Abstract
YLR162W is an uncharacterized Saccharomyces cerevisiae ORF whose transcript level is elevated in cells under environmental stress, during α-factor response and in stationary phase. We obtained a partial cDNA clone of YLR162W by subtractive hybridization cloning of genes that were not expressed in a CoCl2 resistant DNA synthesis mutant but expressed in its wild type counterpart. Our studies demonstrated that YLR162W transcript level was reduced in BY4741 cells upon exposure to the hypoxia mimetic agent CoCl2, and continuous expression of full length YLR162W from a plasmid borne copy of the gene rendered BY4741 cells extremely susceptible to the hypoxic conditions induced by CoCl2. At initial time points following the induction of YLR162W expression, cell cycle progression was inhibited with the emergence of a distinct sub-G1 peak indicative of apoptotic cells, mitochondrial membrane potential was also decreased along with an increase in the fraction of cells permeable to propidium iodide; none of the above was further affected by CoCl2. The up-regulation of Ylr162wp in cells exposed to environmental stress and in non-replicating cells appears to be related to its growth inhibitory properties presented in this report.
Keywords: Saccharomyces cerevisiae, YLR162W, Hypoxia, Apoptosis, Mitochondrial membrane potential
Introduction
YLR162W is an uncharacterized S. cerevisiae ORF that resides ~20 kb upstream of the chromosomal rDNA repeat in chromosome XII and is supposed to have arisen from a duplication event. It’s third half and attached flanking region (a total of 1 kb) is more than 99% similar to the reverse complement of the 25S rRNA coding region. YLR162W encodes a hypothetical membrane protein of a molecular mass of 13,055 Da (NCBI accession no. 568478) containing 118 amino acids with one putative transmembrane domain coded by residues 37–53. Ylr162wp has been classified as a type-2 membrane protein [1] that does not have a cleavable signal sequence and displays an N-terminal extracellular and C- terminal cytoplasmic orientation [2].
Very little experimental data is available on the YLR162W gene product. Its over-expression provides resistance against an anti-microbial peptide (MiAMP1) in a strain of S. cerevisiae that is susceptible to MiAMP1 [3]. Expression studies have shown that YLR162W transcript level was increased by 4.3 folds in cells over-expressing MLH1 [4] i.e., under conditions of elevated spontaneous mutation rate and genomic instability. YLR162W expression was also significantly elevated during high-pressure stress [5], upon Mg2+ starvation [6], during response to α-factor (50 folds) [7] and in stationary phase [8]. Its expression is reduced under both oxidative and reductive stress [9] and during hypoxia followed by reoxygenation in both glucose and galactose [9]. In spite of the available expression data, a specific function is yet to be assigned to Ylr162wp. Available data indicate that YLR162W mRNA level increased in cells under various stress conditions and in non-replicating cells.
Cobalt chloride is a well-established hypoxia mimetic agent in S. cerevisiae [10] and in mammalian cell in culture [11]. It has hence been widely used to study the effects of hypoxia on cellular activities.
In the present communication, we report functional characterization of the YLR162W gene product.
Materials and Methods
Chemicals, Growth Media and Growth Condition
Media components were purchased from Becton Dikinson and Co. and all reagents used were of molecular biology grade. S. cerevisiae cells were grown at 30°C either in SD (synthetic dextrose) or in SG (synthetic galactose) media containing appropriate supplements. Wherever indicated CoCl2 was added to a final concentration of 0.75 mM to the media to induce hypoxia specific gene expression. Hypoxia specific repression of ROX1 and induction of OLE1 was confirmed in control experiments in cells exposed to CoCl2 in parallel with cells exposed to hypobaric hypoxia (Table 1).
Table 1.
Microbial strains and vectors used in this study
| Strains | Genotype | Source |
|---|---|---|
| BJ5416 (ABC709) | MATa ura3-52 lys2-801leu2-D1his3-D200 GAL | Dr. S. Biswas JNU, Delhi, India. |
| BY4741 (ABC733) | MATa his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 | Dr. A. Bachhawat. IMTECH, Chandigarh, India. |
| BY4741pBG1805YLR162W-3xHA | BY4741pBG1805YLR162W-3xHA | This work. |
| Y800OLE1-3xHA | 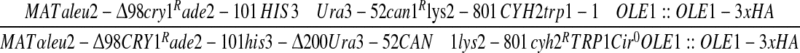 |
Open Biosystems. |
| E. coli XL1-Blue | [12] | Bangalore Genei |
| Bluescript | [12] | Dr. S. Biswas JNU, Delhi, India. |
Cloning Techniques, DNA Sequencing and Identification of YLR162W by Sequence Similarity Search
All cloning experiments were performed according to Sambrook et al. [12]. CoCl2 resistant DNA synthesis mutants were isolated from BJ5416 cells based on their ability to incorporate α-35SdATP into their chromosomal DNA in the presence of CoCl2. Subtractive hybridization [13] was performed with the PCR-select subtractive hybridization kit (BD Biosciences) according to the manual supplied by the manufacturer. Equal amounts of Poly-A+ RNA (1 μg) isolated from the mutant and BJ5416 cells either following exposure to 0.75 mM CoCl2 for 3 h or without any exposure were mixed before cDNA synthesis to ensure equal representation of RNAs expressed under stressed and non-stressed conditions in the two strains. Subtractive hybridization was carried out with cDNA synthesized from Poly-A+ RNA isolated from the mutant as driver and that from BJ5416 cells as tester. The subtracted library was amplified twice by PCR using primers (specific for the tester) supplied in the kit, digested with Rsa I and ligated into EcoR I digested, end-filled Bluescript. The library was transformed into E. coli XL1-Blue cells and transformants containing inserts were identified by blue-white screening. Recombinant plasmids isolated from the transformants were digested with BamH I and Hind III to confirm clones containing inserts. DNA sequencing was performed commercially (Lab India Pvt. Ltd) using both T3 and T7 universal primers and the region between restriction sites BamHI and HindIII was used as a query for similarity search in the Saccharomyces ORF database (SGD).
Cell Survival Assay
Cell survival assays were performed by spotting ten fold serial dilutions (103, 102, 101, 100 cells) of log phase cells of the appropriate strain onto plates containing media and additives as indicated in the respective figures. Plates were incubated at 30°C for three days before being photographed.
Northern Blotting
Northern blotting was performed as per standard procedure [12]. 32P-labeled single stranded probes were prepared by primer extension with the Klenow fragment of E. coli DNA polymerase I using anti-sense primer (YLR162WAS: 5′ GGATCCGAATTCCTA GCAACGGGTGCTCTTGGCGGAAAGGCC 3′) and PCR amplified YLR162W as template in the presence α-32PdATP (Primers YLR162WS: 5′ ATCGATAAGCTT ATATGCAGCACGCTTACCCGGACCGCCTCT 3′ and YLR162W AS).
Protein Isolation and Western Blotting:
Cell lysis was performed as described in [14] and proteins of interest were detected by immuno-blotting using appropriate Antibodies.
Cell Cycle Progression by Flow Cytometry:
Cell cycle progression during growth under optimal conditions and in the presence of 0.75 mM CoCl2 was determined by flow cytometry essentially as described in [15] except that cells were synchronized by overnight incubation in minimal media containing 5 μg/ml α1-mating factor (Sigma). 104 events were acquired per sample and counts versus FL2-A histograms were obtained in a Becton–Dickinson flow cytometer (488 nm argon laser) and further analyzed using Cell Quest software.
Estimation of Cell Death by Propidium Iodide Staining
Log phase cells were shifted to galactose containing media to induce YLR162W expression and either exposed to CoCl2 (0.75 mM) for 3 h or kept at 30°C without CoCl2 as the untreated control. 2.5 × 106 cells from each aliquot were withdrawn, washed with PBS and suspended in 500 μl PBS followed by the addition of 15 μl of 1 mg/ml propidium iodide (PI) solution. After incubation at 30°C for 30 min, aliquots of 104 cells were analyzed by flow cytometry (Becton–Dickinson, 488 nm argon laser) for fluorescence. An acquisition protocol was defined to measure Forward scatter (FSC), Side scatter (SSC) and fluorescence (FL2-H) on a four decades logarithmic scale. Further analysis was done by Cell Quest software.
Measurement of Mitochondrial Membrane Potential
Mitochondrial membrane potential was measured essentially as described in [16]. Briefly, log phase cells in a total volume of 3 ml were shifted to galactose containing media to induce YLR162W expression and were harvested at different intervals of time. NaN3 was added to a final concentration of 20 mM to a separate culture to be used as a control. 106 cells were harvested for each reading, washed twice with ice–cold water and suspended in water at 106 cells/ml, Rhodamine123 (Rh123; Sigma) was then added to a final concentration of 25 nM and incubated for 10 min at 30°C in dark. 2 × 104 cells were analyzed using an acquisition protocol to measure Forward scatter (FSC), Side scatter (SSC) and Green Fluorescence (FL1-H) on a four decade logarithmic scale. Further analysis was done by cell Quest software.
Results and Discussion
Cloning of YLR162W
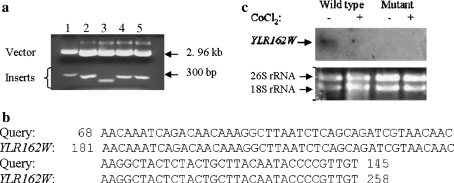
We performed subtractive hybridization cloning of genes that were not expressed in a CoCl2 resistant DNA synthesis mutant in comparison to its wild type counterpart (BJ5416) to identify factors responsible for efficient DNA synthesis in a hypoxic environment. Positive clones were confirmed by restriction digestion and all the inserts obtained were between 200 and 300 bp in length (Fig. 1a). Following sequencing of the inserts and similarity search in the Saccharomyces ORF Database one of the clones was found to be identical to a part of YLR162W–an uncharacterized S. cerevisiae ORF (Fig. 1b and a, Lane 3). Northern blotting experiments indicated that YLR162W transcript level was reduced in BJ5416 cells during exposure to CoCl2 in comparison to growth under optimal conditions. It was undetectable in the mutant both in the presence and absence of CoCl2 (Fig. 1c). Since we had set out to clone genes that were not expressed in the CoCl2 resistant DNA synthesis mutant, the northern blotting data vindicated our subtractive hybridization experiment.
Fig. 1.
YLR162W cloning and expression under CoCl2 induced hypoxia. a Restriction digestion of clones obtained following subtractive hybridization: recombinant plasmid isolated from the clones were digested by BamH I and Hind III and analyzed by agarose gel electrophoresis. b Sequence similarity between the cloned gene (query) and S. cerevisiae ORF YLR162W. cYLR162W transcript level during exposure to CoCl2: 20 μg total RNA isolated from BJ5416 cells and CoCl2 resistant DNA synthesis mutant either following exposure to 0.75 mM CoCl2 for 30 min or without any exposure was analyzed for YLR162W expression by Northern blotting and hybridization with full length YLR162W anti-sense probe. The experiment was carried out with two different populations of total RNA and representative results are shown. c Lower panel: 2 μg of the total RNA used in the above blot was analyzed by agarose gel electrophoresis to determine if the RNA was intact
YLR162W Over-Expression Rendered BY4741 Cells Susceptible to CoCl2
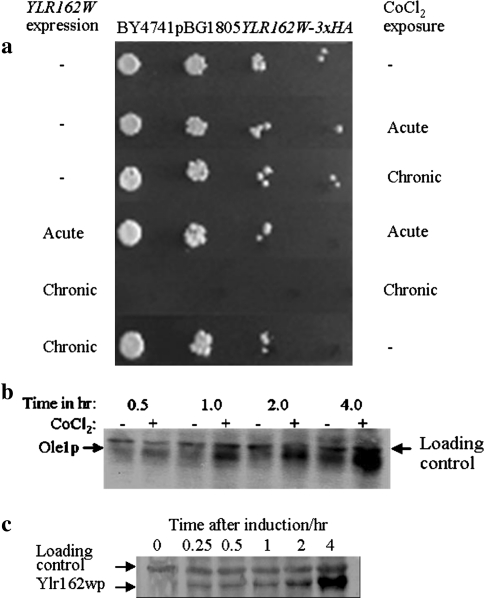
For functional characterization of the YLR162W gene product, we studied the phenotypic effects of its over-expression in BY4741 cells. Continuous expression of YLR162W had a slight inhibitory effect on cell proliferation but rendered cells extremely sensitive to chronic CoCl2 exposure (Fig. 2a). Neither acute exposure to CoCl2 for 2 h during YLR162W expression nor chronic exposure to CoCl2 alone had any significant effect on cell viability (Fig. 2a). Figure 2b and c are controls demonstrating the induction of hypoxia specific Ole1p expression during exposure to CoCl2 (Fig. 2b) and expression of Ylr162wp in media containing galactose (Fig. 2c), respectively.
Fig. 2.
Effect of YLR162W expression on cell viability in the presence and absence of CoCl2: a for acute exposure to CoCl2 in the presence of YLR162W, cells were grown in SG broth containing 0.75 mM CoCl2 for 2 h, exposure to CoCl2 alone was carried out in SD broth. Chronic exposure to CoCl2 was carried out in plates containing 0.75 mM CoCl2, SG plates were used for concurrent expression of YLR162W and SD plates for exposure to CoCl2 alone. Cells were spotted in tenfold serial dilutions and SG plates were incubated for four days at 30°C to compensate for the slower growth rate in galactose containing media. b Induction of OLE1 expression by CoCl2: Ole1p levels were detected by immunoblotting, 40 μg total protein isolated fromY800OLE1-3xHA that were either untreated or treated with 0.75 mM CoCl2 for periods of time as indicated in the figure was electro-blotted onto PVDF membranes and probed with anti-HA Ab and developed with HRP conjugated secondary Ab and chemiluminescent HRP substrate. cYLR162W expression in media containing galactose: 40 μg of total cellular protein isolated at different time points from a log phase culture of BY4741pBG1805YLR162W-3xHA cells following transfer to galactose containing media was analyzed for Ylr162wp levels as described in (b) with anti-HA Ab and alkaline phosphatase conjugated secondary Ab, blots were developed with BCIP and NBT
Cell Cycle Progression was Inhibited in Cells Expressing YLR162W
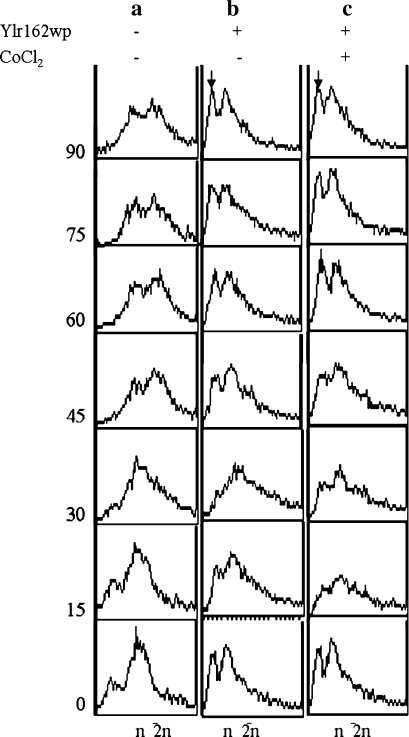
Further characterization of the growth inhibitory properties of Ylr162wp especially in the presence of CoCl2 was carried out by cell cycle progression studies of cells expressing YLR162W in the presence and absence of CoCl2. Results demonstrated that following removal of the α-factor block a fraction of the cells expressing YLR162W entered the S phase (15–30 min, Fig. 3) but did not enter the G2 phase of the cell cycle, instead, the sub-G1 and G1 peaks re-emerged (45 min onwards, Fig. 3). Since a sub-G1 peak is indicative of apoptotic cells [17 and 18] it appears that YLR162W expression induced apoptosis in BY4741 cells. The presence of CoCl2 in the growth media did not have any significant additional effect. Inhibition of cell cycle progression is known to require the activation of checkpoint pathways [19]; we hence determined the response of checkpoint pathway mutants to CoCl2 during YLR162W expression. The response of the chk1Δ, rad9Δ and dun1Δ cells towards exposure to CoCl2 during YLR162W expression was essentially identical to that of BY4741 cells (data not shown; similar to Fig. 2a). Our results suggested that the combined effect of YLR162W and CoCl2 on cell viability was irrespective of checkpoint functions since the absence of key checkpoint proteins could not overcome the above inhibitory effect. Some apoptotic pathways however are known to be independent of checkpoint functions in yeast [20].
Fig. 3.
Cell cycle progression of YLR162W expressing cells. Cell cycle progression of a BY4741 in SD media (Control) b BY4741pBG1805YLR162W-3xHA in SG media and c BY4741pBG1805YLR162W-3xHA in SG media containing 0.75 mM CoCl2 was determined by flow cytometry of aliquots taken out at regular intervals of time following removal of the α-factor block. The sub-G1 peaks indicative of apoptotic cells have been indicated by arrows (↓)
Cell Death and Decreased Mitochondrial Membrane Potential in YLR162W Expressing Cells
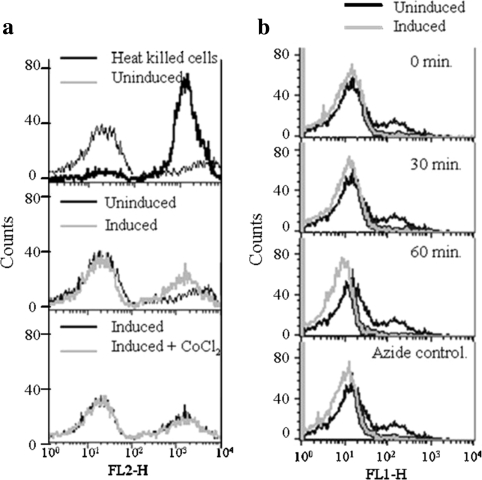
Apoptosis induced by YLR162W over-expression (Fig. 3) was further investigated by determining the percentage of cells that were permeable to propidium iodide (dead cells) at early time points following the induction of YLR162W expression. Results (Fig. 4a) showed that YLR162W overexpression caused an increase in the number of cells permeable to propidium iodide as indicated by the flow cytometry data (Fig. 3). CoCl2 did not have any additional effect over that observed during YLR162W expression alone (as also observed in the flow cytometry data, Fig. 3). Since mitochondrial membrane potential (ψm) is decreased upon initiation of apoptosis [18], we measured ψm in YLR162W expressing cells. Mitochondrial membrane potential began decreasing within 30 min of induction of YLR162W expression and continued to decrease further for the next 30 min (Fig. 4b). The data (Fig. 4a, b) demonstrated that over-expression of YLR162W induced cell death and caused a substantial reduction in ψm.
Fig. 4.
Effect of YLR162W expression on a Cell death and b Mitochondrial membrane potential. Experiments were performed essentially as described in materials and methods
Functional analysis of Ylr162wp based upon phenotypic studies of cells expressing the protein demonstrated that, at early time points following the induction of YLR162W expression in BY4741 cells, cell cycle progression was inhibited (Fig. 3), mitochondrial membrane potential was decreased (Fig. 4b) and cell death was increased (Fig. 4a). Cells expressing YLR162W were rendered extremely susceptible to the hypoxic conditions induced by CoCl2 (Fig. 2a).
The growth inhibitory property of Ylr162wp seems to explain its up-regulation under stress conditions and when cells do not actively proliferate; Ylr162wp may hence be part of the cellular stress response mechanism that inhibits cell proliferation during exposure to stressful conditions and thereby conserves cellular ATP reserves that is required during adaptation to adverse environmental conditions. In addition, the hitherto uncharacterized S. cerevisiae ORF YLR162W or peptides synthesized based upon Ylr162wp may be important pharmaceutical agents for its growth inhibitory properties especially during exposure to hypoxic conditions.
Acknowledgments
We are grateful to Dr. G. Ilavazhagan Director, DIPAS, for his support during the course of this work. We acknowledge Ms. S. Parmar’s (a former summer trainee in the laboratory) work in isolating the CoCl2 resistant DNA synthesis mutants. The work was supported by the Ministry of Defence, Govt. of India. NK was supported by a fellowship from DRDO, Min. of Defence, Govt. of India.
References
- 1.Klein P, Kanehisa M, DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985;815:468–476. doi: 10.1016/0005-2736(85)90375-X. [DOI] [PubMed] [Google Scholar]
- 2.Heijne G, Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988;174:671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]
- 3.Stephens C, Harrison SJ, Kazan K, Smith FWN, Goulter KC, Maclean DJ, Manners JM. Altered fungal sensitivity to a plant antimicrobial peptide through over-expression of yeast cDNAs. Curr Genet. 2005;47:194–201. doi: 10.1007/s00294-005-0562-8. [DOI] [PubMed] [Google Scholar]
- 4.Shcherbakova PV, Hall MC, Lewis MC, Bennett SE, Martin KJ, Bushel PR, Afshari CA, Kunkel TA. Inactivation of DNA mismatch repair by increased expression of yeast MLH1. Mol Cell Biol. 2001;21:940–951. doi: 10.1128/MCB.21.3.940-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuoka H, Suzuki Y, Iwahashi H, Arao T, Suzuki Y, Tamura K. The biological effects of high-pressure on the yeast transcriptome. Braz J Med Biol Res. 2005;38:1267–1272. doi: 10.1590/S0100-879X2005000800016. [DOI] [PubMed] [Google Scholar]
- 6.Wiesenberger G, Steinleitner K, Malli R, Graier WF, Vormann J, Schweyen RJ, Stadler JA. Mg2+ deprivation elicits rapid Ca2+ uptake and activates Ca2+/calceneurin signaling in Saccharomyces cerevisiae. Eukaryot Cell. 2007;6:592–599. doi: 10.1128/EC.00382-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts CJ, Nelson B, Marton MJ, Stoughton R, Meyer MR, Bennett HA, He YD, Dai H, Walker WL, Hughes TR, Tyers M, Boone C, Friend SH. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science. 2000;287:873–880. doi: 10.1126/science.287.5454.873. [DOI] [PubMed] [Google Scholar]
- 8.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai LC, Kosorukoff AL, Burke PV, Kwast KE. Metabolic-state-dependent remodeling of the transcriptome in response to anoxia and subsequent reoxygenation in Saccharomyces cerevisiae. Eukaryotic Cell. 2006;5:1468–1489. doi: 10.1128/EC.00107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwast KE, Lai L-C, Menda N, James DT, III, Aref S, Burke PV. Genomic analyses of anaerobically induced genes in Saccharomyces cerevisiae: functional roles of Rox1 and other factors in mediating the anoxic response. J Bacteriol. 2002;184:250–265. doi: 10.1128/JB.184.1.250-265.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang GL, Semenza GL. Purification and characterization of hypoxia inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. NewYork: CSHL Press; 1989. [Google Scholar]
- 13.Diatchenko L, Lau Y-FC, Campbell AP, Chenchik A, Moqadm F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlot E, Siebert PD. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S-J, Schwartz MF, Duong JK, Stern DF. Rad53 phosphorylation sites clusters are important for Rad53 regulation and signaling. Mol Cell Biol. 2003;23:6300–6314. doi: 10.1128/MCB.23.17.6300-6314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacKAY VJ, Mal B, Waters L, Breeden LL. Early cell cycle box-mediated transcription of CLN3 and SWI4 contributes to the proper timing of the G1-to-S transition in budding yeast. Mol Cell Biol. 2001;21:4140–4148. doi: 10.1128/MCB.21.13.4140-4148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludovico P, Sousa MJ, Silva MT, Leao C, Côrte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 2001;147:2409–2415. doi: 10.1099/00221287-147-9-2409. [DOI] [PubMed] [Google Scholar]
- 17.Kajstura M, Halicka DH, Pryjma J, Darzynkiewicz Z. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete ‘‘Sub-G1’’ peaks on DNA content histograms. Cytometry Part A. 2007;71A:125–131. doi: 10.1002/cyto.a.20357. [DOI] [PubMed] [Google Scholar]
- 18.Nargund AM, Avery SV, Houghton JF. Cadmium induces a heterogeneous and caspase-dependent apoptotic response in Saccharomyces cerevisiae. Apoptosis. 2008;13:811–821. doi: 10.1007/s10495-008-0215-8. [DOI] [PubMed] [Google Scholar]
- 19.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 20.Burhans WC, Weinberger M, Marchetti MA, Ramachandran L, D’Urso G, Huberman JA. Apoptosis-like yeast cell death in response to DNA damage and replication defects. Mutat Res. 2003;532:227–243. doi: 10.1016/j.mrfmmm.2003.08.019. [DOI] [PubMed] [Google Scholar]