Abstract
A study was undertaken to investigate the presence of archaeal diversity in saltpan sediments of Goa, India by 16S rDNA-dependent molecular phylogeny. Small subunit rRNA (16S rDNA) from saltpan sediment metagenome were amplified by polymerase chain reaction (PCR) using primers specific to the domain archaea. 10 unique phylotypes were obtained by PCR based RFLP of 16S rRNA genes using endonuclease Msp 1, which was most suitable to score the genetic diversity. These phylotypes spanned a wide range within the domain archaea including both crenarchaeota and euryarcheaota. None of the retrieved crenarchaeota sequences could be grouped with previously cultured crenarchaeota however; two sequences were related with haloarchaea. Most of the sequences determined were closely related to the sequences that had been previously obtained from metagenome of a variety of marine environments. The phylogenetic study of a site investigated for the first time revealed the presence of low archaeal population but showed yet unclassified species, may specially adapted to the salt pan sediment of Goa.
Keywords: Small subunit rRNA, Phylogeny, Archaea, Saltpan, Hypersaline environment
Introduction
Saltpans and salt lakes have been reported to harbor high number of taxonomically diverse halophilic microorganism, which differ in salt requirement and metabolic capabilities. Their inhabitants make these unique ecosystems fascinating to study. Very little is known about the processes occurring in the saltpans and their importance to adjacent ecosystems. Saltpans are sites where different ions, including metals, become concentrated and halophilic bacteria evolve, suppressing the less halophilic and halotolerant forms. There is hardly a hypersaline niche in nature that is not occupied by some Halophiles [1]. Cultured microorganisms represent only a minor component in the existing diversity of salt pan because of the difficulty in culturing most of the microbial assemblage [2].
Among three major evolutionary domains of life on earth, members of the Archaea domain are the least understood in terms of their diversity, physiology, genetics, and ecology. Archaea domain is divided into four phyla, Euryarcheaota, Crenarchaeota, Korarchaeota, the presence of which has been determined only by environmental DNA sequences [3, 4] and the recently reported Nanoarchaeota [5, 6].
The use of molecular methods to investigate uncultivated microbes from natural environments has revolutionized our views of microbial biodiversity and ecology in recent years. Studies based on the extraction of total community DNA from environmental samples followed by polymerase chain reaction (PCR), cloning, and sequencing of 16S rRNA genes have now become common place, often comprising one of the first steps in studying the microbiology of an environment of interest [7]. Recovery and analysis of 16S rRNA genes directly from environmental DNA provides a means of investigating microbial populations in any habitat, eliminating dependence on isolation of pure cultures [8–12].
The saltpans of Goa are situated along the western coast in the Arabian Sea, which constitute a part of the Indian salt industry. Every year many tons of salt are produced and distributed all over India [13]. This area is under the influence of semidiurnal tides and the surrounding marshy land supports rich mangrove vegetation. It takes approximately 20 days to clean up and prepare a salt pan. Each pan is surrounded by ‘bandhs’ or mud borders on all four sides to prevent siltation and helps regulate the flow of water [14]. In Mumbai saltern, seawater from the Arabian Sea enters the creek through a sluice gate at high tide and during low tide; water from the creek is drained out into the estuary. Although many investigations have been carried out to study archaeal diversity in hypersaline environments and many new lineages have been found [11, 15–23]. The phylogenetic diversity of archaea in saltpans of Goa has not yet been surveyed. Here, we report the results of phylogenetic analysis of cloned 16S ribosomal RNA genes. These results may improve our knowledge of archaea in the saltpans. The retrieved sequence information can then be used to gain further information about the survey of phylotypes from saltpans.
Materials and Methods
Sample Collection
Four subsurface sediment samples were collected from two adjacent saltpans of Arpora, Goa. The sampling site is situated at latitude of 15.567 and longitude of 73.767. The samples were collected from a depth of 2- to 10-cm. At each site, 3 individual soil samples were taken from different locations which were mixed to obtain a composite sample for the site. The area of each site was 60 m × 60 m, and the minimum distance between any of the 3 subsamples was 3 m. The sample was black in color having salinity of 15% as determined by a refractometer. This area is under the influence of semidiurnal tides. The samples were stored on icebox to prevent direct sunlight until processed.
DNA Extraction
The DNA extraction process was done by UltraClean™ Soil DNA isolation Kit (Catalog. No. 12900-10, MoBio laboratories Inc.) with slight modification. Genomic DNA was extracted directly from 10 g each dry weight of soil. The DNA was pooled from three samples and used for PCR amplification of the archaeal 16S RNA gene. The concentration of dsDNA was determined spectrophotometrically at 260 nm Lambda 35 (Perkin Elmer).
PCR Amplification of 16S rRNA
Genomic DNA purified from sediment was used as template for PCR. Amplification of 16S rRNA genes was done by PCR using a primer set specific to Archaea [24]. S-D-Arch-0344-a-S-20 (5′-ACGGGGCGCCAGCAGGCGCGA-3) and Univ-1517-a-A-21 (5-ACGGCTACCTTGTTACGACTT-3) were the primers used, which yielded PCR products of 1120 bp. Oligonucleotide primers were synthesized by M/S Sigma-Aldrich. Amplification was carried out in a 20 μl reaction mixture containing 1.5 mM MgCl2, 0.2 mM each dNTP’S, 10 pmol each primers, 10 ng DNA template and 1 U Taq polymerase (Bangalore genei, India) with reaction buffer supplied by the manufacturer. Hot-start PCR was performed at 95°C prior to addition of DNA Taq polymerase. PCR amplification was performed with a Mastercycler gradient (Eppendorf). The cycling parameters of 3 min denaturation at 94°C followed by 30 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 1 min were employed. Positive control reaction contained 10 ng of Halobacterium salinarium (MTCC 1626) however negative reaction contained no exogenous template and an amplified product of the expected size (1.1 kb) was observed. Amplified products from ten independent reactions using total DNA from template were pooled and purified using a PCR purification kit (MoBio). PCR products were examined by gel electrophoresis on 1% agarose gel in 1× TAE buffer.
Cloning of Environmental PCR Product
Purified PCR products were ligated with pGEMT vector (Promega, USA) following manufacturer’s instructions. Ligation reaction was carried out overnight at 4°C. Ligated mixture was cloned in E. coli JM110 by electroporation (200 Ω, 25 μF and 2.5 kV) using Micropulser (Bio-Rad. USA). Transformants were obtained on ampicillin, X-Gal (5-bromo-4 chloro 3-indolyl-β-D-galactopyranose) and IPTG (isopropyl-beta-D-thio-galactoside) containing LB agar plates. Positive clones were picked by blue/white selection and checked for size of the right insert by PCR. Plasmid DNA was isolated by miniprep kit (Applied Biosystems, USA).
RFLP Screening of rDNA Clones
rDNA inserts from recombinant clones were reamplified by PCR reactions. Primers specific to plasmid or rDNA primers were used. The cycle parameters applied were the same as for the initial amplification of the rDNA. Aliquots (15 μl) of crude reamplified rDNA PCR products were digested with 1 U each of the 4-base specific restriction endonuclease Msp 1 (New England Bio labs, Bevery, Mass) in a final volume of 20 μl, for 3 h at 37°C. Digested products were separated by agarose (2%) gel electrophoresis. Banding pattern was visualized by staining with ethidium bromide and subsequent UV illumination. RFLP (Restriction fragment length polymorphism) pattern of each library were grouped visually and representatives were selected for sequencing.
Nucleotide Sequencing of Cloned SSU rRNA Genes
The nucleotide sequences of the cloned SSU rRNA genes were determined using an automatic DNA Sequencer (310 Genetic Analyser; Applied Biosystems, Foster city, CA). Plasmid DNA was purified with plasmid purification kits (Qiagen inc; chatsworth, CA). The partial sequencing of the 16S rRNA gene was performed making use of 6 μl Big Dye Terminator ABI Prism (Version 3); 4 pmol M13/pUC 1211 forward initiator oligonucleotide (5′-GTAAAACGACGGCCAGT-3′); 30 ng plasmid DNA, and mili-Q (Millipore) H2O for a 20 μl volume. The reactions conditions used were 24 cycles of 10 s. at 96°C, 55°C for 5 s, 60°C for 4 min.
Phylogenetic Analysis of Cloned SSU rRNA Gene Sequences
The closest database-relatives of all sequences generated were compared to 16S rRNA gene sequences available in the National Centre for Biotechnology Information (NCBI). MegAlign program of Lasergene version 5.0 was used for Multiple sequence alignment. Phylogenetic tree was constructed using Clustal W by version 1.80 (DNASTAR Inc., USA). A total of 27 sequences were involved and aligned for Phylogenetic analysis including using reported sequences available in NCBI database.
Nucleotide Sequence Accession Numbers
The sequences reported here have been deposited in the Gene bank database under the accession numbers: EU888601, EU888602, EU888603, EU888604, EU888605, EU888606, EU888607, EU888608, EU888609 and EU888610).
Results
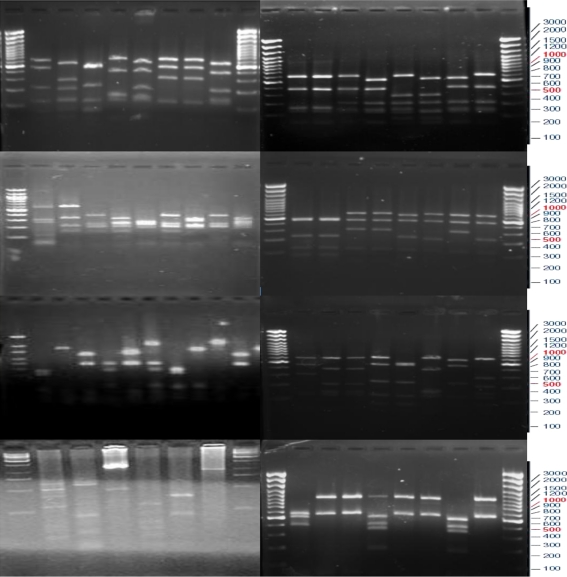
The sediment samples from saltpan were analyzed for the presence and diversity of archaea by molecular phylogenetic analysis. Small subunit (SSU) rRNA gene sequences from archaea were selectively amplified by PCR and cloned. Partial nucleotide sequence information was obtained for ten clones. This information was used to determine the cloned inserts were indeed SSU rRNA gene sequences and further to identify unique SSU rRNA gene sequences within the SSU rDNA library. 60 clones obtained from cloning were used for further analysis by restriction fragment length polymorphism (RFLP) and subsequent sequencing. Ten unique phylotypes were obtained using restriction enzyme Msp1; a four-site cutter endonuclease resulted in 3–6 bands. Based on the RFLP pattern, redundancy of inserts was checked and clones with maximum variability in banding pattern were used for sequencing (Fig. 1). Eight phylotypes were associated with phylum crenarchaeota and two with phylum euryarcheaota of domain archaea.
Fig. 1.
Restriction Fragment Length Polymorphism (RFLP) pattern of archaeal 16S ribosomal RNA gene library using endonuclease Msp 1
Evaluation by the CHECK_CHIMERA programme and inspection of putative secondary structures in all cloned SSU rDNA did not detect the presence of chimeric sequences which have been reported to arise in PCR amplified products using a mixed population of template DNA [25–28].
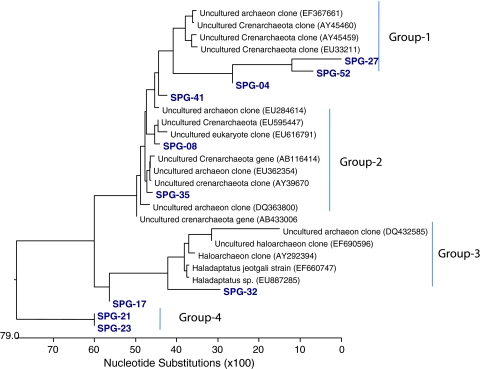
DNA sequences obtained along with retrieved 16S ribosomal sequences of selected archaeal clones were used to generate phylogenetic tree. Phylogenetic analyses revealed that majority of the clones of archaeal domain were closely related to uncultured archaea. In an overview of the tree, the archaeal rDNA sequences obtained were mostly showing similarity to the reported rDNA sequences from estuary sediments and alkaline soils. Phylogenetic assemblages thus emerged, comprised a group of Crenarchaeota and euryarcheaota lineage (Fig. 2, Group 1–4).
Fig. 2.
Phylogenetic tree showing the relationship among archaeal 16S ribosomal RNA gene sequences from salt pan sediment of Goa with reference sequences obtained through BLAST analysis. The phylogenetic tree includes 10 clone library sequences and 17 reference sequences. The archaeal clones are shown by clone name which is given in bold type and gene Bank sequence are shown by species followed by a genBank accession number
8 clones in the archaeal 16SrDNA library were related to crenarchaeota and two clones related to Euryarchaeota. None of the retrieved sequences could be grouped with previously cultured crenarchaeota, however only one clone SPG-17 of phylum Eurarchaeota was related to cultivated haloarchaea. Three sequences of Group-1 namely SPG-04, SPG-27 and SPG-52 were related to the tropical sediments of Mumbai, Goa and Brazil, whereas SPG-08, SPG-35 and SPG-41 of the group-2 were showing affiliation with sediments samples of salt marsh, tidal flat, sea floor and mangrove soil. Two sequences SPG-17 and SPG-32 formed the Group-3 in which SPG-17 was affiliated with Haloarcula, the haloarchaeon that grow at low salinities and SPG-32 showing affiliation with uncultured haloarchaeon clone, isolated from alkaline saline soil of former lake of Mexico. However the two sequences namely SPG-21 and SPG-23 of Group-4 stably formed an independent clade.
Discussion
Artificial solar salterns designed to harvest NaCl from seawater are found worldwide and consist of a set of shallow ponds connected by canals in which seawater is gradually driven to ponds of greater salinities, ranging from that of seawater to sodium chloride saturation and sometimes even beyond. Solar salt pans are well known as a habitat for halophiles and an understanding of the microbial isolates in such hypersaline environments is highly desirable due to their potential applications [29].
The aim of this study was to describe the diversity of archaea in saltpan sediments of Goa by 16S rDNA-dependant molecular phylogeny. Sequence similarity ranges of 84–96% were found in the 16S rDNA library. Our results suggested that rDNA sequences linked to archaeal microflora probably represent many novel groups of archaea within the two main kingdoms of euryarcheaota and crenarchaeota. Eight sequences were showing affiliation with the phylum crenarchaeota isolated from saltpans, tropical estuary, marine, salt marsh, tidal flat, subsea floor sediments and mangrove soil [11, 30–32]. Metabolically, Crenarchaeota are quite diverse, ranging from chemoorganotrophs to chemolithoautotrophs. They are anaerobes, facultative anaerobes or aerobes and many utilize sulfur in some way for energy metabolism. Two clones in the library resembled Haloarchaea, a member of the halophile community in which high salt concentration is required for the growth of the microorganism. Clone SPG-32 showed similarity with Haloarchaeon [33, 34] which require salt concentrations in excess of 2 M (or about 10%) to grow and optimal growth usually occurs at much higher concentrations, typically 20–25%. However, haloarchaea can grow up to saturation (about 37% salts). Whereas, clone SPG-32 showed significant alignment with the haloarchaeon that grow at low salinities. Most of the reported haloarchaeal isolates have been found to be obligate extreme halophiles requiring at least 9% (w/v) NaCl for growth and are typically the dominant heterotrophic organisms in salterns and soda lakes. However, haloarchaeal genotypes with lower requirements for salt (2.5% w/v NaCl) have also been reported in coastal salt marsh sediments [35] suggesting the wider ecological range of these physiologically versatile prokaryotes. Halophilic archaea have also been isolated from saltpans and continental shelf sediments of the west coast of India [2, 36]. It has been suggested that microsites with sufficiently high salt concentrations might be responsible for the growth of halophilic archaea in saline soils [37].
Although quantitative estimates of relative numbers were not carried out in this study, our results indicate that these haloarchaea and crenarchaeotal lineages must be major contributors to the archaeal population in salt pan sediment. However, 10 clones studied may not give the exhaustive diversity of this archaeal community. The archaeal community retrieved from the salt pan sediment of Goa suggests that at least some of the archaeal sequences identified may be unique to this site. Future research considering even deeper soil and involving more sampling may help to further explain the biodiversity of these communities and characterize community structure as well as describe the ecological role of Archaea in the saltpan. Considering their abundance and broad distribution, it is likely that members of the Crenarchaeota phylum have important ecological roles in biogeochemical cycles of sulfur, carbon and other elements. Microorganisms in marine sediments contribute significantly to global cycles of organic and inorganic matters because of their abundance [38]. The archaeal community retrieved from the saltpan sediments of Goa suggests that at least some of the archaeal sequences identified may be unique to this site and their role in sulphur metabolism.
Acknowledgments
Nasier Ahmad wishes to express his gratitude to Council of Scientific and Industrial Research (CSIR), Govt. of India for the awarding of a Senior Research fellowship. The authors are grateful to Rajinder Kumar for excellent technical assistance in sequencing work. This work was financially supported by CSIR, New Delhi, Project Number NWP006.
References
- 1.Oren A. Bioenergetic aspects of halophilism. Microbial Mol Biol Rev. 1999;63:334–348. doi: 10.1128/mmbr.63.2.334-348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghavan TM, Furtado I. Occurrence of extremely halophilic Archaea in sediments from the continental shelf of west coast of India. Curr Sci. 2004;86:1065–1067. [Google Scholar]
- 3.Auchtung TA, Tackacs-Vesbach CD, Cavanaugh CM. 16S rRNA phylogenetic investigation of the candidate division Korarchaeota. Appl Environ Microbiol. 2006;72:5077–50824. doi: 10.1128/AEM.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barns SM, Delwiche CF, Palmer JD, Pace NR. Perspectives on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. Proc Natl Acad Sci. 1996;93:9188–9193. doi: 10.1073/pnas.93.17.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brochier C, Gribaldo S, Zivanovic Y, Confalonieri F, Forterr P. Nanoarchaea: representatives of a novel archaeal phylum or a fast-evolving euryarchaeal lineage related to Thermococcales. Genome Biol. 2005;6:R42. doi: 10.1186/gb-2005-6-5-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber H, Hohn MJ, Rachel R, Fuchs T, Wimmer VC, Stetter KO. A new phylum of archaea represented by a nanosized hyperthermophilic symbiont. Nature. 2002;417:63–67. doi: 10.1038/417063a. [DOI] [PubMed] [Google Scholar]
- 7.Oline DK, Schmidt, Grant M. Biogeography and landscape-scale diversity of the dominant crenarchaeota of soil. Microbial Ecol. 2006;52:480–490. doi: 10.1007/s00248-006-9101-5. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad N, Johri S, Abdin MZ, Qazi GN. Molecular characterization of bacterial population in the forest soil of Kashmir, India. World J Microbiol Biotechnol. 2009;25:107–113. doi: 10.1007/s11274-008-9868-2. [DOI] [Google Scholar]
- 9.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol J Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad N, Sharma S, Khan FG, Kumar R, Johri S, Abdin MZ, Qazi GN. Phylogenetic analyses of archaeal ribosomal DNA Sequences from Salt Pan Sediment of Mumbai, India. Curr Microbiol. 2008;57:145–152. doi: 10.1007/s00284-008-9167-z. [DOI] [PubMed] [Google Scholar]
- 12.Olsen GJ, Lane DJ, Giovannoni SJ, Pace NR, Stahl DA. Microbial ecology and evolution: a ribosomal RNA approach. Annu Rev Microbiol. 1986;40:337–365. doi: 10.1146/annurev.mi.40.100186.002005. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh SK. Isolation of dermatophytes and other keratinophilic fungi from the vicinity of salt pan soils of Mumbai, India. Mycopathologia. 2004;157:265–267. doi: 10.1023/B:MYCO.0000024174.69248.8d. [DOI] [PubMed] [Google Scholar]
- 14.Kerkar S, Lokabharathi PA. Stimulation of sulfate-reducing activity at salt-saturation in the salterns of Ribandar, Goa, India. Geomicrobiol J. 2007;24:101–110. doi: 10.1080/01490450701266597. [DOI] [Google Scholar]
- 15.Qian-fu W, Wei L, Hai Y, Yan-li L, Hai-hua C, Dornmayr-Pfaffenhuemer M, Stan-Lotter H, Guang-qin G. Halococcus qingdaonensis sp. nov., a halophilic archaeon isolated from a crude sea-salt sample. Int J Syst Evol Microbiol. 2007;57:600–604. doi: 10.1099/ijs.0.64673-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasic L, Bartual SG, Ulrih NP, Grabnar M, Velikonja BH. Diversity of halophilic archaea in the crystallizers of an Adriatic solar saltern. FEMS Microbiol Ecol. 2005;54:491–498. doi: 10.1016/j.femsec.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Fourcans A, Bleijswijk JV, Grimalt JO, Kuhl M, Esteve I, Gerard Muyzer, Caumette P, Duran R. Characterization of functional groups in a hypersaline microbial mat community (Salins-de-Giraud, Camargue, France) FEMS Microbiol Ecol. 2004;51:55–70. doi: 10.1016/j.femsec.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Ochsenreiter T, Pelicitas, Schleper C. Diversity of Archaea in hypersaline environments characterized by molecular phylogenetic and cultivation studies. Extremophiles. 2002;6:267–274. doi: 10.1007/s00792-001-0253-4. [DOI] [PubMed] [Google Scholar]
- 19.Radax C, Gruber C, Stan-Lotter H. Novel haloarchaeal 16S rRNA gene sequences from Alpine Permo-Triassic rock salt. Extremophiles. 2001;5:221–228. doi: 10.1007/s007920100192. [DOI] [PubMed] [Google Scholar]
- 20.Cytryn E, Minz D, Oremland RS, Cohen Y. Distribution and diversity of archaea corresponding to the limnological cycle of a hypersaline stratified lake (Solar Lake, Sinai, Egypt) Appl Environ Microbiol. 2000;66:3269–3276. doi: 10.1128/AEM.66.8.3269-3276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eder W, Ludwig W, Huber R. Novel 16S rRNA gene sequences retrieved from highly saline brine sediments of Kebrit Deep, Red Sea. Arch Microbiol. 1999;172:213–218. doi: 10.1007/s002030050762. [DOI] [PubMed] [Google Scholar]
- 22.Munson MA, Nedwell BD, Embley TM. Phylogenetic diversity of archaea in sediment samples from a coastal salt marsh. Appl Environ Microbiol. 1997;63:4729–4733. doi: 10.1128/aem.63.12.4729-4733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benlloch S, Acinas SG, Anton J, Lopez L, Luz SP, Rodriguez-Valera F. Archaeal biodiversity in crystallizer ponds from a solar saltern: culture versus PCR. Microbiol Ecol. 2001;41:12–19. doi: 10.1007/s002480000069. [DOI] [PubMed] [Google Scholar]
- 24.Vetriani C, Reysenbach AL, Dore J. Recovery and phylogenetic analysis of archaeal rRNA sequences from continental shelf sediments. FEMS Microbiol Lett. 1998;161:83–88. doi: 10.1111/j.1574-6968.1998.tb12932.x. [DOI] [PubMed] [Google Scholar]
- 25.Hugenholtz P, Huber T. Chimeric 16S rDNA sequences of diverse origin are accumulating in the public databases. Int J Syst Evol Microbiol. 2003;53:289–293. doi: 10.1099/ijs.0.02441-0. [DOI] [PubMed] [Google Scholar]
- 26.Komatsoulis GA, Waterman MS. A new computational method for detection of chimeric 16S rRNA artifacts generated by PCR amplification from mixed bacterial populations. Appl Environ Microbiol. 1997;63:2338–2346. doi: 10.1128/aem.63.6.2338-2346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopczynski ED, Batson MM, Ward DM. Recognition of chimeric small-subunit ribosomal DNAs composed of genes from uncultivated microorganisms. Appl Environ Microbiol. 1994;60:746–748. doi: 10.1128/aem.60.2.746-748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuldiner AR, Nirula A, Roth J. Hybrid DNA artifact from PCR of closely related target sequences. Nucleic Acids Res. 1989;17:4409. doi: 10.1093/nar/17.11.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsiamis G, Katsaveli K, Ntougias S, Kyrpides N, Andersen G, Piceno Y, Bourtzis K. Prokaryotic community profiles at different operational stages of a Greek solar saltern. Res in microbiol. 2008;159:609–627. doi: 10.1016/j.resmic.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Park SJ, Park BJ, Rhee SK. Comparative analysis of archaeal 16S rRNA and amoA genes to estimate the abundance and diversity of ammonia-oxidizing archaea in marine sediments. Extremphiles. 2008;12:605–615. doi: 10.1007/s00792-008-0165-7. [DOI] [PubMed] [Google Scholar]
- 31.Asami H, Aida M, Watanabe K. Accelerated sulphur cycle in coastal marine sediment beneath areas of intensive shellfish aquaculture. Appl Environ Microbiol. 2005;6:2925–2933. doi: 10.1128/AEM.71.6.2925-2933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim BS, Oh HM, Chun J. Archaeal Diversity in tidal flat as revealed by 16S rRNA analysis. J. Microbiol. 2005;43:144–151. [PubMed] [Google Scholar]
- 33.Valenzuela-Encinas C, Neria-Gonzalez I, Alcantara-Hernandez RJ, Enriquez-Aragon JA, Estrada-Alvarado I, Hernandez-Rodriguez C, Dendooven L, Marsch Phylogenetic analysis of the archaeal community in an alkaline-saline soil of the former lake Texcoco. Extremphiles. 2008;12:247–254. doi: 10.1007/s00792-007-0121-y. [DOI] [PubMed] [Google Scholar]
- 34.Mesbah NM, Abou-Ela SH, Wiegel J. Novel and unexpected prokaryotic diversity in water and sediments of the alkaline, Hypersaline lakes of the Wadi An Natrun, Egypt. Microbial Ecol. 2007;54:598–617. doi: 10.1007/s00248-006-9193-y. [DOI] [PubMed] [Google Scholar]
- 35.Purdy KJ, Cresswell-Maynard TD, Nedwell DB, McGenity TJ, Timmis KN, Embley TM. Isolation of haloarchaea that grow at low salinities. Environ Microbiol. 2004;6:591–595. doi: 10.1111/j.1462-2920.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 36.Khandavilli S, Sequiera F, Furtado I. Metal tolerance of extremely halophilic bacteria isolated from estuaries of Goa, India. Ecol Env Cons. 1999;5:149–152. [Google Scholar]
- 37.Quesada E, Ventosa A, Rodriguez-Valera F, Ramos-Cormenzana A. Types and properties of some bacteria from hypersaline soils. J Appl Bacteriol. 1982;53:155–161. [Google Scholar]
- 38.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]