Abstract
Catechol 1,2-dioxygenase (C12O) was purified to electrophoretic homogeneity from Acinetobacter sp. DS002. The pure enzyme appears to be a homodimer with a molecular mass of 66 kDa. The apparent Km and Vmax for intradiol cleavage of catechol were 1.58 μM and 2 units per mg of protein respectively. Unlike other C12Os reported in the literature, the catechol 1,2-dioxygenase of Acinetobacter showed neither intradiol nor extradiol cleavage activity when substituted catechols were used as substrates. However, it has shown mild intradiol cleavage activity when benzenetriol was used as substrate. As determined by two dimensional electrophoresis (2DE) followed MALDI-TOF/TOF analyses and gel permeation chromatography, no isoforms of C12O was observed in Acinetobacter sp. DS002. Further, the C12O was seen only in cultures grown in benzoate and it was completely absent in succinate grown cultures. Based on the sequence information obtained from MS/MS data, degenerate primers were designed to amplify catA gene from the genomic DNA of Acinetobacter sp. DS002. The sequence of the PCR amplicon and deduced amino acid sequence showed 97% similarity with a catA gene of Acinetobacter baumannii AYE (YP_001713609).
Keywords: Biodegradation, Proteomics, Ortho pathway
Introduction
Aromatic carbon compounds are degraded aerobically and anaerobically. The aerobic degradation of many aromatic compounds was thoroughly investigated in most of the bacteria [1–3]. The degradation of aromatic compounds usually proceeds in two phases. Initially, the aromatic ring is hydroxylated by either monooxygenases or dioxygenases to form reactive, hydroxylated intermediates such as protocatechuate, catechol or gentisate [4, 5]. These intermediates further undergo ring fission to yield metabolites for the TCA cycle. Catechol is the most frequently encountered metabolite and ring fission of catechol is catalyzed by dioxygenases. The intradiol and extradiol cleavages of catechol are termed as ortho and meta cleavages and the reactions are catalyzed by catechol 1,2-dioxygenase and catechol 2,3-dioxygenase respectively. The ortho pathway of catechol degradation is also called as the β-ketoadipate pathway. Catechol 1,2-dioxygenase is the key enzyme in the β-ketoadipate pathway. It has been extensively studied in both gram negative (Pseudomonas and Acinetobacter) and positive bacteria (Arthobacter and Rhodococcus) [6–9]. In majority of cases C12O is a homodimer with a subunit molecular mass of 30–34 kDa. Catechol is the primary substrate of C12O. It catalyzes the ring cleavage reaction by hydrolyzing the C–C bond found between first and second carbon atoms. However, when substituted catechols are used as substrates, C12Os of few pseudomonads, dramatically show extradiol cleavage by hydrolyzing the C–C linkage found between second and third carbon atoms [10, 11].
Serratia sp. DS001 is a soil bacterium, isolated from organophosphate polluted agricultural soils. In our previous reports, we have shown its ability to utilize methyl parathion and p-nitrophenol as source of carbon and energy [12]. Acinetobacter sp. DS002 was found as a co-culture along with Serratia sp. DS001. Acinetobacter sp. DS002 was able to grow on methyl parathion as source of carbon only when it is cultured along with Serratia sp. DS001 which suggests that DS002 is not utilizing methyl parathion but is utilizing break down products of methyl parathion. Acinetobacter sp. DS002 utilizes benzoate as sole source of carbon by degrading it through the ortho pathway. Catechol 1,2-dioxygenase (C12O) is the key enzyme in ortho pathway of catechol degradation. If C12O of Acinetobacter sp. DS002 acts on substituted catechols such as 4-nitrocatechol or 1,2,4-benzenetriol, the degradation products of 4-nitrophenol, then it is possible for DS002 to utilize the breakdown products of 4-nitrophenol as carbon source. In order to gain such insights, we have purified C12O from Acinetobacter sp. DS002 and tested its ability to act on substituted catechols.
Materials and Methods
Bacterial Strain and Media Conditions
Acinetobacter sp. DS002 was grown at 30°C in minimal media prepared by dissolving 4.8 g of K2HPO4; 1.2 g of KH2PO4; 1 g of NH4NO3; 0.2 g of MgSO4 7H2O; 0.04 g of Ca(NO3)2·4H2O and 0.001 g of Fe2(SO4)3 in 1000 ml of distilled water. The medium was autoclaved and stored at room temperature until further use. Minimal media containing benzoate as sole carbon source was prepared by supplementing filter sterilized sodium benzoate (5 mM).
Catechol 1,2-Dioxygenase Assay
Catechol 1,2-dioxygenases (C12O) was assayed spectrophotometrically by following the procedures described elsewhere [13]. Formation of the product, cis-cis muconic acid from catechol was monitored using spectrophotometer at 260 nm. Specific activity was defined as micro mole of cis, cis muconic acid formed per min per mg of protein.
Purification of Catechol 1,2-Dioxygenase
Acinetobacter sp. DS002 grown in minimal medium supplemented with 5 mM sodium benzoate for 16 h and the cells were harvested by centrifuging at 8,000 rpm for 10 min. The cell pellet was washed twice with 20 mM Tris buffer pH 8.0 and resuspended in the 7 volumes of 50 mM Tris buffer (pH 8.0) and the cells were disrupted by sonication for a period of 10 min with a pulse of 30 s. Cell debris was removed by centrifuged at 15,000 rpm for 30 min and the supernatant was again centrifuged at 45,000 rpm for 1 h. The supernatant is fractionated using ammonium sulphate and the proteins that are precipitated in the 40–60% were dialysed against 50 mM Tris–Cl, pH 8.0. As this fraction showed maximum C12O activity, it was loaded on a DEAE Sepharose column pre-equilibrated with buffer A (50 mM Tris–Cl, pH 8.0 containing 0.18 M NaCl). The column was then connected to AKTA basic FPLC system and was washed with 7 column volumes of buffer A. The bound proteins were eluted at a flow rate of 0.4 ml/min with a linear gradient of buffer B (50 mM Tris–Cl, pH 8.0 containing 0.4 M NaCl). Fractions having more than 50% activity are brought to 15% saturation with ammonium sulphate and loaded on a Phenyl Sepharose column pre-equilibrated with buffer C (15% saturated solution of buffer A). The column was washed with 3 column volumes of buffer C and the proteins were eluted using a 50 mM Tris–Cl buffer having 0.18 M NaCl with a negative stepwise gradient of buffer C/buffer A. Fractions having more than 50% activity were pooled and precipitated at 60% ammonium sulphate saturation. The precipitated proteins were separated on a Sephacryl 200 HR column pre-equilibrated with buffer A. The flow rate was 0.4 ml/min and the fractions obtained were assayed for C12O activity and the active fractions were analyzed on a 12.5% SDS PAGE having a 7.5% stacking gel [14]. The fractions that showed single band were pooled and the quantity of the pure protein was determined [15] before using it for further studies.
Kinetics and Substrate Specificity
The activities of C12O at various initial concentrations of catechol ranging from 10 to 120 μM were determined and kinetic parameters were calculated by a non-linear regression analysis using Graphpad Prism software (www.graphpad.com). The substrate specificity of catechol 1,2-dioxygenase for the substrates 3-methyl catechol, 4-methyl catechol, 4-nitrocatechol and 1,2,4-benezenetriol was assayed using oxygraph [7].
Preparation of Protein Sample for IEF
Acinetobacter sp. DS002 was grown in minimal media supplemented with 5 mM benzoate or 50 mM benzoate or 10 mM succinate till they reach mid-log phase. The cell pellet was collected by centrifuging at 8,000 rpm for 10 min and washed with 20 mM Tris pH 8.0. 200 mg of cell pellet was extracted with extraction buffer (40 mM Tris, 8 M Urea, 4% CHAPS, 2 mM DTT, 0.2% Ampholytes) and was precipitated with cold acetone and the pellet was washed with absolute alcohol. The precipitated protein was air dried and dissolved in 0.34 ml of rehydration solution (8 M Urea, 2% CHAPS, 10 mM DTT and 2% Ampholytes). Protein was estimated using Bradford method after TCA–acetone precipitation.
Two Dimensional Gel Electrophoresis
100 μg of protein extracted with the extraction buffer was taken in 350 μl of sample buffer and loaded onto IPG strips (18 cm, pH 3–10) and rehydrated for 12 h at 20 V and IEF was performed on Ettan IPGphor3 system using a four step programme [500 V for 30 min (gradient), 500 V for 30 min (step), 8000 V for 3 h (gradient) and continued till 40,000 Vh]. After focusing, strips were equilibrated with equilibration buffer I (75 mM Tris, 6 M Urea, 2% SDS, 20% glycerol, 2% DTT) and equilibration buffer II (75 mM Tris, 6 M Urea, 2% SDS, 20% glycerol, 2% Idoacetamide) for 15 min each. The second dimension electrophoresis was performed on 12.5% polyacrylamide gels in a Ettan DALTsix system (GE Healthcare Life Sciences, USA).The IPG strips were placed on top of the 12.5% polyacrylamide gels and sealed with 0.5% agarose sealing solution. The gels were run at constant voltage of 200 V until the bromophenol blue had migrated to the bottom of the gel. The gels were stained overnight with coomassie brilliant blue and after destaining the gels were scanned with a resolution of 300 dpi on an Image scanner (GE Healthcare Life Sciences, USA). The Image analysis was done with ImageMaster 2D Platinum software (GE Healthcare Life Sciences, USA).
In-Gel Digestion
The coomassie brilliant blue stained protein spot was excised manually and the gel pieces were then destained with ACN and 25 mM NH4HCO3 in 1:1, dried with 100% ACN and reduced with dithiothreitol (DTT) in 25 mM NH4HCO3 for 1 h at 50°C, alkylated using a 55 mM idoacetamide in 25 mM NH4HCO3 for 45 min at room temperature. The gel pieces were dehydrated with 100% acetonitrile, rehydrated with a minimum volume of 50 mM of NH4HCO3 containing Trypsin (10 ng/μl), and digested at 37°C for 16 h. The peptides were extracted twice with 50% acetonitrile containing 1% trifluoroacetic acid. The peptide mixture was concentrated under vacuum in a Concentrator (Eppendorf) for 1 h at room temperature.
MALDI-MS
The tryptic peptides were dissolved in 2 μl solution of 50% ACN and 1% TFA and mixed with 2 μl of 1% cyano-4-hydroxycinnamic acid (HCCA) dissolved in 50% ACN and 1% and 1 μl of it was applied on the MALDI target plate. Peptides were analyzed using MALDI-TOF Autoflex (Bruker Daltonics, Germany) in reflectron mode. MS/MS of selected peptides were performed by LIFT. The spectra were calibrated by Pepmix (Bruker Daltonics, Germany).
Protein Identification
The spectral data were analyzed using Biotools software and searches were performed for protein identification using MASCOT search engine (www.matrixscience.com) against Swiss-prot and NCBInr databases. The following search parameters were used: trypsin is the enzyme and one missed cleavage was allowed, the peptide tolerance was set at ±0.5 Da, carbamidomethyl and oxidized methionine were set as fixed and variable modifications respectively. MS/MS data analysis was performed using Biotools software.
Cloning of catA gene from Acinetobacter sp. DS002
Sequence tags obtained from the MS/MS data were used to obtain the homologous sequences from the NCBI database using the BLAST (www.ncbi.nlm.nih.gov/blast/BLAST.cgi). Multiple alignments were done using ClustalW (www.ebi.ac.uk/Tools/clustalw) and highly conserved portions in the alignment were selected for designing the degenerate primers using the sequence manipulation suite software [16]. Genomic DNA of Acinetobacter sp. DS002 was used as a template in a 100 μl reaction mixture containing 50 pmol each of the degenerate PCR primers c12ofp2 (5′-ACNCCNMGNACNATHGARGGN-3′) and c12orp (5′-CKNGTNGCRAANGCRAARTCRTC-3′), 100 μM each of deoxynucleoside triphosphate, 1.5 mM MgCl2 and 1 U of Taq DNA polymerase. The PCR programme consisted of 30 cycles of 94°C for 30 s, 52°C for 30 s, 72°C for 30 s and a final extension at 72°C for 10 min. The PCR product was gel eluted and cloned into pGEMT-Easy vector. The amplicon was sequenced commercially and the sequence comparison was done with sequences in the NCBI database using BLAST (www.ncbi.nlm.nih.gov/blast/Blast.cgi).
Results and Discussion
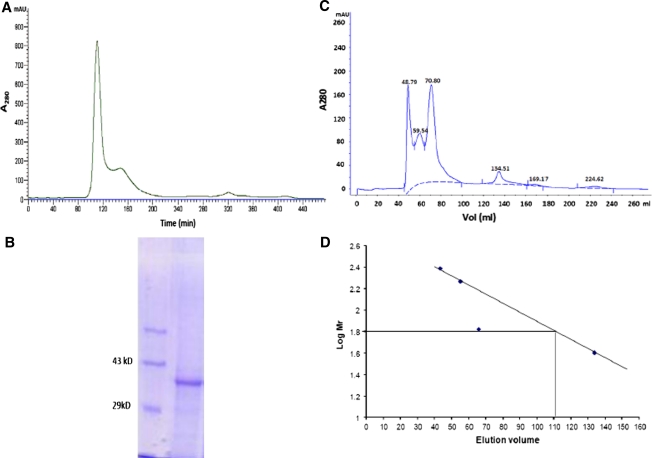
Catechol 1,2-dioxygenase was purified to electrophoretic homogeneity by using anion exchange chromatography, hydrophobic interaction chromatography and gel permeation chromatography (Fig. 1). The purification procedure gave 1 mg of protein from 10 g of cell pellet. While proceeding for purification, fractions containing more than 50% catechol 1,2-dioxygenase activity were pooled and were used in next stage of purification. At each stage of purification the specific activity and fold purification got increased and finally when gel permeation chromatography was performed the enzyme seemed virtually homogenous (Fig. 1). As shown by Fewson and his associates [17] the hydrophobic interaction chromatography did not eliminate impurities from the enzyme. Therefore, we have further performed gel permeation chromatography to increase the purity of the enzyme. The pure enzyme was then used to determine its native mass by employing gel permeation chromatography along with proteins of known molecular weights. As determined by gel permeation chromatography the pure protein showed a native mass of 66 kDa. However, the very same protein when analyzed on SDS-PAGE showed a molecular mass of 34 kDa and thus indicating the C12O of Acinetobacter sp. DS002 as a homodimer. The pure enzyme was then used to determine the Vmax, Km using catechol and substituted catechols. The Km and Vmax of C12O of Acinetobacter sp. DS002 are 1.58 μM and 2 units per mg of protein respectively. On perusal of literature it is evident to have certain C12Os, especially those purified from Rhodococcus, Ralstonia and Pseudomonas arvilla with relaxed substrate specificity. C12Os from these bacteria have even used methyl and halocatechols as substrates [17–19]. Besides having relaxed substrate specificity, these C12Os have been shown to perform meta cleavage when 3-methyl or 3-methoxy catechol were used as substrates [10, 11]. In contrast to the published results, the C12O of Acinetobacter sp. DS002 showed very strict substrate specificity. It has used only catechol as substrate and failed to show any activity either on substituted catechols or on nitrocatechol. However, considerable activity was seen when 1,2,4-benzenetriol was used as substrate (Table 1). In Serratia sp. DS001, p-nitrophenol (PNP) degradation proceeds via formation of 4-nitrocatechol and benzenetriol [12]. As C12O of Acinetobacter DS002 showed weak activity towards 1,2,4 benzenetriol we tempt to speculate convergence between PNP and benzoate degradation pathways.
Fig. 1.
A Purification profile of C12O on Sephacryl S200 column. B SDS PAGE showing purified C12O: Lane 1 represents Mol wt. Marker and lane 2 purified C12O. C Separation profile of protein molecular weight standards (Catalase, 260 kDa; Glucose oxidase, 156 kDa; BSA, 66 kDa and peroxiadase, 40 kDa) and D Calibration curve of the above standards and native mass of C12O
Table 1.
Relative substrate specificities of Catechol 1,2-dioxygenase of Acinetobacter sp. DS002
| Substrate | Relative activity |
|---|---|
| Catechol | 100 |
| 3-methyl catechol | 0 |
| 4-methyl catechol | 0 |
| 4-nitro catechol | 0.3712 |
| 1,2,4-benzene triol | 16 |
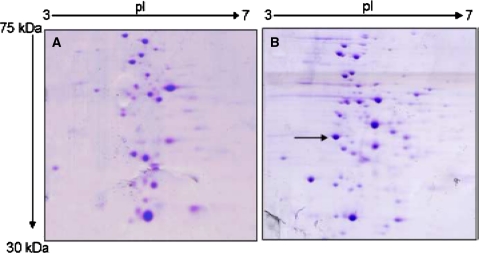
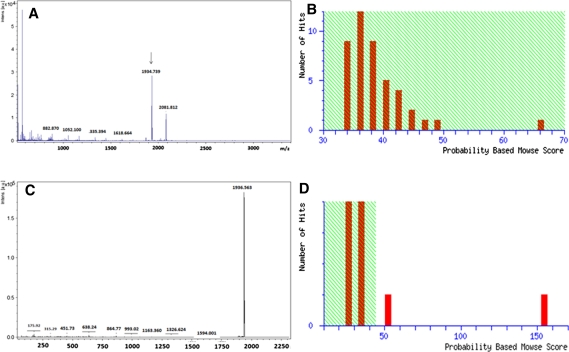
Isoforms of C12Os with unique physical and catalytic properties are known both in gram negative and positive bacteria [8, 20–22]. To detect the isoforms of C12O in Acinetobacter sp. DS002 we have used two independent approaches. In the first approach we have collected entire protein purified by using Phenyl Sepharose column and analyzed on gel permeation chromatography. Every single fraction collected from gel permeation chromatography was analyzed for C12O activity. This approach would generate information on existence of C12Os with different molecular masses. Interestingly, the entire C12O activity was seen in a single peak suggesting absence of C12O isoforms in Acinetobacter sp. DS002 with detectable size difference. To have knowledge on isoforms of C12Os with identical size and different pIs, soluble proteome extracted from benzoate grown cultures of Acinetobacter sp. DS002 was analyzed on 2DE (Fig. 2). The protein spots in the range of 30–40 kDa were independently taken to generate peptide mass fingerprints (PMFs) using MALDI-TOF (Fig. 3). The PMFs were then compared with the PDB database to gain identity. Though 145 spots were taken to generate PMFs only one spot matched with C12O of Acinetobacter baumannii AYE. These results are in agreement with the results of gel permeation chromatography which suggested non existence of isoforms for C12O of Acinetobacter sp. DS002.
Fig. 2.
2DE gels showing the soluble proteome profile of Acinetobacter sp. DS002 extracted from A 10 mM succinate, B 5 mM benzoate. The C12O spot is seen only in 5 mM benzoate grown cultures. The C12O spot is shown with an arrow mark
Fig. 3.
A, B represents the PMF and mowse score of C12O respectively. The peak selected for MS/MS was shown with an arrow mark. C, D The MS/MS profile of m/z peak 1934.7 and its mowse score respectively
On 2DE gels the intensity of C12O spot increased with the increased concentration of benzoate in culture medium (Fig. 2). In order to gain better insights on the expression of C12O in Acinetobacter sp. DS002, 2DE profile was generated for the proteins extracted from the cultures grown in succinate and benzoate. As expected the C12O was seen only in benzoate grown cultures and it was totally absent in the proteome extracted from succinate grown cultures (Fig. 2). Such results are of clear indication to show the inducible nature of C12O in Acinetobacter sp. DS002 and are in good agreement with the earlier reports [23, 24]. Based on the MS/MS data of C12O, we have designed degenerate primers to amplify partial catA gene from Acinetobacter sp. DS002. The sequence of the amplicon and its deduced amino acid sequence showed high similarity to C12O of A. baumannii AYE (YP_001713609) (Fig. 4).
Fig. 4.
Pairwise alignment of C12O nucleotide sequence of Acintobacter sp. DS002 with A. baumannii AYE
Acknowledgments
PEVP is a recipient of Senior Research Fellowship (SRF) from Council of Scientific and Industrial Research (CSIR), New Delhi. Research work in the laboratory of DS is supported by Dept. of Science and Technology (DST), Govt. of India and CSIR, New Delhi.
References
- 1.Warhurst M, Fewson CA. Biotransformations catalyzed by the genus Rhodococcus. Crit Rev Biotechnol. 1994;14:29–73. doi: 10.3109/07388559409079833. [DOI] [PubMed] [Google Scholar]
- 2.Bouwer EJ, Zehnder AJB. Bioremediation of organic compounds—putting microbial metabolism to work. Trends Biotechnol. 1993;11:360–367. doi: 10.1016/0167-7799(93)90159-7. [DOI] [PubMed] [Google Scholar]
- 3.Williams PA, Sayer JR. The evolution of pathways for aromatic hydrocarbon oxidation in Pseudomonas. Biodegradation. 1994;5:195–217. doi: 10.1007/BF00696460. [DOI] [PubMed] [Google Scholar]
- 4.Houghton JE, Shanley MS. Catabolic potential of Pseudomonads: a regulatory perspective. In: Chaudhry GR, editor. Biological degradation and bioremediation of toxic chemicals. Portland: Dioscorides Press; 1994. pp. 11–32. [Google Scholar]
- 5.Johnson BF, Stanier RY. Dissimilation of aromatic compounds by Alcaligenes eutrophus. J Bacteriol. 1971;107:468–475. doi: 10.1128/jb.107.2.468-475.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayaishi O, Katagiri M, Rothberg S. Studies on oxygenases: pyrocatechase. J Biol Chem. 1957;229:905–9207. [PubMed] [Google Scholar]
- 7.Patel RN, Hou CT, Felix A, Lillard MO. Catechol 1,2-dioxygenase from Acinetobacter calcoaceticus: purification and properties. J Bacteriol. 1976;127:536–544. doi: 10.1128/jb.127.1.536-544.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoki K, Konohana T, Shinke R, Nishira H. Purification and characterization of catechol 1,2-dioxygenase from aniline-assimilating Rhodococcus erythropolis AN- 13. Agric Biol Chem. 1984;48:2087–2095. [Google Scholar]
- 9.Aoki K, Konohana T, Shinke R, Nishira H. Two catechol 1,2-dioxygenases from aniline-assimilating bacterium, Frateuria species ANA-18. Agric Biol Chem. 1984;48:2097–2104. [Google Scholar]
- 10.Dorn E, Knackmuss HJ. Chemical structure and biodegradability of halogenated aromatic compounds: two catechol 1,2-dioxygenases from a 3- chlorobenzoate grown pseudomonad. Biochem J. 1978;174:73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujiwara M, Golovleva LA, Saeki Y, Nozaki M, Hayaishi O. Extradiol cleavage of 3-substituted catechols by an intradiol dioxygenase, pyrocatechase, from a pseudomonad. J Biol Chem. 1975;250:4848–4855. [PubMed] [Google Scholar]
- 12.Suresh BP, Purushotham G, Pijari AB, Krovidi RK, Baru R, Yanamandra M, Merrick M, Siddavattam D. Biodegradation of methyl parathion and p-nitrophenol: evidence for the presence of a p-nitrophenol 2–hydroxylase in a Gram negative Serratia sp. strain DS001. Appl Microbiol Biotechnol. 2006;73:1452–1462. doi: 10.1007/s00253-006-0595-z. [DOI] [PubMed] [Google Scholar]
- 13.Briganti Fabrizio, Pessione Enrica, Giunta Carlo, Scozzafava Andrea. Purification, biochemical properties and substrate specificity of a catechol 1,2-dioxygenase from a phenol degrading Acinetobacter radioresistens. FEBS Lett. 1997;416:61–6414. doi: 10.1016/S0014-5793(97)01167-8. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;772:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Stothard P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques. 2000;28:1102–1104. doi: 10.2144/00286ir01. [DOI] [PubMed] [Google Scholar]
- 17.Strachan PD, Freer AA, Fewson CA. Purification and characterization of catechol 1,2-dioxygenase from Rhodococcus rhodochrous NCIMB 13259 and cloning and sequencing of its catA gene. Biochem J. 1998;333(3):741–747. doi: 10.1042/bj3330741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridder L, Briganti F, Boersma MG, Boeren S, Vis EH, Scozzafava A, Veeger C, Rietjens IMCM. Quantitative structure/activity relationship for the rate of conversion of C4-substituted catechols by catechol 1,2-dioxygenase from Pseudomonas putida (arvilla) C1. Eur J Biochem. 1998;257:92–100. doi: 10.1046/j.1432-1327.1998.2570092.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, You S, Wang S. Purification and characterization of a novel catechol 1,2-dioxygenase from Pseudomonas aeruginosa with benzoic acid as a carbon source. Proc Biochem. 2006;41:1594–1601. doi: 10.1016/j.procbio.2006.03.008. [DOI] [Google Scholar]
- 20.Briganti F, Pessione E, Giunta C, Mazzoli R, Scozzafava A. Purification and catalytic properties of two catechol 1,2-dioxygenase isozymes from benzoate grown cells of Acinetobacter radioresistens. J Protein Chem. 2000;19:709–716. doi: 10.1023/A:1007116703991. [DOI] [PubMed] [Google Scholar]
- 21.Murakami S, Wang CL, Naito A, Shinke R, Aoki K. Purification and characterization of four catechol 1,2-dioxygenase isozymes from the benzamide assimilating bacterium Arthrobacter species BA-5–17. Microbiol Res. 1998;153(2):163–171. doi: 10.1016/s0944-5013(98)80036-0. [DOI] [PubMed] [Google Scholar]
- 22.Nakai C, Kagamiyama H, Saeki Y, Nozaki M. Nonidentical subunits of pyrocatechase from Pseudomonas arvilla C-1. Arch Biochem Biophys. 1979;195:12–22. doi: 10.1016/0003-9861(79)90322-9. [DOI] [PubMed] [Google Scholar]
- 23.Canovas JL, Stanier RY. Regulation of the enzymes of the β-ketoadipate pathway in Moraxella calcoaceticus. Eur J Biochem. 1967;1:289–300. doi: 10.1111/j.1432-1033.1967.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 24.Ornston LN. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971;35:87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]