Abstract
The study comprised of 60 Candida spp., 50 isolates from HIV and TB positive individuals (immunocompromised) and 10 isolates from non-HIV and -TB patients (immunocompetent). Among the 60 Candidal isolates, 83.3% were identified as C. albicans, 11.6% as C. glabrata and rest 5% as C. krusei. There is no study in production pattern of extracellular enzymes of Candida spp. isolated from HIV and TB patients in comparison with non-HIV and -TB patients in India. The comparison of phospholipase activities showed that there was a significant difference between the groups at (P = 0.001). The non-HIV and -TB groups of C. glabrata and C. krusei did not show detectable phospholipase activity when compared to the HIV and TB groups. The mean difference in the phospholipase activities of these two groups was significant (P = <0.001). Candida spp. of both the groups do not possess the ability to hydrolyze gelatin. All the strains possessed the ability to show alpha haemolysis. Even though it had shown alpha haemolysis, the significant difference in haemolytic activity was observed only in C. albicans (P = <0.001). None of the isolates from the two groups possessed the ability to hydrolyze gelatin. In the resistance profile of Candida spp., C. albicans of HIV and TB groups had shown resistance to fluconazole, Itraconazole, ketaconazole, nystatin but showed 100% sensitivity towards amphotericin-B. The isolates of C. krusei and C. glabrata showed no resistance to any of the drugs tested. In the case of, non-HIV and -TB patients the resistance pattern was low.
Keywords: Candida, Protease, Lipase, Fluconazole, HIV, TB
Introduction
Fungal infection caused by opportunistic pathogens have become more frequent, of these Candida sp. can cause a wide range of infections from mucocutaneous to systemic infections [1]. Candida is a major fungal pathogen of human capable of invading oral cavities and epithelial surfaces of vagina; in immunocompromised patients, it can be carried through blood to invade the internal organs [2]. Oral candidiasis occurs most frequently in immunocompromised hosts, intensive care patients and patients undergoing chemotherapy [3]. Candidiasis has also been associated with tubercle bacilli infection [4]. Tuberculosis, a major public health problem has turned to be a major threat with the worldwide epidemic of Human Immunodeficiency Virus (HIV). The prevalence of dually infected patients globally has increased greatly in the present century. It has been accounted that there are more than 14 million dually infected persons globally with India accounting for more than one million. There is substantial evidence proving that the consequences of HIV and tuberculosis co-infection are greater than the presence of either of them [5]. It is known that candidiasis is one among the symptoms of AIDS. Hydrolytic enzymes contribute to the virulence factors in Candida albicans. The enzymes produced are proteinase, lipase, and phospholipase; factors which are responsible for the invasiveness, and proliferation of fungi caused by the destruction of host tissues in which the organisms are provided with nutrients by the host tissues [6]. Furthermore, humans do not possess free iron and hence most pathogens obtain this indirectly from haemoglobin [7]. In HIV-positive subjects there might be selection of more virulent C. albicans strains and increased resistance to antifungal drugs, thus contributing to the outbreak of the disease, along with its easily relapsing nature and treatment failure [8]. There has been a rapid spread of antifungal multidrug resistance (MDR), which has become a serious public health problem in recent years [9]. There is no information about the production pattern of extracellular enzymes of Candida spp. isolated from HIV and TB positive patients in comparison with non-HIV and -TB patients in India.
The purpose of this study was to compare the enzymatic activities, haemolysin production and antifungal sensitivity pattern of oral clinical isolates of Candida spp. in HIV and TB positive and non-HIV and -TB patients.
Materials and Methods
The study comprised of 60 Candida spp. isolated from HIV and TB positive individuals (immunocompromised) who were voluntarily enrolled and ten isolates from non-HIV and -TB patients (immunocompetent) from the Holy Hanchanorium Community Care Centre in Tiruchirappalli, Tamil Nadu, India. The collected samples were processed at the Department of Microbiology, Bharathidasan University, Tiruchirappalli, Tamil Nadu, India. The Candida spp. were isolated from the oropharyngeal of male and female HIV and TB and non-HIV and -TB patients in the age group of 18–60 years. 34 strains were from male and 26 were from female patients. The clinical specimen included sampling from buccal mucosa, floor of mouth and dorsal surface of the tongue. The samples were characterized by creamy white, curd like patches, or by typical erythematous lesions on the oral/and or pharyngeal mucosa. All subjects had CD4+ lymphocyte counts of less than 200 cells/mm3, and the signs and symptoms observed among the opportunistic tuberculosis patients were confirmed in the laboratory by demonstrating the bacilli using acid fast staining and by a positive mycobacterium culture. The control groups had mild fever; cough, chills, and they were declared as negative for HIV and TB based on clinical and by means of laboratory investigations and all were in the same age group of the test group. The samples were collected with sterile swabs (Hi-Culture™ Transport swabs, Hi-Media, India). The swabs were transported to the laboratory within 2 h of sampling and agitated for 1 min. 150 μl of swab wash was spread onto Sabouraud’s Dextrose Agar (SDA) and incubated aerobically at 37°C for 2–4 days. After incubation, the colonies were streaked on to Candida differential medium (Hi-Media, Mumbai). Candida spp. were identified using germ tube test, pigment production on niger seed agar, capsule observation, microscopic morphology on cornmeal-Tween 80 agar, and carbohydrate fermentation and assimilation assays comprising seven and 15 sugars. Yeast colonies were identified according to the method described by Kreger-Van Rij [10].
Enzymatic Activity
Phospholipase activity
The phospholipase activity was assayed by egg yolk agar plate method [11]. The medium was slightly modified by enriching SDA with egg yolk as substrate. Egg yolk was added to equal amounts of saline and 5 ml of this was added to the medium. The inoculated plates were incubated at 37°C for 48 h. The precipitation zone around the colony was observed for the production of phospholipase enzyme. Phospholipase activity was measured according to the method described by Price et al. [11].
Proteinase Activity
For testing the proteinase activity of the Candida isolates, caseinase, and gelatinase activity tests were performed.
Caseinase Activity
Caseinase activity was measured by single diffusion technique in SDA agar plates provided with 1% casein [12]. The plates were inoculated and incubated for 48 h at 37°C. The zone of clearance was observed by the addition of 30% Trichloroacetic acid. The zone was measured as per standard procedures. Caseinase assay was performed by adding 1 ml of cell free supernatant to 1 ml of casein in phosphate buffer at pH 7.5 and incubating it for 1 h. 2 ml of 0.2 M TCA was added to stop the reaction. The reaction mixture was then centrifuged at 3,000 rpm for 10 min. Further, 0.5 ml supernatant was added to 2.5 ml of 0.55 M Sodium carbonate and 0.5 ml Folins reagent was added to this. It was then incubated for 15 min and OD values were recorded at 650 nm against control.
Gelatinase Activity
Gelatinase assay was also done for testing the ability of the Candida spp. to produce proteinases. SDA agar plates were prepared with 1% gelatin [13]. Single diffusion technique was applied. The inoculated plates were incubated for 48 h at 37°C. The appearance of inhibition zone was clearly visualized by the addition of 0.1% mercuric chloride. The zone diameter was measured by standard procedures [14].
Lipase Activity
Production of lipase was observed by inoculating the isolates of Candida by single diffusion method in SDA agar provided with 1% tributyrin as substrate. Clear zone formation was found around the colony after 24–48 h incubation at 37°C. The zone diameter was measured by standard procedures [14].
Haemolysin Activity
Haemolytic activity was measured using blood agar plates [15]. The Candida spp. were streaked onto SDA enriched with 5 ml of human blood at pH 5.6 ± 0.2 and incubated for 5 days at 25°C. The plates were analyzed with the aid of a computerized image analysis system (UVP Documentation System, UK) for all the assays and measurement of the zone of haemolytic activity.
Antifungal Sensitivity Pattern
Antifungal sensitivity pattern of the isolates were performed according to National Committee for Clinical Laboratory Standards protocol M27-A2 [16] with antifungal agents such as fluconazole (25 mcg), ketoconazole (10 mcg), itraconazole (10 mcg), nystatin (100 unit), and amphotericin-B (100 unit). C. albicans ATCC-10231 were used as control strains (Hi-Media, Mumbai, India). The antifungal sensitivity pattern in the form of zone formation was observed after incubation for 48 h at 37°C. The diameter of the zone was measured.
Statistical Analysis
The data was analysed using the SPSS package version 11.0. Using one way ANOVA for various enzymatic activities for the two groups were analysed. The significance of the various enzymatic activities of the patients with HIV and TB patients and non-HIV and -TB patients were determined based on the P value. The observed P < 0.001 is referred to as significant difference between two groups.
Results
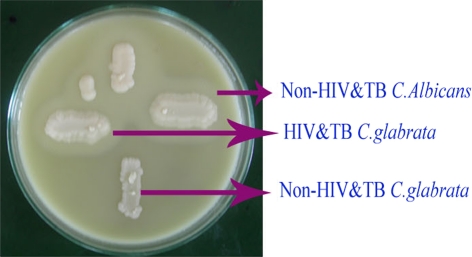
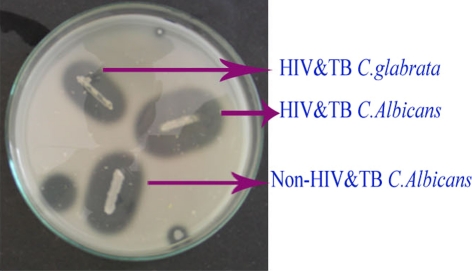
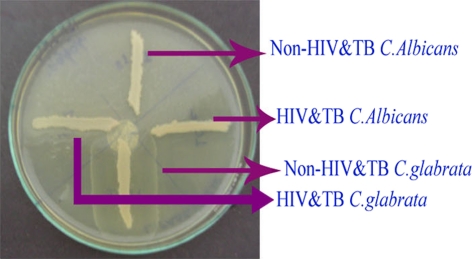
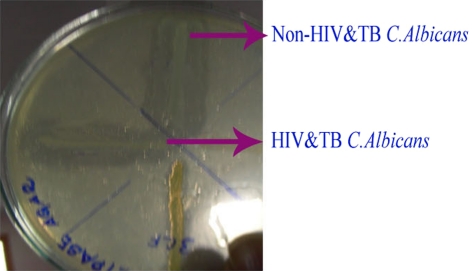
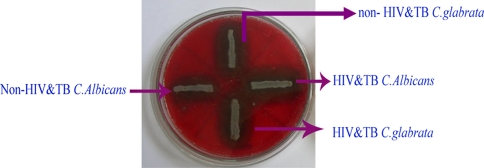
Among the 60 isolates, 83.3% were identified as C. albicans, 11.6% as C. glabrata and 5% as C. krusei using standard procedures (Figs. 1, 2). In addition, the enzymatic activities of the Candida spp. isolated from HIV and TB and non-HIV and -TB groups were determined and a comparative analysis was made (Table 1). The mean phospholipase activity of C. albicans from HIV and TB and non-HIV and -TB groups was recorded as 0.480–0.007 and 0.376–0.024, respectively. The comparison of phospholipase activities showed that there was a significant difference between the groups at 0.001 level (Fig. 3). The non-HIV and -TB groups of C. glabrata and C. krusei did not show detectable phospholipase activity whereas for the HIV and TB groups, the values were 0.428–0.018 and 0.430–0.013, respectively. The mean difference in the phospholipase activities of these two groups was significant (P < 0.001). The caseinase activities of C. albicans, C. glabrata, and C. krusei were 0.0.915–0.011, 0.941–0.009, and 0.970–0.005, respectively in non-HIV and -TB groups. In HIV and TB groups, it was 0.931–0.008, 1.004–0.017, and 0.923–0.008, respectively. The difference in the caseinase activity between the groups was insignificant as with the case of lipase activity (Figs. 4, 5). All the strains possessed the ability to show alpha haemolysis. The mean haemolytic activities of the HIV and TB groups of C. albicans, C. glabrata, and C. krusei were 0.329–0.007, 0.282–0.013, and 0.301–0.022, respectively and in non-HIV and -TB groups, it was 0.173−0.008, 0.165–0.004, and 0.146–0.017, respectively. Even though all the strains had shown alpha haemolysis, the significant difference in haemolytic activity was observed only in C. albicans (P = <0.001) (Fig. 6). None of the isolates from the two groups possessed the ability to hydrolyze gelatin (Fig. 7). In some cases, the difference in the enzymatic activities of two groups was observable but they could not be differentiated statistically. These results go in tune with actual mean rather than statistical mean.
Fig. 1.
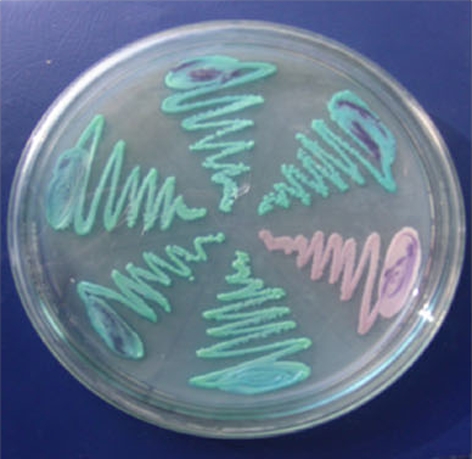
Highchrome agar plate green colour—Candida albicans, brown colour—Candida glabrata
Fig. 2.
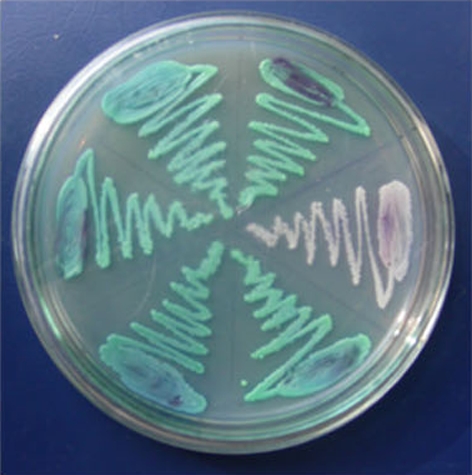
Highchrome agar plate green colour—Candida albicans, white colour—Candida crusi
Table 1.
Comparative analysis of enzymatic activities in Candida spp. isolated from non-HIV and -TB, and HIV and TB patients
| Non-HIV and -TB | HIV and TB | |||||
|---|---|---|---|---|---|---|
| Strains | Enzymes variables | Mean (standard error) | Strains | Enzymes variables | Mean (standard error) | P value |
| C. albicans (n7) | Phospholipase | 0.376 (0.024) | C. albicans (n43) | Phospholipase | 0.480 (0.007) | 0.001 |
| Lipase | 0.760 (0.011) | Lipase | 0.778 (0.005) | NS | ||
| Caseinase | 0.915 (0.011) | Caseinase | 0.931 (0.008) | NS | ||
| Haemolysin | 0.173 (0.008) | Haemolysin | 0.329 (0.007) | 0.001 | ||
| Gelatin | ND | Gelatin | ND | NS | ||
| C. glabrata (n2) | Phospholipase | ND | C. glabrata (n5) | Phospholipase | 0.428 (0.018) | 0.001 |
| Lipase | 0.840 (0.009) | Lipase | 0.747 (0.012) | NS | ||
| Caseinase | 0.941 (0.009) | Caseinase | 1.004 (0.017) | NS | ||
| Haemolysin | 0.165 (0.004) | Haemolysin | 0.282 (0.013) | NS | ||
| Gelatin | ND | Gelatin | ND | NS | ||
| C. krusei (n1) | Phospholipase | ND | C. krusei (n2) | Phospholipase | 0.430 (0.013) | 0.001 |
| Lipase | 0.830 (0.005) | Lipase | 0.813 (0.004) | NS | ||
| Caseinase | 0.970 (0.005) | Caseinase | 0.923 (0.008) | NS | ||
| Haemolysin | 0.146 (0.017) | Haemolysin | 0.301 (0.022) | NS | ||
| Gelatin | ND | Gelatin | ND | NS | ||
ND not detected, n number
Fig. 3.
Phospholipase activity
Fig. 4.
Caseinase activity
Fig. 5.
Gelatinase activity
Fig. 6.
Lipase activity
Fig. 7.
Haemolysin assay
In the resistance profile of Candida spp., C. albicans of HIV and TB group showed resistance to fluconazole (23.5%), Itraconazole (41.1%), ketaconazole (41.1%), nystatin (11.9%) but showed 100% sensitivity towards amphotericin-B (Table 2). The isolates of C. krusei and C. glabrata showed no resistance to any of the tested drugs. In the case of non-HIV and -TB group, C. albicans showed resistance to fluconazole (14.2%), itraconzole (28.5%), ketoconazole (28.5%), nystatin (14.2%), and 100% sensitivity to amphotericin-B. C. krusei and C. glabrata showed no resistance to any of the tested drugs.
Table 2.
Antifungal sensitivity pattern of Candida spp. isolated from non-HIV and -TB, and HIV and TB patients
| Groups | Strains | Antifungal resistance pattern | ||||
|---|---|---|---|---|---|---|
| HIV and TB | Fluconozole (R%) | Itraconozole (R%) | Ketaconazole (R%) | Nystatin (R%) | Amphottericin-B (R%) | |
| C. albicans (n43) | 23.5 | 41.1 | 41.1 | 11.7 | S | |
| C. Glabrata (n5) | S | S | S | S | S | |
| C. krusei (n2) | S | S | S | S | S | |
| Non-HIV and -TB | C. albicans (n7) | 14.2 | 28.5 | 28.5 | 14.2 | 0 |
| C. glabrata (n2) | S | S | S | S | S | |
| C. krusei (n1) | S | S | S | S | S | |
R resistance, S sensitive
Discussion
In the present investigation, 60 strains of Candida isolates were isolated from the oral cavity of HIV and TB patients and non-HIV and -TB patients affected with oral candidiasis. The isolates were characterised based on the colour morphology on HICHROME agar plates (Figs. 1, 2), staining and germ tube method. The species identified by standard methods includes 50 (83.3%) C. albicans, 7 (11.6%) C. glabrata, and 3 (5%) C. krusei. Enzyme analysis and antifungal sensitivity pattern were performed to conclude the virulence and resistance pattern of Candida spp. Secretion of caseinase and lipase showed maximum similarity in strains isolated from both the groups. Phospholipase activities of C. glabrata and C. krusei were similar; it was 100% in HIV and TB positive patients whereas in the non-HIV and -TB patients it was nil. It was found to be higher in C. albicans isolates [17]. Extracellular phospholipases facilitate the ability of Candida to invade the host cells [18]. Phospholipase and protease activity was observed in patients with invasive Candida infection [19].
Protease enzyme as a virulence factor has been studied on substrates such as casein and gelatin [17]. In this study, gelatinolytic activity was found to be nil in case of C. albicans, C. glabrata, and C. krusei in both groups but caseinolytic activity was observed in all of the isolates. Thus, the production of the caseinase may contribute to virulence factor of the Candida isolates. The frequencies of phospholipase and protease in C. albicans were 53.08 and 56.7%, respectively and in non-albicans Candida, 17.07 and 43.9%, respectively. Our results second the view that, of all the hydrolytic enzymes, C. albicans produce highest protease activity [20]. Lipase activity of Candida plays a key role in persistence and virulence in humans [21]. C. albicans showed higher lipase activity in this study and confirmed our findings that C. albicans had higher lipase activity than C. glabrata. Colonization of mucosal surfaces by Candida is considered as a leading risk factor for infection [3]. In this study, all the isolates of Candida spp. showed α-haemolysis (100%), which may contribute to the virulence nature of the isolates. Candida shows hemolytic activity when grown on enriched blood agar [15]. 24 h post inoculation, all strains of the Candida spp. showed only alpha haemolysis surrounding the inoculum sites. Thus, the term alpha and beta haemolysis used here to describe the different patterns of hemolysis in Candida species can only be regarded as descriptive, since the exact nature of these variants and the underlying mechanisms are yet to be explored.[22]. The resistance pattern of HIV and TB positive C. albicans showed resistance towards fluconazole (23.5%), ketoconazole (41.1%), itraconazole (41.1%), and nystatin (11.7%). In the case of non-HIV and -TB positive patients C. albicans showed resistance to fluconazole (14.2%), ketoconazole (39.2%), itraconazole (28.5%), and nystatin (14.2%). C. krusei and C. glabrata of both the groups showed no resistance towards antibiotics tested against them, barring amphotericin-B. Patients with prolonged periods of AIDS are more likely to have received longer periods of antifungal therapy and consequently, related to the emergence of colonization and infection by non-albicans to carry on species or isolates resistance to azoles [23]. Multiple molecular mechanisms may be responsible for the contribution of azole resistance in C. albicans [24]. A study conducted by Kurtaran et al. [25], showed that all the isolates were sensitive to amphotericin-B and that C. albicans and other species were resistant to fluconazole at 4.3 and 15.6%, respectively. Resistance to itraconzole was found to be higher in C. albicans (11.4%) and 26.5% in other species.
Conclusion
Our findings indicate that Candida spp. isolated from HIV and TB (immunocompromised) patients has significantly higher production of phospholipase, caseinase and lipase, than non-HIV and -TB (Immunocompetent) patients. The production of these proteolytic enzymes is responsible for the proliferation and demolition of the host tissues. Alpha haemolytic activity was observable in all the isolates. A statistical significant difference in its production between the groups was also observable. The production of haemolysin in both the groups may be responsible for virulence to the host tissue. If we could find out the nature of the haemolytic factors secreted by Candida spp. it is incredibly uncomplicated to decipher the pathogenic mechanisms to the host. The resistance rate is increasing towards azole drugs by C. albicans of both the groups, so the next drug of choice is amphotericin-B. Thus, it necessitates an urgent need for the proper clinical diagnosis and antifungal therapy for mucosal candidiasis especially in HIV and TB positive individuals. The growing prominence of antifungal drug resistance will probably continue through the decade. The identification of the gene responsible for the colonization may provide novel targets for antifungal prophylaxis.
Acknowledgments
The authors greatly acknowledge Dr. Sys Reetha MD, (Italy) and Dr. M. Santhosh Kumar, Holy Hanchanorium Community Care Centre, Trichy, and Dr. Shanmugasundram, District Project Manager, AIDS Control Board, Trichy.
References
- 1.Chung YL, Emily CDS, Heng FS, Rozita R, Kee PN, Pei PC. Increased expression and hot spot mutations of the multidrug efflux transporter, CDR1 in azole-resistant Candida albicans isolates from vaginitis patients. FEMS Microbiol Lett. 2005;249:283–289. doi: 10.1016/j.femsle.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 2.Calderone RA. Candida and candidiasis. Washington: ASM Press; 2002. [Google Scholar]
- 3.Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immuno suppressed patients. Lancet Infect Dis. 2003;3:685–702. doi: 10.1016/S1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 4.Jain SK, Agarwal RL, Sharma DA, Agarwal MM. Candida in pulmonary tuberculosis. J Postgrad Med. 1982;19:24–28. [PubMed] [Google Scholar]
- 5.Walia K. Current issues in HIV/TB co-infection. Indian J Tub. 2002;49:21. [Google Scholar]
- 6.Naglik J, Albrecht A, Bader O, Hube B. Candida albicans proteinases and host/pathogen interactions. Cell Microbiol. 2004;6:915–926. doi: 10.1111/j.1462-5822.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- 7.Belanger M, Begin C, Jacques M. Ipopolysaccharides of Actinobacillus pleuropneumoniae bind pig hemoglobin. Infect Immun. 1995;63:656–662. doi: 10.1128/iai.63.2.656-662.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boerlin P, Berlin-Petzold F, Durussel C, Addo M, Pagani JL, Chave JP, Bille J. Cluster of oral atypical Candida albicans isolates in a group of human immunodefiency virus-positive drug users. J Clin Microbial. 1995;33:1129–1135. doi: 10.1128/jcm.33.5.1129-1135.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perea S, Patterson TF. Antifungal resistance in pathogenic fungi. Clin Infect Dis. 2002;35:1073–1080. doi: 10.1086/344058. [DOI] [PubMed] [Google Scholar]
- 10.Kreger-Van Rij NJW, editor. The yeasts a taxonomic study (1982) Amsterdam: Elsevier Science Publishers; 1984. [Google Scholar]
- 11.Price MF, Wilkinson ID, Gentry LO. Plate method for the detection of phospholipase activity in Candidaalbicans. Sabouraudia. 1982;20:7–14. doi: 10.1080/00362178285380031. [DOI] [PubMed] [Google Scholar]
- 12.Doroti OG, Jorge T, Marina BM, Wldemar F, Sinto S, Roberto MY. Proteases (caseinase, elastinase), hemolysins, adhesion and susceptibility to antimicrobials of Stenotrophomonas maltophilia isolates obtained from clinical specimens. J Microbiol. 2002;33:157–162. [Google Scholar]
- 13.Jacob MB, Gerstein MJ. Hand book of microbiology. Princeton: D. Van Nostrand Co. Inc.; 1960. p. 61. [Google Scholar]
- 14.Aneeja KR. Experiments in microbiology, plant pathology, tissue culture and mushroom cultivation. II. Delhi: New Age International Publishers; 1996. [Google Scholar]
- 15.Manns JM, Mosser DM, Buckley HR. Production of haemolytic factor by Candida albicans. Infect Immun. 1994;62:5154–5156. doi: 10.1128/iai.62.11.5154-5156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LS NCC. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi: proposed standards. NCCLS document M38-A. Wayne: National Committee for Clinical Laboratory Standards; 2002. [Google Scholar]
- 17.Sukru O, Idris S, Mustafa Y, Aynur G, Tevfik Y, Demet K, Ayse NK. Phospholipase and proteinase activities in different Candida species isolated from anatomically distinct sites of healthy adults. Jpn J Infect Dis. 2007;60:280–283. [PubMed] [Google Scholar]
- 18.Silverman DJ, Santucci LA, Meyers N, Sekeyova Z. Penetration of host cells by Rickettsia rickettsii appears to be mediated by a phospholipase of rickettsial origin. Infect Immun. 1992;60:2733–2740. doi: 10.1128/iai.60.7.2733-2740.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantarcioglu AS, Yucel A. Phospholipase and protease activities in Candida isolates with reference to the sources of strains. Mycoses. 2002;45:160–165. doi: 10.1046/j.1439-0507.2002.00727.x. [DOI] [PubMed] [Google Scholar]
- 20.Kusmarinah B, Masashi Y, Ryoji T, Hideoki O. Comparison of proteinase, lipase and alpha glucosidase activities from the clinical isolates of Candida species. Jpn J Infect Dis. 2006;59(2):73–76. [PubMed] [Google Scholar]
- 21.Martin S, Borelli C, Korting HC, Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48:365–37719. doi: 10.1111/j.1439-0507.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 22.Gang L, Lakshman P, Samaranayake LP, Joyce Y, Yau Y. Candida species exhibit differential in vitro hemolytic activities. J Clin Microbiol. 2001;39:2971–2974. doi: 10.1128/JCM.39.8.2971-2974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eveline PM, Marcelo NB, Esper GK, Olga F, Paulo R, Costa O, Arnaldo LC. Azole resistance among oral Candida species isolates from aids patients under ketoconazole exposure. Diagn Microbiol Infect Dis. 1998;2:211–216. doi: 10.1016/s0732-8893(98)00107-2. [DOI] [PubMed] [Google Scholar]
- 24.Jana C. Resistance mechanisms in fluconazole-resistant Candida albicans isolates from vaginal candidiasis. Int J Antimicrob Agents. 2006;29(2):117–232. doi: 10.1016/j.ijantimicag.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Kurtaran B, Inal AS, Kibar F, Yaman A, Tasova Y, Saltoglu N, Aksu HSZ (2005) The epidemiology and antifungal sensitivity results of nosocomial infections caused by Candida species. 15th congress of clinical microbiology and infectious diseases. Copenhagen, Denmark, 2–5 April 2005