with levels of virus RNA. One goat in group 1 was positive up to day 35 post-challenge. In group 2 (1× with two doses) four out of six goats did not shed the virus whereas two goats were positive on 5 days post-challenge. Three goats from group 3 (4× with single dose) were positive between 5 and 10 days post-challenge. In group 4 (1× with single dose) five out of six goats excreted virus up to 3–5 days post-challenge. Probang samples collected from one goat in this group was positive up to 35 days post-challenge. One goat each from the group 1 and 4 and three unvaccinated goats were declared as carriers on day 35 post-challenge as they were positive by VI and qRT-PCR.
Fig. 2.
Results of qRT-PCR from probang samples from vaccinated and unvaccinated goats on different days post-challenge (dpc)
Serology
All of the unvaccinated control goats were found to be NSP antibody positive from 10 to 15 days post-challenge and continued to be positive up to 35 days post-challenge. One vaccinated goat in group 1 (G11) and two goats from group 4 (G29 and G37) were found to be NSP antibody positive from 10 to 35 days post-challenge.
Serum neutralizing antibody responses in vaccinated (Groups 1, 2, 3 and 4) and unvaccinated (Group 5) goats following vaccination and challenge are shown in Fig. 3. Also presented is the mean antibody titre at each point for each group. One goat (G11) from group 1 did not seroconvert at the time of challenge. A comparison of mean titre of neutralizing antibody between both the vaccinated group and the unvaccinated groups revealed significant difference (P < 0.001) at the time of challenge. At the time of challenge, no significant difference of mean neutralizing antibody titre (P > 0.05) were observed between the different groups. However, goats belonging to G4 showed significantly higher (P < 0.05) higher mean antibody titre than 1× antigen payload with single dose at the time of challenge. Convalescent antibody response was noticed in unvaccinated control goats that declined after 21 days post-challenge.
Fig. 3.
Mean serum neutralising antibody response in goats following vaccination with an oil adjuvant O1 Manisa vaccine and subsequent challenge on day 21 or 28 post challenge. The vaccinated goats in groups 1, 2, 3 and 4 had an average neutralizing antibody titre of 1.65 (95% CI 1.52–1.97), 1.81 (95% CI 1.68–1.94), 1.62 (95% CI 1.43–1.81) and 1.41 (95% CI 1.15–1.66) respectively at the time of challenge. There was no significant difference between the vaccinated groups (P > 0.05) while there was a highly significant difference (P < 0.001) between the vaccinated and unvaccinated groups
Discussion
We studied the effect of vaccines with two different payloads with or without booster vaccination in offering protection against virus challenge, virus replication and persistence in goats. The results showed that vaccination offered complete protection in goats against the clinical disease even with a low antigen pay load of 1.88 μg without booster vaccination. A similar finding was obtained for sheep that were challenged 14 days after vaccination with different payloads of monovalent type O1 Lausanne vaccine [13].
Protection from the clinical disease did not always coincide with prevention of localized, sub-clinical infection. In the first 10 days after challenge, FMDV was found within the oropharynx of 12 out of the 24 vaccinated goats. During the first 10 days after challenge, the amount of virus produced was significantly less (P < 0.001) less in the vaccinated group compared to unvaccinated goats. These findings demonstrate the ability of the vaccine to either prevent or reduce the viral replication shortly after virulent challenge at the site of the primary infection (the oropharynx), thereby limiting the amount of infectious material released into the environment from sub-clinically infected animals.
Virus was isolated from probang samples of two vaccinated goats (G11 and G37) on 35 days post-challenge. Since there is paucity of information regarding vaccination and challenge in goats, the result from the present study were compared with information on sheep. Evidence of persistence of FMDV infection in sheep is well established and the vaccinated sheep becoming carriers has been reported [3]. Orsel et al. [14] showed a difference in the likelihood of becoming a carrier between vaccinated (4.1%) and unvaccinated (50%) sheep exposed to infection and Parida et al. [15] reported that 10–20% of vaccinated sheep and 37.5% of unvaccinated controls became carriers on day 35 post-challenge. In our study 8.3% vaccinated goats and 50% unvaccinated control goats became carriers.
The results showed that the amount of viral RNA present in the vaccinated goats was significantly lower (P < 0.001) than the unvaccinated animals up to 35 days post-vaccination. However, it is difficult to assess the relationship between RNA copy number and infectivity [16]. Results presented here show that correlation between isolation of virus and detection of viral RNA from nasal secretions is variable. The presence of low level of viral RNA in the absence of detectable live virus at 35 days post infection for few vaccinated goats is interesting. The most likely reason for this lack of correlation is the involvement of antibody, either from serum or local production at the mucosa of following sub-clinical infection. It is clear therefore that although detection of FMDV RNA may be useful for the diagnostic purpose, as in determining whether an animal has had contact with virus, RNA copy number alone may not be useful as an indicator for determining whether a persistently infected animal is likely to present a risk for disease transmission. Since transmission from both the vaccinated and unvaccinated animals was not measured directly, the biological significance of this reduction remains unsubstantiated. Clearly future studies need to be designed to measure transmission directly and are the natural progression from the present work. This is the first report to measure the excretion of FMDV RNA in nasal secretions and probang samples of vaccinated and subsequently challenged goats.
NSP antibody responses in challenged animals were apparent in only three vaccinated goats at 35 days post-challenge. However, all of the unvaccinated control goats showed NSP antibodies at 10–35 days post-challenge. This data suggests vaccinated animals (Group 1–4) could resist challenge with live virus.
Protective FMDV neutralizing antibody was present on the day of challenge in animals vaccinated with the two payloads of antigen with or without booster. In our study, most of the vaccinated goats had neutralising antibody titre at the time of challenge while all the unvaccinated animals were sero negative. Cox et al. [17] showed that protection was associated in part with the induction of serum antibody response, though some sheep in spite of having low antibody levels in circulation were protected. This observation explains why one goat (G11) was protected from clinical disease with very little or no serum neutralizing antibody titre.
Overall, the results of the study suggest that the vaccination offers protection from clinical disease even at a low payload of 1.88 μg and one-half of the cattle doses of the current vaccine formulations is sufficient to induce protective immune response in goats. An antigen payload of 1.88 μg may reduce the virus replication at the oropharynx, shedding of virus in nasal secretions and reduce the amount of virus released into the environment subsequent to exposure to live virus. The study also showed that goats that do not respond to vaccination, in the absence of protective immune response can become potential carriers similar to the unvaccinated animals as in the case of sheep.
Contributor Information
M. Madhanmohan, Email: madan@indimmune.com
S. B. Nagendrakumar, Email: nagu@indimmune.com
P. Santhakumar, Email: santhakumar@indimmune.com
D. Thiagarajan, Email: thiagu@indimmune.com
M. Lakshmi Narasu, Email: mangamoori@jntuh.ac.in
V. A. Srinivasan, Phone: +91-40-23000894, FAX: +91-40-23005958, Email: srini@indimmune.com
References
- 1.Madhanmohan M, Tresamol PV, Saseendranath MR. Immune response in goats to two commercial foot-and-mouth disease vaccines and the assessment of maternal immunity in their kids. Transbound Emerg Dis. 2009;56:49–53. doi: 10.1111/j.1865-1682.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- 2.Doel TR. FMD vaccines. Virus Res. 2003;91:81–99. doi: 10.1016/S0168-1702(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 3.Burrows R. The persistence of foot-and-mouth disease virus in sheep. J Hyg (Lond) 1968;66:633–640. doi: 10.1017/S0022172400028369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quan M, Murphy CM, Zhang Z, Alexandersen S. Determinants of early foot-and-mouth disease virus dynamics in pigs. J Comp Pathol. 2004;131:294–307. doi: 10.1016/j.jcpa.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Snowdon WA. Growth of foot-and-mouth disease virus in monolayer cultures of calf thyroid cells. Nature. 1966;210:1079–1080. doi: 10.1038/2101079a0. [DOI] [PubMed] [Google Scholar]
- 6.Ferris NP, Dawson M. Routine application of enzyme-linked immunosorbent assay in comparison with complement fixation for the diagnosis of foot-and-mouth and swine vesicular diseases. Vet Microbiol. 1988;16:201–209. doi: 10.1016/0378-1135(88)90024-7. [DOI] [PubMed] [Google Scholar]
- 7.Shaw AE, Reid SM, Ebert K, Hutchings GH, Ferris NP, King DP. Implementation of a one-step real-time RT-PCR protocol for diagnosis of foot-and-mouth disease. J Virol Methods. 2007;143:81–85. doi: 10.1016/j.jviromet.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Fowler VL, Paton DJ, Rieder E, Barnett PV. Chimeric foot-and-mouth disease viruses: evaluation of their efficacy in cattle and potential as marker vaccines. Vaccine. 2008;26:1982–1989. doi: 10.1016/j.vaccine.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Hema M, Nagendrakumar SB, Yamini R, Chandran Dev, Rajendra L, Thiagarajan D, Parida S, Paton DJ, Srinivasan VA. Chimeric tymovirus-like particles displaying foot-and-mouth disease virus non-structural protein epitopes and its use for detection of FMDV-NSP antibodies. Vaccine. 2007;25:4784–4794. doi: 10.1016/j.vaccine.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Golding SM, Hedger RS, Talbot P. Radial immuno-diffusion and serum neutralisation techniques for the assay of antibodies to swine vesicular disease. Res Vet Sci. 1976;20:142–147. [PubMed] [Google Scholar]
- 11.Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch Exper Pathol Phar-makol. 1931;162:480–487. doi: 10.1007/BF01863914. [DOI] [Google Scholar]
- 12.Snedecor GW, Cochran WG. Statistical methods applied to experiments in agriculture and biology. 6. Ames: Iowa State University Press; 1968. [Google Scholar]
- 13.Barnett PV, Keel P, Reid SM, Armstrong RM, Statham RJ, Voyce C, Aggarwal N, Cox SJ. Evidence that high potency foot-and-mouth disease vaccine inhibits local virus replication and prevents the “carrier” state in sheep. Vaccine. 2004;22:1221–1232. doi: 10.1016/j.vaccine.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Orsel K, Dekker A, Bouma A, Stegeman JA, Jong MC. Quantification of foot and mouth disease virus excretion and transmission within groups of lambs with and without vaccination. Vaccine. 2007;25:2673–2679. doi: 10.1016/j.vaccine.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 15.Parida S, Fleming L, Oh Y, Mahapatra M, Hamblin PA, Gloster J, Paton DJ. Emergency vaccination of sheep against foot-and-mouth disease: significance and detection of subsequent sub-clinical infection. Vaccine. 2008;26:3469–3479. doi: 10.1016/j.vaccine.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Alexandersen S, Zhang Z, Reid SM, Hutchings GH, Donaldson AI. Quantities of infectious virus and viral RNA recovered from sheep and cattle experimentally infected with foot-and-mouth disease virus O UK 2001. J Gen Virol. 2002;83:1915–1923. doi: 10.1099/0022-1317-83-8-1915. [DOI] [PubMed] [Google Scholar]
- 17.Cox SJ, Barnett PV, Dani P, Salt JS. Emergency vaccination of sheep against foot-and-mouth disease: protection against disease and reduction in contact transmission. Vaccine. 1999;17:1858–1868. doi: 10.1016/S0264-410X(98)00486-1. [DOI] [PubMed] [Google Scholar]