Abstract
The objective of this study was to investigate the effects of mangrove tea on salivary bacterial flora in DMBA induced hamster buccal pouch carcinoma. Tea from mangrove plant Ceriops decandra was administered against DMBA induced buccal pouch carcinoma in hamster rats. The chemical constitutions and quality of mangrove tea is similar with the commercial tea Camellia sinensis. The Hamster rats were painted thrice a week with DMBA in their right buccal pouch, and also administrated orally with 1.25% of Ceriops tea extract, on alternate days of the DMBA treatment. Appropriate control animals were maintained. After 14 weeks of treatment, bacterial species in saliva were enumerated, tumor incidences were analyzed using histopathological section and tumor volume in the animals was quantified using water-displaced method. The decreased counts of beneficial bacteria and increased counts of harmful bacteria were associated with increased volume of tumors. The present study concluded that the tea extract from C. decandra prevents the oral cancer incidences and maintain the good health conditions of the animals.
Keywords: Beneficial bacteria, Harmful bacteria, Oral cancer, Ceriops decandra, Mangrove tea, DMBA
Introduction
Oral cancer is the sixth most common malignancy worldwide [1] and is particularly prevalent in developing countries, such as in Southeast Asia, where up to 40% of all malignancies are located within the oral cavity [2]. More than 90% of cancers in mouth are squamous cell carcinomas (SCCs), originating from the oral mucosa [3]. With an average of all stage, 5-year survival rate for oral cancer of less than 50%, the annual mortality figures are comparable to those of carcinoma of the cervix and malignant melanoma [4, 5]. Bacteria are known to associate with cancer tissues. Nagy et al. [6] have demonstrated a difference in the microflora associated with the surface of tumors in comparison to control sites. Patients with oral cancer tend to possess significantly low concentration of beneficial bacteria in their saliva. This is of particular interest because of its potential application as a diagnostic tool to predict oral cancer [7]. Tea that contains many antioxidants is a pleasant and safe drink that is enjoyed by people across the globe. Tea leaves are manufactured as black, green, or oolong. Black tea represents ~78% of total consumed tea in the world, whereas green tea accounts for ~20% of tea consumed. The concept of “use of tea for promotion of human health and prevention and cure of diseases” has become a subject of intense research in the last decade. The health benefits of tea are ranging from a lower risk of certain cancers to weight loss, and protection against infections, like bacterial and viral, to chronic debilitating diseases, including cancer, coronary heart disease, stroke, and osteoporosis. [8]. the mangrove plant Ceriops decandra has traditionally [9, 10] and scientifically rich in medicinal values [11–13]. Our research team attempted to extract black tea from a mangrove species Ceriops decandra which contains large amount of theaflavin (giving flavour) and theambrugin (neurostimulant) [13]. These compounds are produced when polyphenols (present in cytoplasms) are allowed to ferment by polyphenol oxidase (present in cell vacuoles). The tea had no toxicity in mice, and had a better quality than commercial teas, as evident by sensory evaluation tests performed with our centre people. The present study was to determine the effect of mangrove tea on the bacterial flora which associated with saliva of DMBA induced hamster buccal pouch carcinoma.
Materials and Methods
Collection and Preparation of Plant Material
Leaves of the mangrove plant species, Ceriops decandra were collected from the forest of Pichavaram (11° 27′ N; 79° 47′ E) situated in south east coast of India. The specimen was identified and its holotype (No. R90) has been deposited in the herbarium of the Centre of Advanced Study in Marine Biology, Annamalai University, Parangipettai, Tamil Nadu, India. The leaf sample was washed with tap water to remove epiphytes and other external matter.
Preparation of Black Tea Extract
Black tea was extracted from the leaves of Ceriops decandra, adopting the method of [14]. Fresh leaves were spread on a trough and allowed to dry using a warming blender. A known weight of the macerated sample was placed in a piece of cloth and distilled water was continuously trickled over it for 1 h. The fermented sample was dried in a hot air oven at 95°C for 30 min. The tea extract was freshly prepared every day using 1.25 g of Ceriops tea powder in 100 ml boiled water.
Chemicals Used for the Study
Carcinogen Dimethylbenz[a]anthracene (DMBA) was purchased from Sigma Chemical Company, USA and used for this study. All other reagents used were of analytical grade.
Experimental Animals Used
The Syrian hamster cheek pouch epithelium has been a valuable model in studies of chemical carcinogens interactions with oral tissues [15, 16]. Therefore, male Syrian hamsters were used as experimental animals with an age of 8–10 weeks weighing 85–90 g obtained from the National Institute of Nutrition Hyderabad, India. The animals were housed at six per polypropylene cage and provided with standard pellet diet (Mysore Snack Feed Ltd., Mysore, India) and water ad libitum. The animals were maintained at a temperature of 28 ± 2°C with an alternating 12-h light/12-h dark cycle. The animals were maintained as per the norms provided by the Bioethic Committee of Annamalai University (IAEC/CPCSEA 270 Dated 01. 07.2005).
Experimental Design
The animals were randomized into experimental, control groups, and divided into four groups of six animals each. Animals in group I was treated as untreated control. The animals in group II were painted with a 0.5% solution of DMBA in liquid paraffin on the right buccal pouch using a no. 4 brush three times a week for 14 weeks. Each application treated approximately 0.4 mg of DMBA. Group III animals were painted with DMBA as in group II; In addition, the animals were administered with 1.25% of freshly prepared mangrove tea extract twice per day. Group IV animals received the same dose of mangrove tea extract alone. The experiment was terminated at the end of 14th week and animals were sacrificed by cervical dislocation after an over night fast and the fresh tissue were used for estimations.
Isolation and Identification of Bacteria from Saliva
Saliva samples were collected from the buccal region of the animal using sterile Whatman filter paper discs (with a diameter of 5 mm). The sample was serially diluted and plated on culture media deMan Rogosa Sharpe (MRS), streptococcus selection agar (SSA) and modified milk agar and incubated at 37°C for 36 h. All the determinations were carried out in triplicate. The counts are expressed as colony forming unit (CFU) per ml of the sample. Identification was done by following the keys of Bergy’s manual of determinative bacteriology [17, 18].
Histopathological Observations
The specimens were maintained in 10% formalin solution for processing. Embedded in paraffin, the specimens were sectioned and examined under a microscope at 40× magnification, after staining with hematoxylin and eosin.
Statistical Analysis
One-way ANOVA and Duncan multiple range tests were used to compare mean values at 0.05 probabilities.
Results
Cancer Incidences
Tea from mangrove plant Ceriops decandra effectively prevented the DMBA induced carcinogenesis. The DMBA treated animals showed 100% squamous cell carcinoma where as the tea extract treated animals showed hyperplasia alone. We did not observed any cancer incidences in both untreated control and tea extract alone treated animals.
Enumeration of Bacteria in Saliva
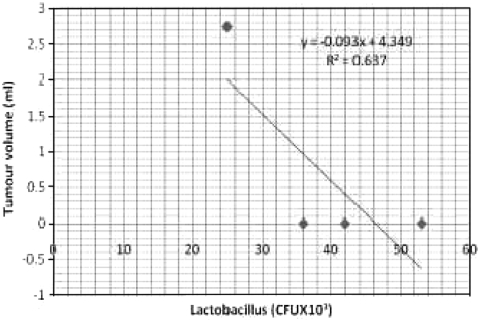
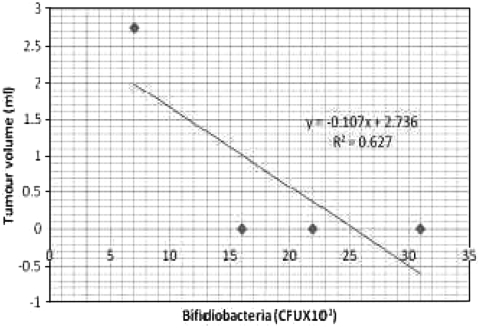
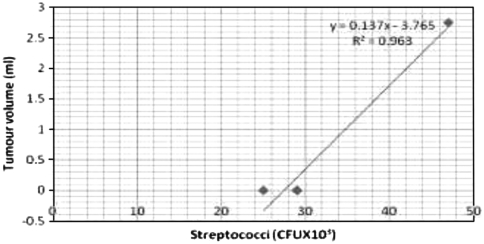
Bacterial counts in the hamsters induced with oral carcinoma are given in the Table 1. Animals in control group exhibited high counts of lactobacilli (42 ± 11 × 103 CFU ml-1) followed by streptococci (29 ± 17 × 103 CFU ml-1) and bifidobacteria (22 ± 17 × 103 CFU ml-1). The tea extract alone treated animals were also rich in lactobacilli and bifidobacterial counts (53 ± 18 × 103, 31 ± 15 × 103 CFU ml-1 respectively) and reduced counts of streptococci (25 ± 13 × 103 CFU ml-1). The DMBA treated groups of animals exhibited low counts of lactobacilli (25 ± 10 × 103 CFU ml-1) and bifidobacteria (07 ± 1.0 × 103 CFU ml-1) however, the animals showed high counts of streptococci (47 ± 17 × 103 CFU ml-1). Whereas the tea extract in the DMBA-treated animals showed increased counts of lactobacilli and bifidobacteria (37 ± 10 × 103; 17 ± 1.1 × 103 CFU ml-1 respectively) and decreased counts of streptococci (32 ± 15 × 103 CFU ml-1). Counts of lactobacilli and bifidobacteria were negatively correlated with tumor size, whereas streptococcus was positively correlated to tumor size (Figs. 1, 2, 3). The ratio between beneficial and harmful bacteria of 0.68 coincided with the incidence of tumor, whereas there was no tumor incidence when the ratio was equal or greater than 1.6 (Table 5).
Table 1.
Effect of tea extract of C. decandra on salivary bacterial counts and tumour volume of experimental animals
| Treatment | Lactobacillus (×103 CFU ml−1) | Bifidobacteria (×103 CFU ml−1) | Streptococci (×103 CFUml−1) | Tumour volume (ml) |
|---|---|---|---|---|
| Control | 42 ± 11a | 22 ± 05a | 29 ± 17a | 0.00a |
| DMBA | 25 ± 10b | 07 ± 1.0b | 47 ± 17b | 2.45 ± 0.2b |
| DMBA+ Tea extract | 36 ± 9c | 16 ± 1.1c | 29 ± 11a | 0.00a |
| Tea extracts alone (1.25%) | 53 ± 18d | 31 ± 18d | 25 ± 13a | 0.00a |
Values are mean ± standard error from three replicates in each group of animals maintained with six each
Values not sharing a common superscript are differ significantly at P > 0.05
Fig. 1.
Correlation between Lactobacilli (CFU × 103.ml−1) count and tumour volume
Fig. 2.
Correlation between Bifidiobacteria (CFU × 103.ml−1) count and tumour volume
Fig. 3.
Correlation between Streptococcus (CFU × 103.ml−1) count and tumour volume.
Table 5.
Ratio between beneficial (lactobacilli, bifidobacteria) and harmful (streptococcus) bacteria and incidence of tumour volume in buccal pouch of Hamsters
| Treatment | Ratio between beneficial and harmful bacteria(CFU ×103.ml−1) | Tumor volume (ml) |
|---|---|---|
| Control | 1.93 ± 0.15a | 0.00a |
| DMBA | 0.68 ± 0.05b | 2.45 ± 0.2b |
| DMBA+ Tea extract (1.25%) | 1.60 ± 0.13a | 0.00a |
| Tea extracts alone (1.25%) | 3.14 ± 0.28a | 0.00a |
Values not sharing a common superscript are differ significantly at P > 0.05
Bacterial Species in Saliva
Bacterial species were identified in saliva based on morphological and biochemical characteristics as shown in Table 2. There were five species of lactobacilli (Lactobacillus acidophilus, L. lactis, L. jensenii, L. casei and L. brevis), two species of bifidobacteria (Bifidobcterium bifidium and B. longum) and two species of streptococci (Streptococcus mutan and S. mitis) identified. The characteristics of the bacterial species are given in Tables 2, 3, 4.
Table 2.
Biochemical reactions of the species of genus Lactobacillus
| Biochemical test | Species of lactobacilli | ||||
|---|---|---|---|---|---|
| L. acidophilus | L. lactis | L. casei | L. jensenii | L. brevis | |
| Fructose | + | + | + | + | + |
| Galactose | + | + | + | + | + |
| Glucose (acid) | + | + | + | + | + |
| Glucose (gas) | − | − | − | − | + |
| Gluconate | − | − | + | − | + |
| Lactose | + | + | − | − | + |
| Maltose | + | − | d | + | + |
| Mannitol | − | − | + | − | + |
| Melizitose | − | − | + | − | − |
| Melibiose | − | − | − | − | + |
| Raffinose | − | − | − | − | − |
| Ribose | − | − | + | − | − |
| Xylose | − | − | − | − | d |
| Esculin | + | − | + | − | d |
| Oxidase | − | − | − | − | − |
| Catalase | − | − | − | − | − |
| Gelatin liquified | + | + | + | + | + |
| Nitrate reduction | − | − | − | − | − |
| Casein hydrolysis | − | − | − | − | − |
| Indole | − | − | − | − | − |
| H2S | − | − | − | − | − |
+ Positive,− Negative, d Variable
Table 3.
Carbohydrate reactions of the species of genus Bifidobacterium
| Species | Sugars | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Arabinose | Xylose | Ribose | Glucose | Cellulose | Lactose | Mannitol | Melibiose | Starch | |
| Bifidobacterium bifidium | − | − | − | − | + | + | − | − | − |
| Bifidobacterium longum | + | + | + | − | − | + | − | + | − |
Table 4.
Some important tests used to differentiate Streptococcus species
| Species | Growth | Hae | Raf | Suc | Inu | ||||
|---|---|---|---|---|---|---|---|---|---|
| 10°C | 45°C | 2% NaCl | 6.5% NaCl | pH 9.5 | |||||
| S. mitis | − | + | + | − | − | D | + | + | + |
| S. mutans | − | − | + | − | − | D | − | − | − |
Hae Haemolysis, Raf Raffinose, Suc Sucrose, Inu Inulin
Histological Observations
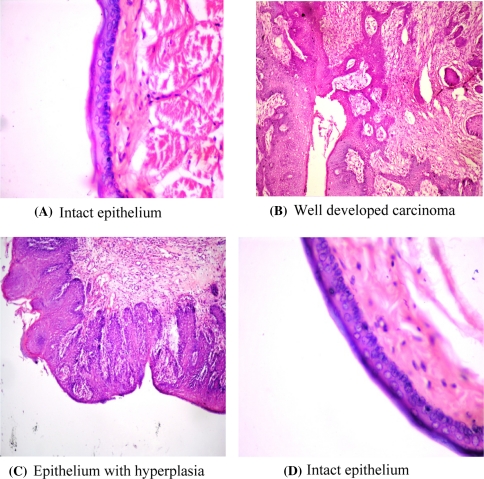
The tumour formation is evident by histological observations. There was well developed squamous cell carcinoma, along with well-defined epithelial and keratin pearls in the connective tissue with cellular pleomorphism (Fig. 4). However, the animals treated with DMBA + mangrove tea extract, exhibited only hyperplasia. The histological studies proved the anti-cancer effect of the mangrove tea extract at the dose of 1.25% twice per day. No pathological observations were noted either in control animals or the animals treated with mangrove tea extract alone. Fig. 4a, d show the histological structure of mucosa extracted from control and mangrove tea extract alone treated animals which are showing normal and intact epithelium. Fig. 4b exhibits the abnormal epithelium intruded into the connective tissue leading to the formation of spherical pearl’ like structures in the mucosa extracted from DMBA–treated animals. Fig. 4c exhibits only multi layer of epithelial cells (hyperplasia) in the mucosa of animal treated with both DMBA + mangrove tea extract. Here there is no abnormal intrusion of epithelium and formation of pearl like structures.
Fig. 4.
Histological changes in the mucus tissue of Hamster buccal pouch. Untreated control (A), treated with DMBA (B) treated with DMBA + mangrove tea (C) and mangrove tea alone (D) (magnification 40×)
Discussions
The results of present study indicated that the tea extract from Ceriops decandra effectively prevented the DMBA induced carcinogenesis. The available salivary bacterial species of the test animals clearly indicated the health status of the host animals. The predominant types of bacteria isolated from the saliva, tongue, dorsum and buccal mucosa of rates were Streptococcus spp., Lactobacillus spp., and Bifidobacteria [19]. Therefore these three types of bacteria were studied in the present investigation. Lactic acid bacteria and bifidobacteria are two well-known groups of beneficial bacteria which constitute an integral part of the health condition. They impart nutritional and therapeutic benefits to their host. The vitamins and enzymes produced by the lactic acid bacteria contribute to host metabolism. The antimicrobial substances produced by these bacteria control the proliferation of undesired pathogens. Lactic acid bacteria produce a soluble compound which may interact directly with oral tumor cells in culture and inhibit their growth [20]. Singh et al. [21] have observed that Bifidobacterim longum exerts a strong anti-tumor activity against colon cancer. Data from epidemiological and experimental studies indicate that ingestion of lactobacilli and bifidobacteria and their fermented products reduce the risk of certain types of cancer and inhibit tumor growth [22, 23]. In the present work, the counts of lactobacilli and bifidobacteria were high in normal and tea extract treated animals when compared to streptococci which were low in number (Table 1).
Pathogenic forms of oral cavity bacteria have recently been known to associate with cancer tissues. The development of illness such as cancer is associated with significant shifts in the number of gram negative bacteria detectable in oral samples [24]. Nagy et al. [6] have demonstrated a difference in the micro-flora associated with the surface of tumors in comparison to control. Patients with oral cancer tend to possess significantly high concentration of certain bacteria in their saliva. Many bacterial species have been similarly tested for their carcinogenic potential in monkeys, rats, hamsters, rodents and mice. The most carcinogenic bacteria are streptococci including Streptococcus mutans, S. sobrinus, S. cricetus, and S. rattus; and other carcinogenic bacterial species include Actinomyces naeslundii, (formerly A. viscosus), S. salivarius, S. sanguis, and Entrococcus faecalis [15]. The mutants of streptococcus (S. cricetus) are known to cause “caries” [25]. The present study recorded high counts of streptococci in oral system of tumor bearing Hamsters (Table 1). The contribution of probiotic bacteria, such as lactobacilli and bifidobacteria are mainly in the control of pathogenic microbes, that cause various diseases through production of antibacterial protein namely bacteriocin [26, 27] and anti-cancer substances [28]. The dietary supplements of lactobacilli are reportedly decreased the induction of experimental colon cancer [29]. They stimulate and modulate the mucosal immune system by reducing the production of pro-inflammatory cytokines through actions on NFκB pathways, increasing production of anti-inflammatory cytokines such as IL-10 and host defense peptides such as β-defensin 2, enhancing IgA defenses and influencing dendritic cell maturation. Modulation of cell proliferation and apoptosis through cell responses to, for example, microbially produced short chain fatty acids [30]. The present study expected the DMBA induced oral cancer could change the chemistry of the mouth, allowing the bacteria to flourish. It may be due to the cellular leakage of cancer tissues. The types of bacteria present in the saliva are depending upon the conditions of the saliva. The alkaline condition of the saliva was observed in DMBA treated tumor bearing animals, whereas acetic and near neutral pH was observed in DMBA + tea treated animals. So the lactobacillus and bifidiobacteria cannot survive in the tumor bearing animals as they grow in acetic condition. It is inferred that the reduction in the beneficial bacterial counts increased the pathogenic bacterial counts in buccal pouch of Hamsters, and this situation made the animal weak and susceptible to incidence of tumor (Tables 1 and 5). The changes in bacterial flora are not the direct effects of DMBA treatment as proved by anti microbial method using disk diffusion method. DMBA showed no antimicrobial activity (data not shown). Therefore the DMBA effects on bacterial flora are indirect perhaps through metabolic changes induced in the test animals.
Acknowledgments
The authors are thankful to the authorities of Annamalai University for having provided with facilities.
References
- 1.Sugerman PB, Joseph BK, Savage NW. The role of oncogenes, tumor suppressor genes and growth factors in oral squamous cell carcinoma: a case of apoptosis versus proliferation. Oral Dis. 1995;1:172–188. doi: 10.1111/j.1601-0825.1995.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues VC, Moss SM, Tuomainen H. Oral cancer in the UK: to screen or not to screen. Oral Oncol. 1998;34:454–465. doi: 10.1016/S1368-8375(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 3.Chen AY, Myers JN. Cancer of the oral cavity. Dis Mon. 2001;47:274–361. doi: 10.1016/S0011-5029(01)90005-7. [DOI] [PubMed] [Google Scholar]
- 4.Brown AE, Langdon JD. Management of oral cancer. Ann R Coll Surg Engl. 1995;77:404–408. [PMC free article] [PubMed] [Google Scholar]
- 5.Zakrzewska JM. Fortnightly review: oral cancer. BMJ. 1999;318:1051–1054. doi: 10.1136/bmj.318.7190.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagy KN, Sondoki I, Szoke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34:304–308. [PubMed] [Google Scholar]
- 7.Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3:1–8. doi: 10.1186/1479-5876-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman M, Mackey BE, Kim HJ. Structure-activity relationships of tea compounds against human cancer cells. J Agric Food Chem. 2007;55:243–253. doi: 10.1021/jf062276h. [DOI] [PubMed] [Google Scholar]
- 9.Bandaranayake WM. Traditional medicinal uses of mangroves. Mang Salt Mars. 1998;2:133–148. doi: 10.1023/A:1009988607044. [DOI] [Google Scholar]
- 10.Bandaranayake WM. Bioactivities: bioactive compounds and chemical constituents of mangrove plants. Wetl Ecol Manag. 2002;10:421–452. doi: 10.1023/A:1021397624349. [DOI] [Google Scholar]
- 11.Padmakumar K (1988) Bioactive substances from marine algae and mangroves. Ph.D Thesis, Annamalai University, Parangipettai p. 81
- 12.Sakagami H, Kashimita M, Toguchi M, Satoh K, Odanaka Y, Ida Y, Premanathan M, Arakaki R, Kathiresan K, Nakashma H, Komatsu N, Fujimarki M, Yoshihara M. Radical modulation activity of lignins from a mangrove plant, Ceriops decandra (Griff.) Ding Hou In vivo. 1998;12:327–332. [PubMed] [Google Scholar]
- 13.Kathiresan K, Veera Ravi A. Seasonal changes in tannin content of mangrove leaves. Ind For. 1990;116:390–392. [Google Scholar]
- 14.Kathiresan K. Studies on tea from mangrove leaves. Environ Ecol. 1995;13:321–323. [Google Scholar]
- 15.Lurie AD, Nintzal BB, Rippey RM. Vascular volume and perfusion in hamster cheek pouches. Can Res. 1977;37:3484–3489. [PubMed] [Google Scholar]
- 16.Shklar G. Experimental oral pathology in the Syrian hamster. Prog Exp Tumor Res. 1972;16:518–538. doi: 10.1159/000393387. [DOI] [PubMed] [Google Scholar]
- 17.Holt JG, Krieg RN, Sneath PHA, Staley JT, Williams ST. Bergey’s Manual System. Bacteriology. 1983;4:440. [Google Scholar]
- 18.Oliver JD. Instruments and methods: taxonomic scheme for the identification of marine bacteria. Deep Sea Res. 1982;29:795–798. doi: 10.1016/0198-0149(82)90007-3. [DOI] [Google Scholar]
- 19.Zeldow BJ. Studies on the antibacterial action of human saliva: II observations on the mode of action of a lactobacillus bacteriocin. J Dent Res. 1961;40:446–453. doi: 10.1177/00220345610400031001. [DOI] [Google Scholar]
- 20.Hirayama K, Rafter J. The role of probiotic bacteria in cancer prevention. Micro Infec. 2000;2:681–686. doi: 10.1016/S1286-4579(00)00357-9. [DOI] [PubMed] [Google Scholar]
- 21.Singh J, Rivenson A, Tomita M, Shimamura S, Ishibashi N, Reddy BS. Bifidobacterium longum, a lactic acid-producing intestinal bacterium inhibits colon cancer and modulates the intermediate biomarkers of colon carcinogenesis. Carcinogenesis. 1997;4:833–841. doi: 10.1093/carcin/18.4.833. [DOI] [PubMed] [Google Scholar]
- 22.Bodana AR, Rao DR. Antimutagenic activity of milk fermented by Streptococcus thermophilus and Lactobacillus bulgaricus. J Dairy Sci. 1990;73:3379. doi: 10.3168/jds.S0022-0302(90)79033-9. [DOI] [PubMed] [Google Scholar]
- 23.Mitsuoka T. Bifidobacteria and their role in human health. J Ind Microbiol. 1990;6:263–267. doi: 10.1007/BF01575871. [DOI] [Google Scholar]
- 24.Spijkervet FKL, Saene JJ, Saene HK, Panders AK, Vermey A, Feilder V.Chlorohexidine inactivation by saliva Oral Surg Oral Med Oral Pathol 199069437–444. 10.1016/0030-4220(90)90377-52326035 [DOI] [Google Scholar]
- 25.Keyes PH. The infectious and transmissible nature of experimental dental caries. Findings and implications. Arch Oral Biol. 1960;1:304–320. doi: 10.1016/0003-9969(60)90091-1. [DOI] [PubMed] [Google Scholar]
- 26.DeVugst L, Vandamme EJ. Bacteriocins of lactic acid bacteria. Microbiol Genet Appl London. 1994;75:140174–140179. [Google Scholar]
- 27.Kathiresan K, Thiruneelakandan G. Prospects of lactic acid bacteria of marine origin. Ind J Biotech. 2008;7:170–177. [Google Scholar]
- 28.Wollowski I, Rechkemmer G, Pool-Zobel BL. Protective role of probiotics and prebiotics in colon cancer. Am J Clin Nutri. 2001;73:451–455. doi: 10.1093/ajcn/73.2.451s. [DOI] [PubMed] [Google Scholar]
- 29.Goldin BR, Gorbach SL. Probiotics for humans. In: Fuller R, editor. Probiotics. London: Champ Hall; 1992. pp. 355–376. [Google Scholar]
- 30.Devine DA, Marsh PD. Prospects for the development of probiotics and prebiotics for oral applications. J Oral Microbiol. 2009;1:1–11. doi: 10.3402/jom.v1i0.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]