Abstract
Pma1p is an essential plasma membrane H+-pump in Saccharomyces cerevisiae that pumps out H+ at the expense of cellular ATP. Its activity is induced by glucose at 30°C and is inhibited by Hsp30 during exposure to heat shock conditions. To further investigate the regulation of Pma1 function by glucose and Hsp30 during exposure to thermal stress, we estimated Pma1 activity, its protein levels and ser-phosphorylation status in membrane fractions isolated from BY4741 and hsp30Δ cells grown in dextrose and sorbitol at 30°C, and following exposure at 40°C for 30 min. Our results demonstrate that Pma1 activity and protein levels were reduced in Hsp30+ cells following exposure to thermal stress in dextrose media. The above was not observed in hsp30Δ cells wherein Pma1 activity did not decrease following exposure to similar conditions. Although Pma1p levels decreased in heat-shocked hsp30Δ cells, it was lower compared to that observed in Hsp30+ cells. Total ser-phosphorylation of Pma1 also showed a decrease following exposure to heat shock condition in dextrose media in both BY4741 and hsp30Δ cells. Its levels were also reduced in BY4741 cells upon heat shock treatment in sorbitol unlike that observed in hsp30Δ cells wherein it was increased. Taken together the above indicate that heat shock induced reduction in Pma1 activity and protein levels in dextrose media required Hsp30. To examine functional interactions between dextrose utilization, Hsp30 and the regulation of various aspects of Pma1, we determined if dextrose regulated other functions attributed to Hsp30. Results demonstrate that the deletion of HSP30 rendered cells dependent on dextrose utilization for survival during exposure to lethal heat stress. Our study has hence been able to establish a functional relationship between glucose utilization, Hsp30 function and the regulation of Pma1 activity. Finally, since the deletion of HSP30 renders Pma1p levels and its activity unresponsive to thermal stress in dextrose media, we concluded that Hsp30 is necessary to maintain Pma1 in a regulation competent conformation. Hsp30 may thus act as a chaperone in the S. cerevisiae plasma membrane.
Keywords: Saccharomyces cerevisiae, Pma1, Dextrose, Hsp30, Chaperone
Plasma membrane H+-ATPase (Pma1) is an essential protein in S. cerevisiae, it pumps out H+ to maintain intracellular pH homeostasis as well as to generate an electrochemical gradient required to drive the import of metabolites into the cell [1]. The above activity of Pma1 utilizes a significant fraction of total cellular ATP. Its activity and expression are induced by glucose [2, 3]. Glucose induced phosphorylation of Pma1 in Ser-899, Thr-912 and Ser-911 [4, 5] correlated with increase in Pma1 catalytic activity. In glucose starved cells Pma1 is negatively regulated by Casein kinase I (Yck1p and Yck2p) phosphorylation [6], which is reduced in the presence of glucose. The mechanism of glucose-induced activation of Pma1 function is not clear at the present point in time; it is thought to involve phosphorylation of Ser-899 and Ser-911 and dephosphorylation of serine residues in Casein kinase I phosphorylation sites. Pma1 activation by glucose requires the participation of the glucose sensor Snf3p, the G-protein Gpa2p and transport and phosphorylation of glucose [7]. Pma1 expression induces oncogenic transformation of NIH 3T3 cells and causes increase in intracellular pH [8]. In recent years, Pma1 has attracted much attention as a probable target for the development of anti-fungal agents [9], its suitability as a biomarker for toxicant induced stress [10] and as a model for the study of membrane protein biogenesis [11].
Pma1 activity is negatively regulated by Hsp30 during exposure to heat shock conditions. Hsp30 thus contributes towards thermo-tolerance by lowering cellular ATP consumption during stress response [12]. S. cerevisiae Hsp30 is a plasma membrane heat shock protein. It has seven predicted trans-membrane domains and may belong to Family A of the G protein coupled receptor superfamily of proteins [13]. Hsp30 expression is induced during exposure to a variety of stress conditions including heat shock, exposure to weak organic acids, hyper-osmotic stress, oxidative stress, glucose limitation, exposure to alcohol and entry into stationary phase [14, 15]. Its role in providing resistance against multiple environmental stress conditions remains to be elucidated. HSP30 expression is independent of Hsf1 and partially dependent on Msn2 and Msn4 [14]. Two other activators of HSP30 expression namely Pci8 [16] and Sfl1p [17] have also been identified. It is part of the Pci8 induced stress regulon that is constituted of genes induced by various stress conditions.
In the present communication, we report on the regulation of Pma1 activity by dextrose and Hsp30 during exposure to thermal stress.
Materials and Methods
Growth Media, Growth Conditions and Strains
Unless otherwise stated yeast cells were grown at 30°C in YPD. S. cerevisiae strains used in the present study were BY4741 (Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0), BY4741 hsp30Δ::LEU2 (constructed in this laboratory) and BY4741 hsp30Δ::kanMX (Open Biosystems).
Exposure of Yeast Cells to Lethal Heat Stress and Cell Survival Assay
Log phase cultures of BY4741 or BY4741hsp30Δ in minimal media containing appropriate supplements were subjected to lethal heat stress at either 50°C for different intervals of time or left untreated at 30°C (control). Cells were then spotted in 10 fold serial dilutions followed by incubation at 30°C for 3 days before photography.
Isolation of Total Membrane Fraction
Total membrane fractions were isolated from 25 ml log phase cultures of BY4741 and BY4741hsp30Δ grown either at 30°C or following exposure to either 40°C for 30 min as described earlier [18, 19]. Briefly, cell lysates prepared by the glass bead method in buffer L [30 mM Tris–HCl (8.5), 5 mM EDTA, 1 mM PMSF, 1 µM Pepstatin and 6 µM Leupeptin] were clarified by centrifugation at 700 g for 10 min and the supernatant was centrifuged at 20,000 g for 30 min to precipitate the membrane fraction. The crude membrane fraction was suspended in buffer S [10 mM Tris–HCl (7.5), 0.2 mM EDTA, 0.2 mM DTT and 20% (v/v) glycerol]. For isolation of membrane fraction used to estimate Pma1 phosphorylation, BY4741 and hsp30Δ cells were grown to log phase in YPD containing 4% dextrose, cells were precipitated, washed in sterile water and incubated at 30°C in sterile water for 1 h [5] followed by growth in either 250 mM sorbitol (glucose starved) or 4% (w/v) dextrose for 1 h. Cells were then subjected to heat shock at 40°C for 30 min or left untreated at 30°C and membrane fractions were isolated from all the cultures as above except that all buffers contained 2 mM NaF and 1 mM ammonium molybdate in addition to all the other components.
Immunoblotting
Immunoblotting was performed as described earlier [20]. Anti-Pma1 (yN-20) Ab (Santa Cruz Biotechnology, Inc.), alkaline phosphatase conjugated anti Rabbit-IgG and anti Goat-IgG (Santa Cruz Biotechnology, Inc.), Monoclonal anti-phospho-serine Ab (abcam) and HRP conjugated anti-mouse IgG (Thermoscientific) were used in dilutions recommended by the manufacturer. Pma1p blots were developed with alkaline phosphatase substrate BCIP and NBT and phospho-serine blots were developed with HRP substrate.
Assay for Pma1-ATPase Activity
Pma1 ATPase activity in total membrane fraction was assayed as described in [21]. The assay was performed in the presence and absence of diethylstilbestrol—a specific inhibitor of Pma1-ATPase to determine the contribution of Pma1 towards total membrane ATPase activity. One unit of Pma1 ATPase activity generates 1 µmol Pi per min by hydrolysis of ATP.
Measurement of Extracellular Acidification
Log phase BY4741 and hsp30Δ cells were precipitated, washed with water and re-suspended in sterile water at a concentration of 150 mg cells (wet weight) per 10 ml water. Dextrose or sorbitol was then added to final concentrations of 2% (w/v) and 250 mM respectively to the cell suspension and incubated at either 30 or 40°C; pH was measured at 1 min intervals by a pH meter [22].
Results and Discussion
To investigate the regulation of Pma1 function by dextrose and Hsp30 during exposure to heat shock conditions, we estimated Pma1 activity its protein levels and ser-phosphorylation status in membrane fractions isolated from BY4741 and hsp30Δ cells grown either in dextrose or in sorbitol at 30°C and following exposure to 40°C for 30 min.
Regulation of Pma1 H+-ATPase Activity
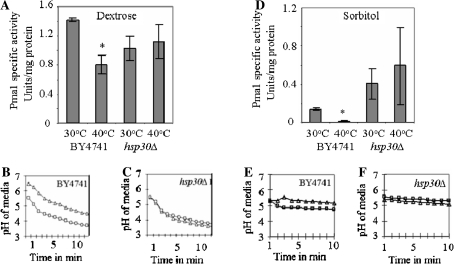
Pma1 ATPase specific activity was reduced in membrane fractions isolated from heat shocked BY4741 cells grown in dextrose media. The same was not reduced in hsp30Δ cells exposed to similar conditions (Fig. 1a). Thermal stress induced alterations in ATPase activity correlated with changes in extracellular pH. In agreement with decreased ATPase activity, BY4741 cells showed increased extracellular pH when incubated at 40°C (Fig. 1b). In contrast, the hsp30Δ strain showed a slight decrease in extracellular pH upon incubation at 40°C (Fig. 1c). Under conditions of dextrose starvation, Pma1 ATPase activity was low in BY4741 cells but increased in hsp30Δ cells (Fig. 1d). Extracellular pH of BY4741 and hsp30Δ cells in sorbitol did not show any alteration at either 30 or 40°C, which suggested that cellular H+ pumping activity, was reduced in dextrose-starved cells (Fig. 1e, f). Pma1 activity was hence regulated primarily by dextrose, which was further influenced by Hsp30 during exposure to thermal stress in BY4741 cells.
Fig. 1.
The effect of dextrose, heat shock and Hsp30 on Pma1 activity. a and d Pma1-ATPase specific activity in strains and conditions as indicated in the figure was determined by the method of Serrano (21). Average data ± SD are shown. * Indicates P < 0.05. Alteration in extra-cellular pH of BY4741 and hsp30Δ cells grown in dextrose [(b) and (c)] and in sorbitol [(e) and (f)] at 30°C (open squares) and 40°C (open triangles), experiments were performed as described in “Materials and Methods” section
Regulation of Pma1 Protein Levels
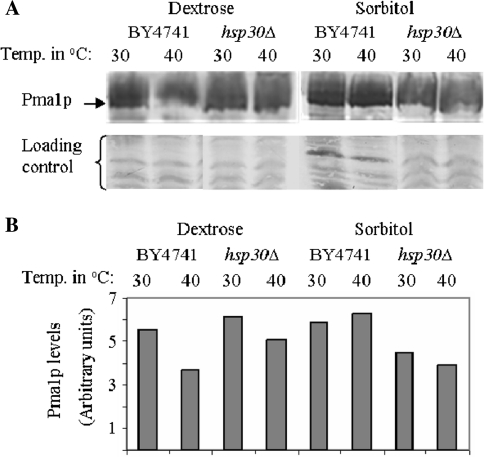
Pma1 protein levels were down-regulated in BY4741 cells during exposure to heat shock conditions in media containing dextrose. In hsp30Δ cells, Pma1 protein levels were also reduced under similar conditions (Fig. 2a, b) but to a lesser extent. A comparison of the effect of dextrose on Pma1p levels in BY4741 and hsp30Δ cells indicated that its levels were reduced in the presence of dextrose in Hsp30+ cells but induced in hsp30Δ cells (Fig. 2; Table 1). Its levels were however higher in dextrose starved Hsp30+ cells compared to that in hsp30Δ cells under similar conditions.
Fig. 2.
Regulation of Pma1p levels by dextrose, heat stress and Hsp30. a Pma1 protein levels were determined in 40 µg membrane fraction isolated from strains and conditions as indicated in the figure by western blotting using anti-Pma1 Ab. The blot was initially used to detect ser-phosphorylation levels of Pma1 by anti-phospho-serine Ab, stripped and re-probed with anti-Pma1 Ab. Lower panel non-specific bands recognized by anti-Pma1 Ab was used as a loading control. b Densitometric scans of a using ImageJ software
Table 1.
Summary of results
| Parameters measured | Environmental conditions | |||||||
|---|---|---|---|---|---|---|---|---|
| Dextrose | Sorbitol | |||||||
| BY4741 | hsp30Δ | BY4741 | hsp30Δ | |||||
| 30°C | 40°C | 30°C | 40°C | 30°C | 40°C | 30°C | 40°C | |
| % Pma1 specific activity | 100 | 56.4 | 72.3 | 78.5 | 0.1 | 0.01 | 28.4 | 34 |
| Pma1p levelsa | 100 | 68 | 110 | 92 | 106 | 114 | 80 | 70 |
| Pma1-P: Pma1pa | 100 | 77 | 126 | 98 | 183 | 177 | 168 | 187 |
| Dextrose requirement during lethal heat stress | Necessary but not essential | Essential | ||||||
Cellular Pma1p levels were hence controlled by a number of factors. It was regulated by thermal stress in both Hsp30+ and hsp30Δ cells, by dextrose availability in hsp30Δ cells and by Hsp30 during dextrose starvation.
Regulation of Pma1 Ser-phosphorylation
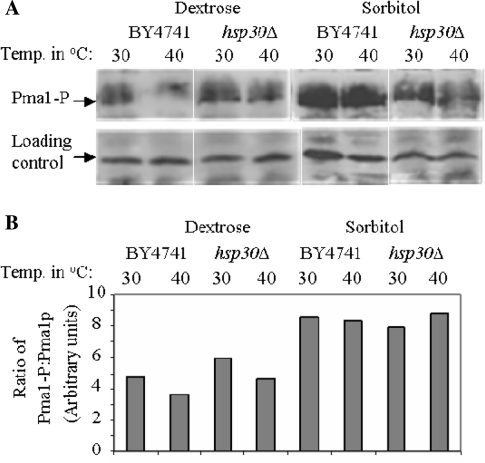
Total ser-phosphorylation of Pma1 as estimated by the ratio of phosphorylated Pma1 to total Pma1 protein was reduced in the presence of dextrose in both BY4741 and hsp30Δ cells (Fig. 3a, b). It was however higher in hsp30Δ cells at both 30°C and following exposure to heat shock conditions compared to Hsp30+ cells (Fig. 3) in dextrose media. Overall ser-phosphorylation of Pma1 was hence regulated primarily by dextrose that was independent of Hsp30.
Fig. 3.
Regulation of ser-phosphorylation of Pma1 by dextrose. a Ser-phosphorylation levels of Pma1 were determined in 40 µg membrane fractions isolated from strains and conditions as indicated in the figure by immunoblotting using monoclonal anti-phospho-serine Ab. Lower panel a non-specific band recognized by the anti-phospho-serine Ab was used as a loading control. b Densitometric scans of a using ImageJ software
Saccharomyces cerevisiae hsp30Δ cells were More Sensitive to Heat Stress in the Presence of 2-Deoxyglucose (2-dg)
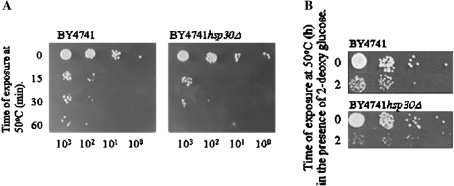
Results presented above demonstrate that Hsp30 influenced Pma1 activity, protein levels and phosphorylation in the presence of dextrose. To examine possible relationships between glucose, Hsp30 and regulation of various aspects of Pma1 we determined if dextrose regulated other functions attributed to Hsp30. We determined cell survival of BY4741 and hsp30Δ cells in the presence and absence of 2-dg following an exposure to lethal heat stress at 50°C for different intervals of time. The hsp30Δ mutant was slightly more sensitive to an exposure at 50°C as compared to BY4741 cells (Fig. 4a). The deletion mutant was however considerably more sensitive to 50°C in the presence of 2-dg even in YPD (Fig. 4b), thus indicating a requirement for dextrose utilization in hsp30Δ cells for survival during exposure to thermal stress. Taken together the results (Fig. 4a, b) indicated a relationship between Hsp30 and cellular glucose utilization, the later in turn regulates Pma1 activation.
Fig. 4.
Cell survival of BY4741 and hsp30Δ cells following exposure at 50°C. a Log phase BY4741 and hsp30Δ cells were exposed to 50°C in minimal media for intervals of time as indicated in the figure and spotted in tenfold serial dilutions, plates were further incubated at 30°C for 3 days before being photographed. b Cell survival in the presence of 2-dg: log phase BY4741 and hsp30Δ cells were spotted in tenfold serial dilutions on YPD plates containing 200 µg/ml 2-dg and either exposed to 50°C for 2 h or left untreated; cells were then allowed to grow at 30°C for 3 days before being photographed
In conclusion, results summarized in Table 1 demonstrate that, Pma1 activity and protein levels were reduced in Hsp30+ cells upon exposure to thermal stress in dextrose unlike that observed in hsp30Δ cells wherein Pma1 activity and protein levels were rendered unresponsive to heat shock treatment. The above suggested that Hsp30 was necessary for the regulation of different aspects of Pma1 during exposure to heat-shock conditions. We observed increased Pma1 activity in membrane fractions isolated from hsp30Δ cells grown in sorbitol compared to its wild type counterpart grown under similar conditions. The data suggest that Hsp30 inhibited Pma1 activity in dextrose-starved cells at 30°C and following heat shock treatment. A comparison of the effect of dextrose on various aspects of Pma1 (Table 1) indicate that Pma1 activity, protein levels and phosphorylation were regulated differently in hsp30Δ cells compared to Hsp30+ cells in response to dextrose. While dextrose regulation of Pma1 activity was quantitatively different in hsp30Δ cells compared to its wild type counterpart, the regulation of Pma1p levels and phosphorylation status by dextrose in hsp30Δ cells was qualitatively different compared to Hsp30+ cells. We hence concluded that the deletion of HSP30 renders the above parameters refractory to regulation by dextrose.
Pma1 phosphorylation in ser residues was reduced in BY4741 cells following heat-shock treatment in both dextrose as well as in sorbitol media and in hsp30Δ cells in dextrose media. It did not show a similar decrease in hsp30Δ cells in sorbitol wherein Pma1 phosphorylation was increased upon heat shock treatment (Table 1). Pma1 activity is regulated by multiple phosphorylation events and its activation by glucose is thought to involve phosphorylation of Ser-899 and Ser-911 and dephosphorylation of serine residues in Casein kinase I phosphorylation sites. Our data on Pma1 phosphorylation is limited to net ser-phosphorylation of Pma1 under different conditions and does not reflect the effect of ser-phosphorylation of Pma1 on its activity. However, it demonstrates increased phosphorylation of Pma1 in hsp30Δ cells in the presence and absence of dextrose as compared to Hsp30+ cells. The above suggests that ser-phosphorylation of Pma1 is regulated by Hsp30 in addition to its regulation by glucose.
Given the above along with the requirement of dextrose as a carbon source by hsp30Δ cells for survival during exposure to lethal heat stress, our study has been able to establish a functional relationship between glucose utilization, Hsp30 function and the regulation of Pma1 activity. Since the deletion of HSP30 renders Pma1 activity, its protein levels and phosphorylation comparatively refractory to heat shock and dextrose availability, it is possible that Pma1p undergoes structural alterations in the absence of Hsp30 that makes it non-responsive to regulatory events. Hsp30 may thus act as a plasma membrane chaperone in S. cerevisiae.
Acknowledgments
We are grateful to Dr. G. Ilavazhagan Director, DIPAS, for his support during the course of this work. S. cerevisiae BY4741 was obtained from the laboratory of Dr. A. K. Bachhawat, Institute of Microbial Technology, Chandigarh. The work was supported by the Ministry of Defence, Govt. of India. ST a former research fellow was supported by a fellowship from DRDO.
References
- 1.Ambesi A, Miranda M, Petrov VV, Slayman CW. Biogenesis and function of the yeast plasma-membrane H(+)-ATPase. J Exp Biol. 2000;203(Pt 1):155–160. doi: 10.1242/jeb.203.1.155. [DOI] [PubMed] [Google Scholar]
- 2.Serrano R. In vivo activation of the yeast plasma membrane ATPase. FEBS Lett. 1983;156:11–14. doi: 10.1016/0014-5793(83)80237-3. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Arranz M, Maldonado AM, Mazon MJ, Portillo F. Transcriptional control of yeast plasma membrane H(+)-ATPase by glucose. Cloning and characterization of a new gene involved in this regulation. J Biol Chem. 1994;269:18076–18082. [PubMed] [Google Scholar]
- 4.Eraso P, Mazon MJ, Portillo F. Yeast protein kinase Ptk2 localizes at the plasma membrane and phosphorylates in vitro the C-terminal peptide of the H+-ATPase. Biochim Biophys Acta. 2006;1758:164–170. doi: 10.1016/j.bbamem.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Lecchi S, Nelson CJ, Allen KE, Swaney DL, Thompson KL, Coon JJ, Sussman MR, Slayman CW. Tandem phosphorylation of Ser-911 and Thr-912 at the C terminus of yeast plasma membrane H+-ATPase leads to glucose-dependent activation. J Biol Chem. 2007;282:35471–35481. doi: 10.1074/jbc.M706094200. [DOI] [PubMed] [Google Scholar]
- 6.Estrada E, Agostinis P, Vandenheede JR, Goris J, Merlevede W, Goffeau JFA, Ghislain J. Phosphorylation of yeast plasma membrane H1-ATPase by casein kinase I. J Biol Chem. 1996;271:32064–32072. doi: 10.1074/jbc.271.50.32064. [DOI] [PubMed] [Google Scholar]
- 7.Souza MAA, Tropia MJ, Brandao RL. New aspects of the glucose activation of the H+-ATPase in the yeast Saccharomyces cerevisiae. Microbiology. 2001;147:2849–2855. doi: 10.1099/00221287-147-10-2849. [DOI] [PubMed] [Google Scholar]
- 8.Perona R, Serrano R. Increased pH and tumorigenicity of fibroblasts expressing yeast proton pump. Nature. 1988;334:438–440. doi: 10.1038/334438a0. [DOI] [PubMed] [Google Scholar]
- 9.Soteropoulos P, Vaz T, Santangelo R, Paderu P, Huang DY, Tamas MJ, Perlin DS. Molecular characterization of the plasma membrane H+-ATPase, an antifungal target in Cryptococcus neoformans. Antimicrob Agents Chemother. 2000;44:2349–2355. doi: 10.1128/AAC.44.9.2349-2355.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt M, Schwanewilm P, Ludwig J, Lichtenberg-Farte H. Use of PMA1 as a housekeeping biomarker for assessment of toxicant-induced stress in Saccharomyces cerevisiae. Appl Environ Microbiol. 2006;72:1515–1522. doi: 10.1128/AEM.72.2.1515-1522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira T, Brett Mason A, Pypaert M, Allen KE, Slayman CW. Quality control in the yeast secretory pathway: a misfolded PMA1+-ATPase reveals two checkpoints. J Biol Chem. 2006;277:21027–21040. doi: 10.1074/jbc.M112281200. [DOI] [PubMed] [Google Scholar]
- 12.Piper PW, Ortiz-Calderon C, Holyoak C, Coote P, Cole M. Hsp30, the integral plasma membrane heat shock protein of Saccharomyces cerevisiae, is a stress-inducible regulator of plasma membrane H+-ATPase. Cell Stress Chaperones. 1997;2:12–24. doi: 10.1379/1466-1268(1997)002<0012:HTIPMH>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saccharomyces Genome Database. www.yeastgenome.org
- 14.Seymour IJ, Piper PW. Stress induction of HSP30, the plasma membrane heat shock protein gene of Saccharomyces cerevisiae, appears not to use known stress-regulated transcription factors. Microbiology. 1999;145:231–239. doi: 10.1099/13500872-145-1-231. [DOI] [PubMed] [Google Scholar]
- 15.Panaretou B, Piper P. The plasma membrane of yeast acquires a novel heat-shock protein (hsp30) and displays a decline in proton pumping ATPase levels in response to both heat shock and entry to stationary phase. Eur J Biochem. 1992;206:635–640. doi: 10.1111/j.1432-1033.1992.tb16968.x. [DOI] [PubMed] [Google Scholar]
- 16.Shalev A, Valasek L, Pise-Masison CA, Radonovich M, Phan L, Clayton J, He H, Brady JN, Hinnebusch AG, Asano K. Saccharomyces cerevisiae protein Pci8p and human protein eIF3/Int-6 interact with eIF3 core complex by binding to eIF3 core complex subunits. J Biol Chem. 2001;276:34948–34957. doi: 10.1074/jbc.M102161200. [DOI] [PubMed] [Google Scholar]
- 17.Galeote VA, Alexandre H, Bach B, Delobel P, Dequin S, Blondin B. Sfl1p as an activator of HSP30 gene in Saccharomyces cerevisiae. Curr Genet. 2007;52:55–63. doi: 10.1007/s00294-007-0136-z. [DOI] [PubMed] [Google Scholar]
- 18.Sorin A, Rosas G, Rao R. PMR1, a Ca2+-ATPase in yeast golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J Biol Chem. 1997;272:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- 19.Perzov N, Nelson H, Nelson N. Altered distribution of the yeast plasma membrane H+-ATPase as a feature of H+-ATPase null mutants. J Biol Chem. 2000;275:40088–40095. doi: 10.1074/jbc.M007011200. [DOI] [PubMed] [Google Scholar]
- 20.Kumar N, Meena RC, Chakrabarti A (2009) Over-expression of YLR162W in Saccharomyces cerevisiae inhibits cell proliferation and renders cells susceptible to the hypoxic conditions induced by cobalt chloride. Indian J Microbiol (in press) [DOI] [PMC free article] [PubMed]
- 21.Serrano R. H+-ATPase from plasma membrane of Saccharomyces cerevisiae and Avena sativa roots: purification and reconstitution. Methods Enzymol. 1988;157:533–544. doi: 10.1016/0076-6879(88)57102-1. [DOI] [PubMed] [Google Scholar]
- 22.Pearce DA, Ferea T, Nosel AS, Das B, Sherman F. Action of BTN1, the yeast orthologue of the mutated in Batten disease. Nat Genet. 1999;22:55–58. doi: 10.1038/8861. [DOI] [PubMed] [Google Scholar]