Abstract
In present study in vitro phytopathogen suppression activity of siderophoregenic preparations of Ni and Mn resistant Alcaligenes sp. STC1 and Pseudomonas aeruginosa RZS3 SH-94B isolated from soil were found superior over the chemical pesticide. Siderophore rich culture broth and siderophore rich supernatant exerted antifungal activity against Aspergillus niger NCIM 1025, Aspergillus flavus NCIM 650, Fusarium oxysporum NCIM 1281, Alternaria alternata ARI 715, Cercospora arachichola, Metarhizium anisopliae NCIM 1311 and Pseudomonas solanacerum NCIM 5103. Siderophore rich broth and supernatant exhibited potent antifungal activity vis-à-vis oraganophosphorus chemical fungicide; kitazine. The minimum fungicidal concentration required was 25 μl for Aspergillus niger, Aspergillus flavus, Fusarium oxysporum, Cercospora arachichola, Metarhizium anisopliae, Pseudomonas solanacerum and 75 μl for A. alternata.
Keywords: Alcaligenes sp., Pseudomonas aeruginosa RZS3, Siderophores, Phytopathogen, Biocontrol, PGPR
Introduction
The growing cost of pesticides and consumer demand for pesticide-free food has led to a search for substitutes for these products. In this regards siderophore producing plant growth promoting rhizobacteria (PGPR) has been recognized as effective biocontrol agent against plant pathogens. Though the siderophores are specific ferric ion chelator, but they can also bind other metals. Thus, heavy metal contaminated soil can be largely influenced by siderophores.
Heavy metals are metals with a density above 5 g/cm3 [1]. Presence of heavy metals even in traces is toxic and detrimental to both flora and fauna. PGPR capable of growing in presence of variety of heavy metals are seen as potent bioinoculants [2]. Bioabsorption is one of the most important biological mechanisms which involve the ability of microorganisms to accumulate heavy metals from contaminated site through metabolically mediated pathway [3].
Every year, severe global economic losses to agricultural crops are encountered due to plant diseases caused by more than sixty pathogens leading to the loss of 30% crop yield amounting 416 million US dollars [4]. Since agricultural fields due to the uncontrolled use of chemical pesticides and fertilizers are most contaminated, search for PGPR having potential of adsorbing heavy metals from agriculture field will have triple advantage of bioremediation, plant growth promotion and disease management [5]. Biocontrol through siderophore-mediated competition for iron have merged as a sustainable approach for integrated plant disease management [6–10].
Siderophores are also found to complex with heavy metals like cadmium, lead, nickel, arsenic (III, V), aluminium, magnesium zinc, copper, cobalt, and strontium other than iron [11, 12]. Under iron stress conditions, rhizobacteria produce siderophores that chelate the available iron and prevent the iron nutrition of respective phytopathogen [13] and there by restrict the proliferation and root colonization by phytopathogen. Siderophore producing rhizobacteria are also known to impart induced systemic resistance (ISRs) to the plants [14, 15] and suppressiveness to the soil [16] and have been implicated in the biocontrol of several plant diseases [17]. Siderophore based biological control agents (BCAs) are gaining commercial significance as they are safer, do not lead to biomagnification, their self-replication circumvents repeated application and target organisms do not develop pesticide resistance [6]. They also provide iron nutrition to the crops thereby promote the plant growth [18, 19].
The present work focuses on the antifungal activity of siderophore producing heavy metal resistant Alcaligenes sp. and Pseudomonas aeruginosa RZS3, against some common phytopathogenic fungi and bacterial strain.
Materials and Methods
Sources of Cultures
Two isolates were obtained from local soil and were labeled as Alcaligenes sp. Pseudomonas aeruginosa RZS3. Fungal cultures like Aspergillus niger NCIM 1025; responsible for causing crown rot and collar rot in groundnut and other crops, Aspergillus flavus NCIM 650; causing afla-root and aflatoxin production in groundnut, Fusarium oxysporum NCIM 1008; causing vascular wilt in radish, cucumber and onion, Metazhizium anisophliae NCIM 1311, and the bacterial strain Pseudomonas solanacerum NCIM 5103 were procured from National Center for Industrial Microorganisms [NCIM], NCL, Pune, India, Alternaria alternata IARI 715 was procured from Indian Agricultural Research Institute [IARI], New Delhi, India, Cercospora arachichola was isolated from infected groundnut. Alcaligenes sp., Pseudomonas aeruginosa RZS3 and Pseudomonas solanacerum were routinely maintained on nutrient agar and fungal cultures were maintained on potato dextrose agar (PDA). All the cultures were preserved at 4°C.
Heavy Metal Resistance
To obtain maximum heavy metal resistance level for Alcaligenes sp. and Pseudomonas aeruginosa RZS3 they were stepwise inoculated on nutrient agar plate with increasing grades of heavy metal concentrations of MnCl2 and NiCl2.
Screening for Siderophore Production
In order to screen siderophore production ability, Alcaligenes sp. and Pseudomonas aeruginosa RZS3 were inoculated into sterile succinic acid medium (SAM) containing (gl−1 in distilled water): K2HPO4, 6.0; KH2PO4, 3.0; (NH4)2SO4, 1.0; MgSO4·7H2O, 0.2; C4H4Na2O4·6H2O, 4.0; and pH.7, at 28 ± 2°C at 120 rpm for 24–48 h [20].
Partial Identification and 16 s rRNA Sequencing
The isolates were subjected to various biochemical tests as per the procedure and protocols of Bergey’s manual of systematic bacteriology [21]. The pre-sterilized Hi-carbohydrate biochemical kits (KB 002 and KB 009, Hi Media, Mumbai, India) were used for biochemicals identification of the isolate. Further this partially identified culture was subjected to 16 s rRNA gene profile. Standard phenol–chloroform methods were used for genomic DNA extraction [22]. The 16S rRNA genes of the isolate were amplified by PCR [23] and sequenced directly on an automated DNA sequencer (ABI377) using the Big Dye terminator kit (Applied Biosystems) [24]. Results were compared with the public databases (NCBI) to determine the identity and homology of the isolate.
Siderophore Production, Detection and Estimation
Growth and siderophore production was carried out in 500 ml Erlenmeyer flask containing 100 ml of modified SAM [20]. For this purpose, Alcaligenes sp. and Pseudomonas aeruginosa RZS3 [6 × 106 cells ml−1] were grown independently in SAM at 28 ± 2°C at 120 rpm for 24–48 h. After the incubation, cell density was measured at 620 nm by using double beam UV–Visible spectrophotometer [1240, Shimadzu, Japan]. The detection and estimation of siderophores was performed following the centrifugation at 15 min 5,000×g cm at 4°C and cell free supernatant was assayed for the presence of siderophore by using Chrome Azurol Sulphonate (CAS) test [25]. CAS shuttle assay [26, 27] was used to measure siderophores produced by the Alcaligenes sp. and Pseudomonas aeruginosa RZS3.
In Vitro Interaction with Phytopathogenic Fungi
In vitro phytopathogen suppression activity of siderophore and siderophoregenic culture preparations of Alcaligenes sp. and Pseudomonas aeruginosa RZS3 was directed against Aspergillus niger, Aspergillus flavus, Fusarium oxysporum, Alternaria alternata, Cercospora arachichola, Metarhizium anisopliae and Pseudomonas solanacerum. These strains are known to be common phytopathogens capable of causing major damages to the groundnut and other crops. In vitro antifungal activity was based on the principle of diffusion in which spore suspension [6 × 106 spores ml−1] of above mentioned fungal sp. was separately mixed with molten PDA and poured in sterile petri plate. After hardening of medium, three wells each of about 10 mm, were bored in each plate and were separately added with 25, 50, 75 and 100 μl, each of culture broth [6 × 106 cell ml−1], cell free supernatant and kitazin. While the antibacterial activity was checked by spreading the culture of Pseudomonas solanacerum [6 × 106 cells ml−1] on the nutrient agar plates, three wells each of about 10 mm, were bored in each plate and were separately added with 25, 50, 75 and 100 μl, each of culture broth [6 × 106 cell ml−1], cell free supernatant and kitazin. Control was prepared by removing siderophore through the addition of 8 hydroxyquinone. Inoculated plates were kept for diffusion at 4°C for 15 min, incubated at 29°C for 48 h and were observed for the inhibition of fungal and bacterial growth. Antifungal and antibacterial potential of various preparations was determined by measuring the zone diameter of reduced fungal and bacterial growth. Zone diameter of more than 8 mm was considered as an indication of growth inhibition.
Determination of Minimum Inhibition Concentration (MIC)
In order to determine the MIC of the preparations, various preparations like culture broth [6 × 106 cells ml−1], cell free supernatant and kitazin were separately taken in the range of 20–100 μl. Each preparation was separately added into PDA previously seeded with fungal pathogen [one fungus per plate]. While the antibacterial activity was checked by spreading the culture of Pseudomonas solanacerum [6 × 106 cells ml−1] on the nutrient agar plates, these plates were allowed to diffuse at 4°C and incubated at 29 ± 1°C for 48 h.
Results and Discussion
Heavy Metal Resistance
Maximum metal resistance level for bacterial Alcaligenes sp. and Pseudomonas aeruginosa RZS3 were observed on nutrient agar with different concentrations of MnCl2 and NiCl2. Pseudomonas aeruginosa RZS3 showed resistant to MnCl2 salt up till 3 mg during step by step repeated culturing on nutrient agar. Similarly resistance of Alcaligenes sp. was obtained upto 1 mg of NiCl2. Most bacterial strain accumulates metal by employing physico-chemical mechanisms and transport system of varying specificity. However, both essential and non-essential metals in concentrations, higher than optimal level, prove toxic to organisms. Under such conditions, these organisms may activate and adapt a mechanism of detoxification to ensure survival. In this study we were successful in developing Ni and Mn resistant strains by step-by-step repeated culture and selection on the medium containing increasing concentration of Ni and Mn.
Partial Identification and 16 s rRNA Sequencing
Preliminary phenotypic characterization showed that the isolates were a Gram-negative straight, motile rod, presented a fermentative metabolism on a wide range of sugars. (Table 1) and synthesized a fluorescent pigments when grown on nutrient agar. The isolates were named as Alcaligenes sp. and Pseudomonas aeruginosa RZS3. 16S ribosomal RNA partial gene sequencing of this isolates showed close i.e. 98 and 99% relationship with Alcaligenes sp. STC1 and Pseudomonas aeruginosa RZS3 SH-94B, respectively, therefore, this isolates were named as Alcaligenes sp. STC1 and Pseudomonas aeruginosa RZS3 SH-94B.
Table 1.
Preliminary identification of Alcaligenes sp. and Pseudomonas aeruginosa RZS3
| Sl no. | Characteristics | Results | |
|---|---|---|---|
| Alcaligenes sp. | Pseudomonas aeruginosa RZS3 | ||
| 1 | Gram staining | Negative | Negative |
| 3 | Phosphate solubilization | Negative | Positive |
| 4 | Detection of siderophore | Positive | Positive |
| 5 | Motility | Motile | Motile |
| 6 | Starch hydrolysis | Negative | Positive |
| 7 | Utilization of carbohydrates | ||
| a. Glucose | Positive | Positive | |
| b. Fructose | Negative | Negative | |
| c. Sucrose | Negative | Negative | |
| d. Raffinose | Negative | Negative | |
| e. l-Arrabinose | Positive | Positive | |
| f. Mannose | Positive | Positive | |
| g. Ribose | Positive | Positive | |
| 12 | Citrate-utilization | Positive | Positive |
| 13 | Nitrate reduction | Negative | Negative |
Screening for Siderophore and Siderophore Production and Detection
In the shake flask studies, change in the color of SAM from colorless to fluorescent green after 24 h, indicated siderophore production. Production of siderophore was confirmed by CAS test, where addition of CAS to cell free supernatant changed the blue color of CAS to orange while no color change occurred in control (un-inoculated). Change in the color of CAS reagent was due to the fact that siderophore present in the supernatant chelates the iron from CAS reagent and results in color change from blue to orange red [25–27].
Alcaligenes sp. excreted highest amount (92.61%) of siderophore while Pseudomonas aeruginosa RZS3 produced less amount (43.22%) of siderophore.
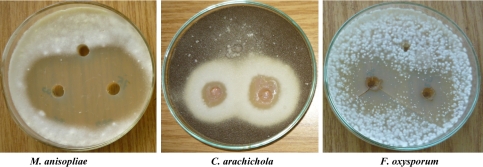
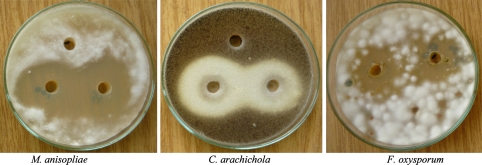
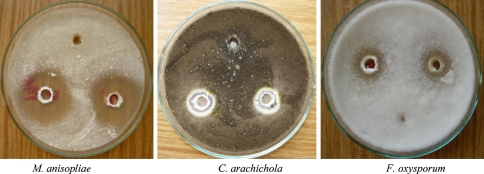
Siderophores produced by rhizobacteria chelate available iron and therefore create artificial shortage of iron to the respective phytopathogens thereby limiting their growth [13]. In vitro phytopathogen suppression by Alcaligenes sp., Pseudomonas aeruginosa RZS3 indicated their biocontrol potential. Both siderophore rich culture broth as well as cell free supernatant were found to inhibit the growth of phytopathogenic fungi namely A. niger, A. flavus, F. oxysporum, A. alternata, C. arachichola, M. anisopliae and P. solanacereum. Control preparation [free of any siderophore activity] did not inhibit the growth of any of the fungal sp. under study. These results suggested that, cell free culture supernatant as well as siderophore rich culture broth have the biocontrol potential against phytopathogenic fungi and P. solanacereum. However, siderophore rich culture broth (Tables 2, 3 and Fig. 1) proved to be potent inhibitor of fungal pathogens than cell free culture supernatant (Tables 2, 3 and Fig. 2) and the chemical fungicide kitazin (Table 4, Fig. 3). The presence of siderophoregenic rhizobacteria around root zone of plants is known to protect the plant from phytopathogen infestations by preventing its iron nutrition [10, 28, 29].
Table 2.
MIC of siderophore based preparation using Alcaligenes sp. against some common phytopathogenic fungi and bacterial strain
| Preparation | Amount (μl) |
Diameter of zone of inhibition (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| A. niger | A. flavus | F. oxysporum | A. alternata | C. arachichola | M. anisopliae | P. solanacerum | ||
| NCIM 1025 | NCIM 650 | NCIM 1281 | IARI 715 | arachichola | NCIM 1311 | NCIM 5103 | ||
| Culture broth (6 × 106 cell ml−1) | 25 | 24.0 | 27.0 | 23.0 | 13.0 | 40.0 | 30.0 | 18.0 |
| 50 | 27.0 | 35.0 | 28.0 | 15.0 | 35.0 | 35.0 | 20.0 | |
| 75 | 30.0 | 30.0 | 29.0 | 27.0 | 33.0 | 40.0 | 22.0 | |
| 100 | 35.0 | 32.0 | 30.0 | 29.0 | 45.0 | 45.0 | 24.0 | |
| Culture | 25 | 22.0 | 35.0 | 20.0 | 19.0 | 29.0 | 30.0 | 11.0 |
| Supernatant | 50 | 25.0 | 38.0 | 22.0 | 22.0 | 35.0 | 35.0 | 16.0 |
| 75 | 40.0 | 45.0 | 24.0 | 25.0 | 32.0 | 32.0 | 15.0 | |
| 100 | 40.0 | 47.0 | 25.0 | 28.0 | 39.0 | 39.0 | 19.0 | |
Table 3.
MIC of siderophore based preparation using Pseudomonas aeruginosa RZS3 against some common phytopathogenic fungi and bacterial strain
| Preparation | Amount (μl) |
Diameter of zone of inhibition (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| A. niger | A. flavus | F. oxysporum | A. alternata | C. arachichola | M. anisopliae | P. solanacerum | ||
| NCIM 1025 | NCIM 650 | NCIM 1281 | IARI 715 | NCIM 1311 | NCIM 5103 | |||
| Culture broth (6 × 106 cell ml−1) | 25 | 23.0 | 26.0 | 25.0 | 15.0 | 28.0 | 23.0 | 16.0 |
| 50 | 26.0 | 28.0 | 32.0 | 18.0 | 32.0 | 25.0 | 18.0 | |
| 75 | 35.0 | 25.0 | 35.0 | 19.0 | 37.0 | 27.0 | 20.0 | |
| 100 | 37.0 | 32.0 | 40.0 | 17.0 | 40.0 | 30.0 | 22.0 | |
| Culture | 25 | 25.0 | 20.0 | 32.0 | 15.0 | 28.0 | 20.0 | 11.0 |
| Supernatant | 50 | 25.0 | 22.0 | 35.0 | 18.0 | 32.0 | 22.0 | 14.0 |
| 75 | 28.0 | 35.0 | 38.0 | 19.0 | 37.0 | 24.0 | 16.0 | |
| 100 | 29.0 | 42.0 | 40.0 | 17.0 | 40.0 | 25.0 | 18.0 | |
Fig. 1.
Antifungal activity of siderophore rich broth of Alcaligenes sp.
Fig. 2.
Antifungal activity of siderophore rich supernatant of Alcaligenes sp.
Table 4.
MIC of Kitazin (fungicide) against some common phytopathogenic fungi and bacterial strain
| Preparation | Amount (μl) |
Diameter of zone of inhibition (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| A. niger | A. flavus | F. oxysporum | A. alternata | Cercospora arachichola | M. anisopliae | P. solanacerum | ||
| NCIM 1025 | NCIM 650 | NCIM 1281 | IARI 715 | NCIM 1311 | NCIM 5103 | |||
| Kitazin | 25 | 0.7 | 1.5 | 15.0 | 20.0 | 15.0 | 18.0 | 15.0 |
| 50 | 0.7 | 1.5 | 20.0 | 25.0 | 15.0 | 20.0 | 12.0 | |
| 75 | 1.0 | 2.0 | 24.0 | 30.0 | 15.0 | 22.0 | 11.0 | |
| 100 | 1.0 | 2.5 | 25.0 | 35.0 | 15.0 | 22.0 | 11.0 | |
Fig. 3.
Antifungal activity of chemical fungicide (kitazin)
Determination of MIC
As depicted in Table 2 and 3, the MIC of culture broth containing siderophoregenic Alcaligenes sp. and Pseudomonas aeruginosa RZS3, respectively, was 25 μl for A. niger, F. oxysporum, A. flavus, C. arachichola and P. solanacerum 75 μl for A. alternata. The MIC of cell free supernatant was 75 μl for A. niger, A. alternata, A. flavus, F. oxysporum, C. arachichola and P. solanacerum. As per the Table 4 the MIC of kitazin (fungicide) was 75 μl for A. niger and A. flavus while 100 μl for F. oxysporum, A. alternata, C. arachichola, M. anisopliae and P. solanacerum.
Antifungal Activity Against Phytopathogenic Fungi
Siderophores produced by heavy metal resistant isolates have been implicated in the biocontrol of several diseases, like vascular wilts caused by F. oxysporum and stem rot of pea nut caused by Rhizoctonia solani [17]. It have been reported that siderophore producing Pseudomonas aeruginosa was capable of inhibiting the growth of A. niger, A. flavus, F. oxysporum and A. alternata [30]. It have been also reported that hydroxamate type of siderophore producing A. chrococcum RRLJ 203 inhibited the growth of F. oxysporum and other phytopathogenic fungi [31].
The siderophoregenic culture broth proved to be potent inhibitor of fungal pathogens than kitazin and cell free culture supernatant indicating the role of other secondary metabolites along with siderophores in the growth inhibition of pathogenic fungi.
Evaluation of Safety for Useful Soil Rhizobia
To be an ideal antagonist, the BCA should be effective against a wide range of pathogens but should not harm the useful soil rhizobia. It have been reported that siderophore producing P. aeruginosa did not cause the growth inhibition of A. vinelandii, R. meliotii and B. japonicum [30]. Rhizobacteria are known to possesses iron regulated outer membrane protein receptors on their cell surface that transport ferric iron complex to the respective cognate membrane [10] and are not deprived of their iron nutrition by the siderophore producing rhizobacteria and thus their growth is not affected [32]. These results provide evidence for eco-friendly role of siderophoregenic Alcaligenes sp. and Pseudomonas aeruginosa RZS3 as a potent BCA.
Conclusion
Siderophore rich broth and supernatant are ecofriendly bio-control agents and have greater antifungal potential than chemical fungicide kitazin. Research into the mechanisms of plant growth promotion by Alcaligenes sp. and Pseudomonas aeruginosa RZS3 have provided a greater understanding of the multiple facets of disease suppression by these biocontrol agents.
Acknowledgment
Financial assistance to the corresponding author from BRNS, BARC under the scheme of DAE Young Scientist research Award Scheme is greatly acknowledged.
References
- 1.Nies DH. Microbial heavy-metal resistance. Appl Microbiol Biotechnol. 1999;51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- 2.Nilanjana D, Vimala R, Karthika P. Biosorption of heavy metals—an overview. Indian J Biotechnol. 2008;7:19–169. [Google Scholar]
- 3.Rafia A, Uzma, Fahim U. Biosorption of toxic metals from solid sewage by marine green algae. Asian J Plant Sci. 2007;6(1):42–45. doi: 10.3923/ajps.2007.42.45. [DOI] [Google Scholar]
- 4.Nehl DB, Allen SJ, Brown JF. Deleterious rhizosphere bacteria: an integrating prospective. Appl Soil Ecol. 1996;5:1–20. doi: 10.1016/S0929-1393(96)00124-2. [DOI] [Google Scholar]
- 5.Tripathi P, Srivastava S. Development and characterization of nickel accumulating mutants of Aspergillus nidulans. Indian J Microbiol. 2007;47:241–250. doi: 10.1007/s12088-007-0045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sayyed RZ, Naphade BS, Chincholkar SB. Ecologically competent rhizobacteria for plant growth promotion and disease management. In: Rai MK, Chikhale NJ, Thakare PV, Wadegaonkar PA, Ramteke AP, editors. Recent trends in biotechnology. Jodhpur: Scientific Publisher; 2005. pp. 1–16. [Google Scholar]
- 7.Sayyed RZ, Chincholkar SB. Siderophore producing A. feacalis more biocontrol potential vis-a-vis chemical fungicide. Curr Microbiol. 2009;58(1):47–51. doi: 10.1007/s00284-008-9264-z. [DOI] [PubMed] [Google Scholar]
- 8.Lugtenberg BJ, Thomas FC, Woeng AC, Blomberg GV. Microbe-plant interactions: principles and mechanisms. Antonie van Leeuwenhock. 2002;81:373–383. doi: 10.1023/A:1020596903142. [DOI] [PubMed] [Google Scholar]
- 9.Thrane C, Harder NI, Neiendam NM, Sorensen, Olson JS. Visconiamide producing Pseudomonas fluorescence DR 54 exerts a biocontrol effect on Pythium ultimum in sugar beet. FEMS Microbiol Ecol. 2000;33:39–46. doi: 10.1111/j.1574-6941.2000.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 10.Johri BN, Sharma A, Virdi JS (2003) Rhizobacterial diversity in India and its influence on plant health. In: Ghosh TK, Ghosh P (eds), Advances in biochemical engineering biotechnology, Springer Verlag, Berlin, p 49–89 [DOI] [PubMed]
- 11.Nair A, Juwarkar AA, Singh SK. Production and characterization of siderophores and application in arsenic removal from contaminated soil. Water Air Soil Pollut. 2006;180:199–212. doi: 10.1007/s11270-006-9263-2. [DOI] [Google Scholar]
- 12.Sayyed RZ and Chincholkar SB (2010). Growth and siderophore production Alcaligenes faecalis is influenced by heavy metals. Indian J Microbiol 50(2):179–182 [DOI] [PMC free article] [PubMed]
- 13.Lemanceau P, Albouvette A. Suppression of Fusarium wilts by fluorescent pseudomondas: mechanism and applications. Biocontrol Sci Tech. 1993;3:219–234. doi: 10.1080/09583159309355278. [DOI] [Google Scholar]
- 14.Wees SCM, Swart EAM, Pelt JA, Loon LC, Pietersen CMJ. Enhancement of induced disease resistance by simultaneous activation of salyclate & jasmonate dependent defense pathway in Arabdopsis thaliana. Proc Natl Acad Sci USA. 2000;97:8711–8716. doi: 10.1073/pnas.130425197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pieterse CMJ, Pelt JA, Wees SCM, Ton J, L-Kloosterziel KM, Keurentjes JJB, Verhagen BMW. Rhizobacteria mediated induced systemic resistance: triggering, signaling and expression. Eur J Plant Pathol. 2001;107:51–61. doi: 10.1023/A:1008747926678. [DOI] [Google Scholar]
- 16.Mazzola M. Mechanism of natural soil suppressiveness to soil borne diseases. Antonie van Leeuwenhock. 2002;81:557–564. doi: 10.1023/A:1020557523557. [DOI] [PubMed] [Google Scholar]
- 17.Sindhu SS, Suneja S, Dadarwal KR. Plant growth promoting rhizobacteria and their role in crop productivity. In: Dadarwal KR, editor. Biotechnological approaches in soil microorganisms for sustainable crop production. Jodhpur: Scientific Publisher; 1997. pp. 149–193. [Google Scholar]
- 18.Sayyed RZ, Naphade BS, Chincholklar SB. Siderophore producing A. feacalis promoted the growth of Safed musali and Ashwagandha. J Med Aromat Plants. 2007;29:1–5. [Google Scholar]
- 19.Sayyed RZ, Patel DC, Patel PR. Plant growth promoting potential of P solubilizing Pseudomonas sp. occuring in acidic soil of Jalgaon. Asian J Microbiol Biotechnol Environ Sci. 2007;4:925–928. [Google Scholar]
- 20.Meyer JM, Abdallah MA. The fluorescent pigments of Fluorescent pseudomonas: biosynthesis, purification and physicochemical properties. J Gen Microbiol. 1978;107:319–328. [Google Scholar]
- 21.Kersters K, De Ley J (1984) Genus Alcaligenes. In: Krieg NR, Holt JG (eds) Bergey’s manual of systematic bacteriology, 2nd ed, vol 2, William and Wilkins, Baltimore, pp 361–373
- 22.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. New York: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 23.Pidiyar V, Adam KA, Badri NN, Patole M, Shouche YS. Aeromonas culicicola sp. nov., from the midgut of Culex quinquefasciatus. Int J Syst Evol Microbiol. 2002;52:1723–1728. doi: 10.1099/ijs.0.02019-0. [DOI] [PubMed] [Google Scholar]
- 24.Hauben L, Vauterin L, Swings J, Moore ERB. Comparison of 16S ribosomal DNA sequences of all Xanthomonas species. Int J Syst Bacteriol. 1997;47:328–335. doi: 10.1099/00207713-47-2-328. [DOI] [PubMed] [Google Scholar]
- 25.Schwyn R, Neilands JB. Universal chemical assay for detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 26.Payne SM (1994) Detection, isolation and characterization of siderophores. Methods Enzymol 235:329–344 [DOI] [PubMed]
- 27.Milagres AMF, Machuca A, Napoleao D. Detection of siderophore production from several fungi and bacteria by a modification of chrome Azurol S (CAS) agar plate assay. J Microbiol Method. 1999;37:1–6. doi: 10.1016/S0167-7012(99)00028-7. [DOI] [PubMed] [Google Scholar]
- 28.Estrella AF, Chet I. Biocontrol of bacteria and phytopathogenic fungi. In: Altman A, editor. Agriculture Biotechnology. New York: Marcel Deckker Inc.; 1998. pp. 263–282. [Google Scholar]
- 29.Bloemberg GV, Lugtenberg BJJ. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol. 2001;4:343–350. doi: 10.1016/S1369-5266(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 30.Manwar AV, Vaigankar PD, Bhonge LS, Chincholkar SB. In vitro suppression of plant pathogens by siderophores of fluorescent pseudomonads. Indian J Microbiol. 2000;40:109–112. [Google Scholar]
- 31.Saikia N, Bezbruah B. Iron dependent plant pathogen inhibition through Azotobacter RRL J203 isolated from iron rich acid soil. Indian J Exp Biol. 1995;35:571–575. [Google Scholar]
- 32.Plessner O, Klapatch T, Guerinot ML. Siderophore utilization by Bradyrhizobium japonicum. Appl Environ Microbiol. 1995;59:1688–1690. doi: 10.1128/aem.59.5.1688-1690.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]