Abstract
Xanthan is a important biopolymer for commercial purpose and it is produced in two stages by Xanthomonas campestris. In the first one, the bacterium is cultivated in the complex medium enriched in nitrogen and the biomass produced is used as inoculum for the next stage in which the gum is produced in another medium. In this work a new medium for the first stage is proposed in place of currently used YM medium. Different formulated growth media were studied and the correspondent biomass produced was analysed as inoculum for the second stage. The inoculum and gum were produced by batch process in shaker at 27°C in pH 6.0 and at 30°C in pH 7.0, respectively. The gum was precipitated with ethanol (3:1 v/v). The dryed biomass and xathan gum produced were determined by drying in oven at 105 and 40°C, respectively. The viscosity of the fermentation broth and 1% gum solution in water were determined in Brookfield viscometer. The formulated medium presented the increase in gum production (30%), broth (136%) and 1% gum solution viscosity (60%) compared to YM, besides the inferior cost. The results showed the importance of the quality of the inoculum from the first stage of the culture which influenced on the gum viscosity in the second stage.
Keywords: Development, Growth medium, Xanthomonas campestris, Xanthan, Viscosity
Introduction
The xanthan gum, a biopolymer synthesized by Xanthomonas campestris, is mainly used as thickener, stabilizer and friction reducer for respectively food, pharmaceutical and petroleum industry [1]. The xanthan market is about US $270 million and it is expected to reach US $400 million with a production of 80,000 tons/year in 2015 [2]. In the industrial production is used 2–4% of carbon source as sucrose or glucose, and 0.05–0.1% of nitrogen source as yeast extract, peptone, ammonium nitrate or urea [3]. The cost, rheological properties and yield of this gum depends on the culture medium used in the fermentation process [4–9]. Some authors reported that sucrose is the best carbon source [10, 11]. There are controversies about the best nitrogen source to be organic or inorganic for the growth of X. campestris and xanthan production. Cadmus et al. [12] produced gum more viscous using (NH4)2HPO4, while Souw and Demain [11] and Garcia-Ochoa et al. [10] used glutamate.
The ratio C/N should also be considered in the process of production of the biopolymer. The biomass is limited by concentration of available nitrogen and the xanthan production rate is influenced by concentration of carbon [13]. Therefore, the requested nutrients by Xanthomonas campestris pv. campestris are different during the bioprocess, becoming necessary its division in two stages: cell growth and gum production. It was verified the importance of using a concentration of yeast extract relatively high in the medium to reach a high cellular density before culture to enter in the stationary phase. Several reports verified that the yield and the rate of production of the biopolymer are favored with the increase of the proportion glucose/yeast extract in the medium [14–16]. Some organic acids have been used as nutrients for the bacterium Xanthomonas campestris. Souw and Demain [11] studied the addition of succinate, pyruvate, and α-ketoglutarate and verified that excess concentrations of these organic acids inhibited xanthan formation. The same authors concluded that citrate (0.09–0.18%) stimulates the production of xanthan when the pH is not controlled in defined media [17].
Many nutritional studies have been focused in the production stage to improve yield and viscosity gum, but not in the first stage where the inoculum is produced. The YM medium is internationally described as growth medium for X. campestris [10–12, 18–20]. However it is expensive (US $248.00/Kg) to be used in the production of xanthan gum wich costs on average US $5.5/kg [21, 22].
In this study is evaluated a new culture medium as alternative to YM to produce an inoculum resulting in cost reduction and improve in xanthan technology.
Materials and Methods
Microorganism and Fermentation
Xanthomonas campestris pv. campestris FCLA n. 26 was obtained from Culture Collection of the University of São Paulo State-UNESP, in Assis, SP, Brazil. This strain was maintained by plating in YM agar [23]. The experiments were conducted in shaker in four fermentations and each one with two stages and in three replications. In the first stage was produced the inoculum for the second one in which xanthan was produced.
The Inoculum Production
The culture of Xanthomonas campestris pv. campestris was started with a aseptic inoculation in plates containing YM agar medium and incubated in stove at 27°C for 24–48 h. From these plates, a cell suspension was obtained mixing the biomass with water solution. The cell concentration (107 cells/ml) was adjusted by sterilized water dilution and quantification by microscopy in a Neubauer Chamber. This cells suspension, an aliquot of 7.5 ml was inoculated in 75 ml of each formulated medium in 500 ml Erlenmeyer flask, and incubated in orbital shaker (Mod. TE 421-Tecnal, Piracicaba, Brazil) at 27°C and 160 rev/min for 24 h.
Formulated Medium
The growth medium was formulated testing different concentrations of the following nutrients: nitrogen source, sucrose, citrate and glutamate, in this sequence. It was selected for each experiment the condition in which was obtained the best quality of the gum through the viscosity in 1% gum solution. In the Table 1 is described the formulated medium for nitrogen source and the other nutrients. Sucrose with several concentrations (0, 1.0, 1.5, 2.0, 2.5 and 2.5% w/v) was studied using 0.5% yeast extract and the other nutrients used in the nitrogen source test. Citrate and glutamate with the same concentrations (0.05, 0.1, 0.15, 0.20% w/v) were formulated as the same previous reported to the others. All media also contained 0.01% MgSO4·7H2O and 0.15% K2HPO4. The media pHs were adjusted to 6.0 with 1 M sodium hydroxide solution before sterilization at 121°C for 15 mim.
Table 1.
Formulation of culture media of Xanthomonas campestr pv. campestris with different nitrogen sources
| Mediuma | Yeast extract (%) | (NH4)2HPO4 (%) |
|---|---|---|
| 1 | 0.30 | 0 |
| 2 | 0.30 | 0.25 |
| 3 | 0.50 | 0 |
| 4 | 0.50 | 0.25 |
| 5 | 0.70 | 0 |
| 6 | 0.70 | 0.25 |
aPercentage (w/v)—all culture media including 2.5% sucrose, 0.01% MgSO4·7H2O and 0.15% K2HPO4
Gum Production
In the second stage of the fermentation process, 25 ml of the culture from the first stage were inoculated aseptically in 300 ml of medium described by Souw and Demain [11] in 1,000 ml Erlenmeyer flask. The culture was incubated in orbital shaker for 72 h at 30°C and 160 rev/min. In all media the pH was adjusted to 7.0 with 1 M sodium hydroxide solution before sterilization by autoclaving at 121°C for 15 min.
Analytical Methods
The cell and xanthan concentration were determined by dry weight. The samples were taken of the fermented broth at initial and final time of the first stage (0 and 24 h) and second one (0 and 72 h). These samples were centrifuged under 5310×g for 40 min at 4°C for cells separation. The washed biomass was obtained after mixed cells and distilled water and other centrifugation. The dryed biomass was determined by drying in oven at 105°C until constant weight. Supernatant of the first centrifugation was mixed with ethanol (1:3 v/v) to precipitate the gum. The biopolymer was separated and dried in oven at 40°C until constant weight. The dried gum was ground with mortar and pistil and its weight determined.
The viscosity of the fermentation broth and 1% gum solution in water were determined in Brookfield viscometer (model LVDVII +) at 25°C. The total reduced sugars were analyzed by Nelson’s method [24], after biopolymer recovery in initial and final time of growth and production stages. Cell yield and xanthan yield were calculated according Pirt [25].
Results and Discussion
Influence of Nitrogen Source in Xanthan and Biomass Production
The nitrogen source in the first stage of fermentation influenced all parameters studied (Table 2). The cell yield was superior (Yx/s = 0.06 g cell/g sugar) in medium 4 with yeast extract (0.5%) and (NH4)2HPO4 (0.25%). The medium 3 showed superior gum viscosity (245 cP) and it was intermediate in nitrogen source (0.5% yeast extract). Media with higher values showed inferior viscosity (ex. medium 5 and 6). The same occurred with YM medium, with more nitrogen sources (0.5% peptone, 0.3% malt extract and 0.3% yeast extract). The quality of the inoculum produced in the first stage influenced the gum production and its viscosity. Flores and Deckwer [26] observed that low concentrations of NH4Cl improved rheological properties of xanthan. According Souw and Demain [11] this fact could be explained due to nitrogen excess to stimulate the metabolism of cellular growth in detriment of the xanthan production.
Table 2.
Influence of different nitrogen source on xanthan production and viscosity, cell and biopolymer yield
| Media | Gum (g/l) (24 h) 
|
Gum (g/l) (72 h) 
|
Cell yielda (g/g) 
|
Xanthan yieldb (g/g) 
|
Gum viscosity (cP) 
|
|---|---|---|---|---|---|
| YM | 1.1 ± 0.2 | 8.4 ± 0.9 | 0.015 ± 0.001 | 0.206 ± 0.079 | 124 ± 19 |
| 1 | 5.3 ± 1.0 | 9.2 ± 1.5 | 0.043 ± 0.006 | 0.162 ± 0.037 | 194 ± 72 |
| 2 | 4.8 ± 0.5 | 9.8 ± 1.4 | 0.024 ± 0.002 | 0.404 ± 0.204 | 207 ± 94 |
| 3 | 5.1 ± 2.3 | 9.9 ± 1.8 | 0.027 ± 0.002 | 0.345 ± 0.236 | 245 ± 109 |
| 4 | 3.6 ± 0.5 | 9.6 ± 1.4 | 0.060 ± 0.007 | 0.212 ± 0.094 | 171 ± 66 |
| 5 | 3.7 ± 1.0 | 7.1 ± 2.9 | 0.037 ± 0.005 | 0.154 ± 0.097 | 121 ± 109 |
| 6 | 3.3 ± 1.2 | 9.5 ± 0.6 | 0.038 ± 0.004 | 0.334 ± 0.179 | 124 ± 53 |
A Average 24 h e 72 h correspond to the growth and production phases, respectively
ag biomass/g sucrose
bg xanthan/g sucrose
Influence of Sucrose and Organic Acid on Xanthan and Biomass Production
The effect of the sucrose and organic acids on biomass and gum production in the first and second stage of fermentation is showed (Table 3). A direct correlation between sucrose concentration and produced biomass was verified. The maximum value of 1.26 g/l was obtained with 2.0% (w/v) sucrose. This value was 72.6% superior to YM medium. Citrate in the growth medium not only serves as a buffering agent but also improve the production of biomass as demonstrated in this work (Table 3). In the fermentation with 0.2% citrate and 2.5% sucrose in the growth medium was obtained higher level of biomass (2.44 g/l), causing further increase in the difference in relation to YM medium (3.3 times superior). Whereas the glutamate did not cause increase in the biomass comparing with the medium without this nutrient.
Table 3.
Influence of sucrose, citrate and glutamate in the formulated medium for growth of Xanthomonas campestris pv. campestris and biomass and xanthan production
| Nutrients (%) | Growth step (g/l) | Gum production step (g/l) | ||
|---|---|---|---|---|
| Biomass | Gum | Biomass | Gum | |
 |
 |
 |
 |
|
| Sucrosea | ||||
| 0.0 | 0.34 ± 0.06 | 0.56 ± 0.28 | 1.58 ± 0.51 | 6.2 ± 1.2 |
| 1.0 | 0.76 ± 0.14 | 1.80 ± 0.40 | 1.39 ± 0.22 | 5.7 ± 1.6 |
| 1.5 | 1.19 ± 0.19 | 2.10 ± 0.44 | 1.34 ± 0.43 | 7.5 ± 0.9 |
| 2.0 | 1.26 ± 0.22 | 3.49 ± 1.18 | 2.83 ± 0.69 | 7.7 ± 0.9 |
| 2.5 | 1.25 ± 0.16 | 3.28 ± 0.89 | 3.03 ± 0.54 | 10.0 ± 1.4 |
| Citrateb | ||||
| 0.0 | 1.25 ± 0.16 | 3.28 ± 0.89 | 3.03 ± 0.54 | 10.0 ± 1.4 |
| 0.05 | 2.16 ± 0.13 | 2.86 ± 0.43 | 1.70 ± 0.33 | 10.4 ± 0.7 |
| 0.10 | 1.94 ± 0.21 | 2.76 ± 0.43 | 1.78 ± 0.33 | 11.7 ± 1.8 |
| 0.15 | 2.35 ± 0.45 | 2.73 ± 0.73 | 2.10 ± 0.27 | 10.4 ± 1.8 |
| 0.20 | 2.44 ± 0.21 | 2.35 ± 0.36 | 2.24 ± 0.41 | 10.9 ± 0.3 |
| Glutamatec | ||||
| 0.0 | 2.16 ± 0.13 | 2.86 ± 0.43 | 1.70 ± 0.33 | 10.4 ± 0.7 |
| 0.05 | 1.87 ± 0.31 | 2.47 ± 0.31 | 2.11 ± 0.56 | 10.6 ± 1.3 |
| 0.10 | 2.02 ± 0.29 | 2.45 ± 0.19 | 1.98 ± 0.21 | 10.4 ± 2.3 |
| 0.15 | 2.09 ± 0.47 | 2.86 ± 0.51 | 2.22 ± 0.21 | 11.5 ± 0.3 |
| 0.20 | 1.92 ± 1.02 | 2.07 ± 1.26 | 2.08 ± 0.42 | 10.4 ± 1.0 |
| YM Medium | 0.73 ± 0.23 | 1.54 ± 0.41 | 1.96 ± 0.38 | 8.7 ± 1.3 |
aIncluding: 0.5% (w/v) yeast extract, 0.15% (w/v) K2HPO4, 0,01% (w/v) MgSO4·7H2O
bIncluding 2.5% sucrose
cIncluding 0.05% citrate
Increasing sucrose concentration in the medium also stimulated xanthan production in both stages of fermentation (Table 3). There was a gum increase of 5.86 times in the first stage and 61.3% in the second stage, when the medium with 2.5% sucrose was compared with the condition without sucrose addition, and 14.9% in the second stage when it was compared with YM medium. A linear correlation between sucrose and produced gum in the first stage of growth (R = 0.9772 and P <0.0001) and second stage (R = 0.8575 P < 0.01) were determined showing the importance of these concentrations in gum production (Fig. 1).
Fig. 1.
Effect of sucrose concentration in the formulated medium on the gum production in the first (24 h) and second stage (72 h) of fermentation
According to Gandhi et al. [27] the carbon source influences both cell growth and the production of the biopolymer; however, given carbon source can support a good growth without significant production of polysaccharide. In this work it was observed that 2.5% sucrose in the medium of the first stage provided a more efficient inoculum for the second stage improving biomass and gum production.
The addition of the 0.05% citrate in the formulation of the medium increased the amount of gum produced especially in the gum production step, about 9% superior to the medium without citrate and 25.3% superior to YM medium. For this reason citrate was incorporated into the growth medium for X. campestris pv. campestris and it was used in the test of glutamate. The glutamate did not contribute to an expressive increase in xanthan production in none of the steps (Table 3).
Influence of the Nutrients in the Viscosity of Biopolymer
An increase of 70% of the viscosity was observed in the medium with 2.5% sucrose compared with medium without sugar. It was observed (Fig. 2) that the formulation containing 2.5% sucrose significantly (Tukey P < 0.01) increased the viscosity of the fermentation broth (532 cP) and 1% gum solution (1,464 cP) in relation to culture with YM medium (1% glucose), respectively increase of 111 and 92.6%.
Fig. 2.
Viscosity of the fermentation broth and xanthan solution (1%) from inoculum obtained in medium with different sucrose concentration (25°C)
Comparative studies of glucose and sucrose showed that the highest yields and viscosities were obtained in fermentation using sucrose as carbon source [11]. The direct correlation verified in sucrose and gum viscosity in the first stage in the present work was not observed with the increase of sucrose in the medium of second stage with X. campestris pv. pruni [28]. In the medium of gum production (second stage) with sucrose concentrations of 4, 6, 8 and 10% (w/v) in fermentation of Xanthomonas campestris pv. campestris occurred decrease of the viscosity and gum production with the increase of sugar [29]. According to the author, the increase of osmotic pressure due to the increase in sucrose concentration may have inhibited the enzymes of xanthan biosynthesis and consequently the viscosity.
In this present work, a higher osmotic pressure caused by increased sucrose concentration (2.5%) in the medium influenced positively the gum production and viscosity. Probably the increase in the osmotic pressure due to higher sucrose concentration in the first stage of fermentation may be responsible for induction of biosynthesis of gum enzymes in the produced biomass, which are responsible for the production and quality of xanthan gum.
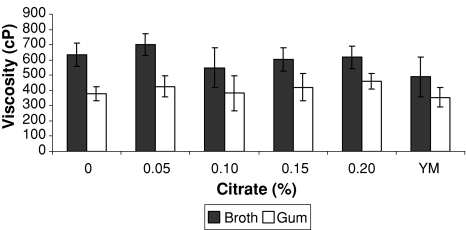
The addition of citrate in the medium did not contribute to increase significantly (Anova P > 0.05) in the viscosity of the gum (Fig. 3). However with 0.05% (w/v) citrate in the medium provided a superior broth viscosity (702 cP). Also as previously reported this increase in the broth viscosity was not due to concentration of the gum because it did not increase (Table 3). So it can be attributed to other factors such as the presence of salts in the medium influencing the behavior of viscosity.
Fig. 3.
The viscosity of the fermentation broth and xanthan solution (1%) from inoculum obtained in medium with different citrate concentration (25°C)
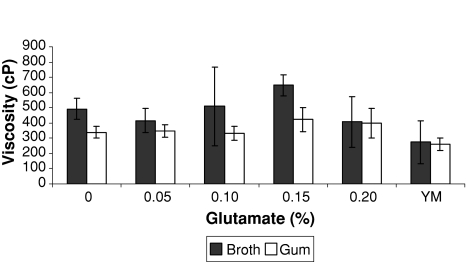
The addition of 0.15% (w/v) sodium glutamate in the culture medium to the growth of X. campestris pv. campestris improved the quality of the produced gum. There was an increase of 24.6% (Fig. 4) in the viscosity of 1% gum solution compared with the medium without glutamate. A significant (Anova P < 0.05) difference was verified among the formulated medium containing 0.15% glutamate (422 cP) and YM medium (263 cP).
Fig. 4.
Viscosity of the fermentation broth and xanthan solution (1%) from inoculum obtained in medium with different glutamate concentration (25°C)
The proposed medium with 2.5% sucrose, 0.05% (w/v) citrate, 0.15% (w/v) glutamate, 0.5% (w/v) yeast extract, 0.15% (w/v) K2HPO4 and 0.01% (w/v) MgSO4·7H2O contributed to the formation of gum with a chemical structure in favor of higher viscosity, which is probably associated with high molecular mass and with favorable chemical composition. The literature shows that the conformation and molecular size influence gum viscosity. Xanthan with higher molecular mass shows higher viscosity [4, 26]. Acetyl and pyruvate present in the xanthan may also have influence on the viscosity. Casas et al. [4] demonstrated that xanthan gum with high levels of this groups had higher viscosity. According Shatwell et al. [30] the rates of acetyl and pyruvate affect the stability of the chain. Pyruvate creates an effect of instability in the helix due to increased repulsion between the internal chemical ramifications. According to these authors, the acetyl influence on stability due to apolar interactions between acetyl and methyl groups or because the acetyl group act as hydrogen acceptor. Thus, probably, the chemical structure of gum produced in the medium formulated contributed to an adequate intramolecular interaction between the gum and the aqueous solution.
The Table 4 shows a comparision for the new formulated medium and YM. The formulated medium shows smaller cost (57%) than YM for the growth of Xanthomonas campestris pv. campestris. Besides this new medium shows superior quality of the gum (60%), increase in broth viscosity (136%), biomass (135%) and gum production (30%). These results indicate the proposed medium can contribute for the improvement of the xanthan technology by Xanthomonas campestris.
Table 4.
Comparision of YM and formulated medium—cost medium per liter and fermentation parameters for gum production
| Reagents | Formulated medium | YM medium | Difference (%) | |||
|---|---|---|---|---|---|---|
| Weight (g) | Cost (US $) | Weight (g) | Cost (US $) | |||
| Components | ||||||
| Yeast extract | 5 | 3.58 | 3 | 2.15 | ||
| K2HPO4 | 1.5 | 0.090 | ||||
| MgSO4·7H2O | 0.1 | 0.002 | ||||
| Sucrose | 25 | 0.45 | ||||
| Citrate | 0.5 | 0.010 | ||||
| Glutamate | 1.5 | 0.086 | ||||
| Malt extract | 3 | 1.43 | ||||
| Peptone | 5 | 3.51 | ||||
| Glucose | 10 | 0.34 | ||||
| Total cost | 4.21 | 7.43 | −57 | |||
| Fermentation parameters | ||||||
| Biomass (g/l) | 2.09 | 0.89 | 135 | |||
| Gum (g/l) | 11.5 | 8.8 | 30 | |||
| Broth viscosity (cP) | 648 | 274 | 136 | |||
| Gum viscosity (cP) | 422 | 263 | 60 | |||
Search: Induslab-Com. Prod. Lab., Vetec Química Fina LTDA and Nuclear (SP, Brazil)
Conclusions
This work showed the importance of the quality of the inoculum from the first step of the culture which influenced in the gum and viscosity produced on the second step.
The growth medium used for production of inoculum is a factor impacting the gum production. A relatively high concentration of sucrose (2.5%), acetate (0.05%), glutamate (0.15%) and yeast extract (0.5%) produced an appropriated biomass as inoculum for the xanthan production. This new medium for the growth of Xanthomonas campestris pv. campestris was superior to YM technically and economically.
References
- 1.Rosalam S, England R. Review of xanthan gum production from unmodified starches by Xanthomonas campestris sp. Enzym Microbial Technol. 2006;39:197–207. doi: 10.1016/j.enzmictec.2005.10.019. [DOI] [Google Scholar]
- 2.Faria S, Vieira PA, Resende MM, França FP, Cardoso VL. A Comparison between shaker and bioreactor performance based on the kinetic parameters of xanthan gum production. Appl Biochem Biotechnol. 2009;156:475–488. doi: 10.1007/s12010-008-8485-8. [DOI] [PubMed] [Google Scholar]
- 3.Papagianni M, Psomas SK, Batsilas L, Paras SV, Kyriakidis DA, Liakopouloukyriakides M. Xanthan production by Xanthomonas campestris in batch cultures. Process Biochem. 2001;37:73–80. doi: 10.1016/S0032-9592(01)00174-1. [DOI] [Google Scholar]
- 4.Casas JA, Santos VE, Garcia-Ochoa F. Xanthan gum production under several operational conditions: molecular structure and Rheological properties. Enzym Microb Technol. 2000;26:282–291. doi: 10.1016/S0141-0229(99)00160-X. [DOI] [PubMed] [Google Scholar]
- 5.Druzian JI, Pagliarini AP. Xanthan gum production by fermentation from residue of apple juice. Ciência e Tecnologia de Alimentos. 2007;27:26–31. doi: 10.1590/S0101-20612007000100005. [DOI] [Google Scholar]
- 6.Garcia-Ochoa F, Santos VE, Fritsch AP. Nutritional study of Xanthomonas campestris in xanthan gum production by factorial design of experiments. Enzym Microb Technol. 1992;14:991–996. doi: 10.1016/0141-0229(92)90083-Z. [DOI] [Google Scholar]
- 7.Kalogiannis S, Iakovidou G, Liakopoulou-Kyriakides M. Optimization of xanthan gum production by Xanthomonas campestris grown in molasses. Process Biochem. 2003;39:249–256. doi: 10.1016/S0032-9592(03)00067-0. [DOI] [Google Scholar]
- 8.Letisse F, Chevallereau P, Simon J, Lindley N. The influence of metabolic network structures and energy requirements on xanthan gum yields. J Biotechnol. 2002;99:307–317. doi: 10.1016/S0168-1656(02)00221-3. [DOI] [PubMed] [Google Scholar]
- 9.Souza DM. Fermentative production of extracellular polysaccharides by bacteria. Semin Agric Sci. 2004;25:331–340. [Google Scholar]
- 10.Garcia-Ochoa F, Santos VE, Casas JA, Gómez E. Xanthan gum: production, recovery, and properties. Biotechnol Adv. 2000;18:549–579. doi: 10.1016/S0734-9750(00)00050-1. [DOI] [PubMed] [Google Scholar]
- 11.Souw P, Demain AL. Nutritional studies on xanthan production by Xanthomonas campestris NRRL-B-1459. Appl Environ Microbiol. 1979;37:1186–1192. doi: 10.1128/aem.37.6.1186-1192.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadmus MC, Knutson CA, Lagoda A. Synthetic media for production of quality xanthan gum in 20 liter fermentors. Biotech Bioeng. 1978;20:1003–1014. doi: 10.1002/bit.260200703. [DOI] [Google Scholar]
- 13.Nitschke M, Rodrigues V, Schinatto LF. Formulação de meios de cultivo à base de soro de leite para produção de goma xantana por X. campestris C7L. Ciência e Tecnologia de Alimentos. 2001;21:82–85. doi: 10.1590/S0101-20612001000100018. [DOI] [Google Scholar]
- 14.Vuyst L, Loo J, Vandame EJ. Two steps fermentation process for improved xanthan production by X. campestris NRRL-B-1459. J Chem Technol Biotechnol. 1987;39:263–273. doi: 10.1002/jctb.280390407. [DOI] [Google Scholar]
- 15.Funahashi H, Machara M, Tagushi H, Yoshida T. Effect of glucose concentration on xanthan gum production by Xanthomonas campestris. J Chem Eng Jpn. 1987;65:603–606. [Google Scholar]
- 16.Lo YM, Yang ST, Min DB. Effects of yeast extract and glucose on xanthan production and cell with in batch culture of Xanthomonas campestris. Appl Microbiol Biotechnol. 1997;47:689–694. doi: 10.1007/s002530050996. [DOI] [Google Scholar]
- 17.Souw P, Demain AL. Role of citrate in xanthan production by Xanthomonas campestris. J Ferment Technol. 1980;58:411–416. [Google Scholar]
- 18.Esgalhado ME, Caldeira AT, Roseiro JC. Sublethal acid stress and uncoupling effects on cell growth and product formation in Xanthomonas campestris cultures. Biochem Eng J. 2002;12:181–192. doi: 10.1016/S1369-703X(02)00070-0. [DOI] [Google Scholar]
- 19.Galindo E, Salcedo G, Flores C, Ramírez E. Improved shake-flask test for the screening of xanthan producing microorganisms. World J Microb Biotechnol. 1993;9:122–124. doi: 10.1007/BF00656533. [DOI] [PubMed] [Google Scholar]
- 20.Kurbanoglu EB, Kurbanoglu NI. Ram horn hydrolysate as enhacer of xanthan production in batch culture of Xanthomonas campestris EBK-4 isolate. Process Biochem. 2007;42:1146–1149. doi: 10.1016/j.procbio.2007.04.010. [DOI] [Google Scholar]
- 21.Available at: http://www.bacteria.us.com/Difco-Yeast-Nitrogen-Base-Bacto-YPD-Mold-Agar-YPD-Difco.htm. Accessed 09 February 2011
- 22.Available at: http://www.kaycircle.com/What-Is-The-Average-Cost-Of-Xanthan-Gum-Per-Pound-Ton-Average-Xanthan-Gum-Price. Accessed 09 Feb 2011
- 23.Jeanes A, et al. Polysaccharide xanthan of Xanthomonas campestris NRRL B-1459: culture of maintenance procedures polysaccharide production and purification and analysis. ARS-NC-51. Agricultural Research Service. Peoria: U.S. Department of Agriculture; 1976. [Google Scholar]
- 24.Nelson MA. Photometric adaptation of the Somogy method for the determination of glucose. J Biol Chem. 1944;153:379–380. [Google Scholar]
- 25.Pirt JS. Principles of microbe and cell cultivation. Oxford: BlackWell Scientific Publications Ltd; 1975. [Google Scholar]
- 26.Flores Deckwer. Effect of the nitrogen source on pyruvate content and rheological properties of xanthan. Biotechnol Prog. 1999;15:446–452. doi: 10.1021/bp990028i. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi HP, Ray RM, Patel RM. Exopolymer production by Bacillus species. Carbohydr Polym. 1997;34:323–327. doi: 10.1016/S0144-8617(97)00132-X. [DOI] [Google Scholar]
- 28.Diaz PS (2000) Determination of the viscosity of polymers obtained with different concentrations of sucrose in the medium of production, vol 1. In:Proceedings of the International Congress of Nutrition, Food and Technology, p. 330–338
- 29.Oliveira LHS, Dias FG, Duarte ICS, Oliva-Neto P, Cruz R, Moreira AS, Vendruscolo CT. Isolamento e caracterização de bactérias produtoras de goma xantana. Plural. 2000;1:115–120. [Google Scholar]
- 30.Shatwell KP, Sutherland IW, Ross-Murphy SB. Influence of acetyl and pyruvate substituents on the solution properties of xanthan polysaccharide. Int J Biol Macromol. 1990;12:71–78. doi: 10.1016/0141-8130(90)90056-G. [DOI] [PubMed] [Google Scholar]