Abstract
Parasite infections are common during the critical developmental period in children. The occurrences of intestinal parasites are also common in orphanage, nurseries and schools in Turkey. The study was carried out to determine the percentage of microsporidium and intestinal parasites in children from Malatya, Turkey. This study was carried out at the Department of Parasitology of Inonu University, Turgut Ozal Medical Center, during January–December 2006. Totally, 1,181 stool samples were examined using the native-Lugol, sedimentation-techniques, modified trichrome (MTS), acid-fast-trichrome stain and calcofluor staining methods. In addition, perianal region material was taken from the children to examine with cellophane tape method. Power analyses were performed for statistical analyses used. Microsporidia were found in 92 (7.8%) of the samples, and also intestinal parasites were detected in 329 (27.8%). The numbers of infections according to the species were as follows: 69 (5.8%) Entamoeba coli, 7 (0.6%) Blastocystis hominis, 114 (9.7%) Giardia intestinalis, 15 (1.3%) Iodomoeba butchlii, 8 (0.7%) Dientamoeba fragilis, 7 (0.6%) Taenia spp. 70 (5.9%) Enterobius vermicularis, 11 (0.9%) Hymenolepis nana, 25 (2.1%) Trichomonas intestinalis, 1 (0.1%) Ascaris lumbricoides and 2 (0.2%) Chilomastix mesnilii. Also, greater than 90% power values were achieved for statistical analyses. Whereas the detection rates of microsporidium and intestinal parasites were found to be low, it was concluded that in addition to intestinal parasites, microsporidium should be also searched for in children with complaints of intestinal system.
Keywords: Children, Microsporidia, Intestinal parasites
Introduction
Microsporidia cause infections in people, depending on which species cause the infection. It may proceed to heal spontaneously. People with a normal immune system typically have no symptoms, but it may induce species associated with clinical symptoms including diarrhea, abdominal pain, muscle aches, eye irritation and cornea ulcers [1, 2].
Primary site of Enterocytozoon bieneusi infection in humans is usually limited to the enterocytes of the small intestine. Intestinal E. bieneusi causes chronic diarrhea which have milder course than cryptosporidial diarrhea. Common symptoms of intestinal microsporidiosis include chronic diarrhea, anorexia and weight loss. The diarrhea is characterized as watery stool without blood and leukocytes. E. bieneusi infections are associated with an increase in frequency of defecation, greater than 20 times passing stool per day. In addition, E. bieneusi infections may also associate with abdominal pain, nausea, vomiting, fewer and malnutrition [3–6]. Infections with Encephalitozoon species have overlapping gastrointestinal clinical symptoms with E. bieneusi, which includes diarrhea, malabsorption and weight loss [18].
The prevalence of intestinal microsporidiosis is not well known yet. Bretagne et al. [7] conducted a study in Ugandan children with diarrhea for a longer period than 72 h using polymerase chain reaction (PCR). They reported prevalence for E. bieneusi of 17.4%. Termmathurapoj et al. [8] reported prevalence for Microsporidia of 1.3% in children in Thailand, detected by calcofluor and Gram-chromotrope stain.
To our knowledge, no previous studies have been conducted on the prevalence of E. bieneusi in Turkey. However, microsporidia have been detected by Yazar et al. [9] in a cancer patient and by Buget et al. [10] in an AIDS patient. Additionally, some studies have also been reported on Nosema apis of honey bees by Ozkirim and Keskin [11], Encephalitozoon cuniculi infection in rabbit colonies by Eroksuz et al. [12], and Nosema in Coleoptera by Yaman and Radek [13].
The aim of this study was to determine the rates of microsporidia and other intestinal parasites in children in Malatya, Turkey. Totally, 1,181 stool samples were examined using the native-Lugol, sedimentation techniques, modified trichrome, acid-fast-trichrome stain and calcofluor staining methods. In addition, perianal region material will be taken from the children to examine with cellophane tape method.
Materials and Methods
This study was carried out at the Department of Parasitology of Inonu University, Turgut Ozal Medical Center, during January–December 2006. The protocol of this study was approved by the Institutional Ethics Committee. Children admitted in our hospital with complaints of gastrointestinal system associated with no underlying immunosuppression or immunodeficiency disorders were included in this study. Following obtaining the informed consent, the patient’s full history and complaints were taken.
Stool specimens were collected from a total of 1,181 children. The specimens were coded. Then, the specimens were assessed for the presence of microsporidia and intestinal parasites by using the native-lugol, sedimentation-techniques, modified trichrome (MTS), acid-fast-trichrome stain and calcofluor staining methods. Additionally, perianal region material was taken from all children to examine with cellophane tape method. In addition to methods of sedimentation and the native-lugol, cellophane tape method was also used for the purpose of diagnosis of helminthes since cellophane tape method is used for the diagnosis of helminths, especially Enterobius vermicularis.
Statistical Analysis
In the present study, normality assumption was tested by Kolmogorov–Smirnov Z test for parametric independent samples t test. Statistical evaluations were carried out by independent samples t test, Pearson χ2 and Yates’ corrected χ2 tests as appropriate [14]. Greater than 90% power values were achieved for statistical analyses used. Power analyses were performed by NCSS for the tests. P < 0.05 values were considered statistically significant.
Results
The mean age of the children was 8.13 ± 3.95 years, of which 557 (47.2%) male and 624 (52.8%) female. The mean ages of the children with or without Microsporidia were 8.63 ± 3.84 year (n = 92) and 8.09 ± 3.95 (n = 1,089) year, respectively, and no significant difference was found (P > 0.05). Microsporidia were detected in 92 (7.8%) of the samples (Figs. 1, 2). Also, intestinal parasites other than microsporidia were detected in 329 (27.8%) of the samples from patients. The overall infection rates of intestinal parasite according to the species were as follows: 69 (5.8%) Entamoeba coli, 7 (0.6%) Blastocystis hominis, 114 (9.7%) Giardia intestinalis, 15 (1.3%) Iodomoeba butchlii, 8 (0.7%) Dientamoeba fragilis, 7 (0.6%) Taenia spp., 70 (5.9%) Enterobius vermicularis, 11 (0.9%) Hymenolepis nana, 25 (2.1%) Trichomonas intestinalis, 1(0.1%) Ascaris lumbricoides and 2 (0.2%) Chilomastix mesnilii. Table 1 summarizes these results in correspondence with the prevalence of every parasite species.
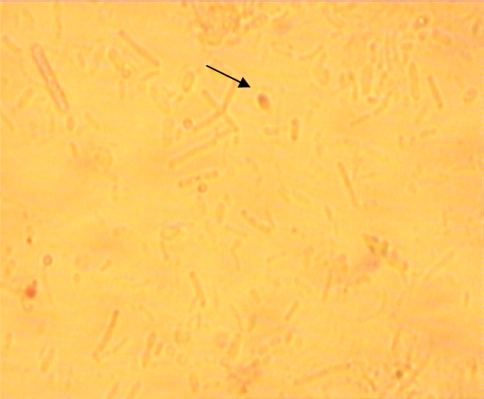
Fig. 1.
Microsporidia spores in positive sample ×100 (modified trichrome—MTS)
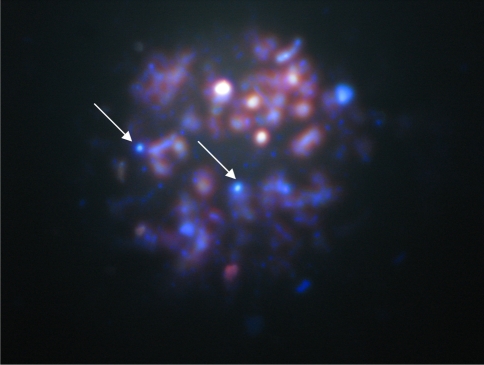
Fig. 2.
Microsporidia spores in positive sample ×100 (calcofluor)
Table 1.
Prevalence of infectivity with different species of intestinal parasites in 1,181 children of Malatya, eastern Turkey (January–December 2006)
| Parasite | Non-infected | Infected | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Microsporidia | 1,089 | 92.2 | 92 | 7.8 | 1,181 | 100 |
| Entamoeba coli | 1,112 | 94.2 | 69 | 5.8 | 1,181 | 100 |
| Blastocystis hominis | 1,174 | 99.4 | 7 | 0.6 | 1,181 | 100 |
| Giardia intestinalis | 1,067 | 90.3 | 114 | 9.7 | 1,181 | 100 |
| Iodomoeba butchlii | 1,166 | 98.7 | 15 | 1.3 | 1,181 | 100 |
| Dientamoeba fragilis | 1,173 | 99.3 | 8 | 0.7 | 1,181 | 100 |
| Taenia spp. | 1,174 | 99.4 | 7 | 0.6 | 1,181 | 100 |
| Enter. vermicularis | 1,111 | 94.1 | 70 | 5.9 | 1,181 | 100 |
| Hymenolepis nana | 1,170 | 99.1 | 11 | 0.9 | 1,181 | 100 |
| Trich. intestinalis | 1,156 | 97.9 | 25 | 2.1 | 1,181 | 100 |
| Ascaris lumbricoides | 1,180 | 99.9 | 1 | 0.1 | 1,181 | 100 |
| Chilomastix mesnilii | 1,179 | 99.8 | 2 | 0.2 | 1,181 | 100 |
The rate of occurrence of infections with microsporidia in children was found to be smaller than the rate of non-infections. Similarly, the occurrence rates of other intestinal parasites other than microsporidia were smaller than the rates of non-infections.
The distribution of the Microsporidia with regard to gender was presented in Table 2. There was no significant relationship between gender and the Microsporidia (P > 0.05).
Table 2.
The distribution of the microsporidia with regard to gender
| Gender | Non-infected | Infected | Total | |||
|---|---|---|---|---|---|---|
| n | %a | n | %a | n | %a | |
| Male | 520 | 93.4 | 37 | 6.6 | 557 | 100 |
| Female | 569 | 91.2 | 55 | 8.8 | 624 | 100 |
| Total | 1,089 | 92.2 | 92 | 7.8 | 1,181 | 100 |
aLine percentage
As can be seen in Table 3, there is a relationship between the infection with the microsporidia and infection with other intestinal parasites (P < 0.05).
Table 3.
The distribution of the microsporidia and other intestinal parasites
| Microsporidia | Other intestinal parasites | |||||
|---|---|---|---|---|---|---|
| Non-infected | Infected | Total | ||||
| n | %a | n | %a | n | %a | |
| Non-infected | 794 | 72.9 | 295 | 27.1 | 1,089 | 100 |
| Infected | 58 | 63.0 | 34 | 37.0 | 92 | 100 |
| Total | 852 | 72.1 | 329 | 27.9 | 1,181 | 100 |
aLine percentage
Discussion
Although the parasite infections are widespread throughout the world, affecting every member of the society children are particularly susceptible. Additionally, chronic parasitic infections can lead to retardation of mental and physical development [15].
The present study was performed to determine prevalence of microsporidia and intestinal parasites. Based on the obtained results of this study, it was found that infections were prevalent (7.8% for microsporidia and 27.8% for intestinal parasites other than microsporidia) in children admitted with gastrointestinal health complaints.
The obtained positive rate (7.8%) of microsporidia might be due to the staining methods. Available literature implicates the importance of the utilized staining methods for assessment of the parasites in stool specimens. Kokoskin et al. [16] described another modification of Weber’s stain using a higher staining temperature (from room temperature to 50°) and a shorter staining time (10 min) for optimal results.
Degirolami et al. [17] performed a comparative study for the sensitivity of Weber’s MTS and identified that 18.6% of the patients were positive for microsporidia. All 44 samples positive by MTS were also positive by Uvitex 2B, concluding that both stains are useful and reliable methods for detection of microsporidia spores in stools. Raynaud et al. [18] also identified E. intestinalis by using MTS and Uvitex 2B staining. In addition, Carter et al. [19] suggested that MTS staining is sensitive for diagnosis of microsporidia.
The first case of human microsporidiosis was reported in 1959. Microsporidiosis is mainly seen in immunocompromised people as opportunistic infections [20]. In Leelayoova et al.’s study [21] the parasite was determined as infectious pathogen in 7.1% of the children with acute and chronic diarrhea.
Encephalitozoon spores were detected in cerebrospinal fluid from a child patient exposed to farm animals suffered from cerebral symptoms such as fever, headache and convulsion. Two additional cases were reported on immunocompromised infant with atypical aplasia in 1973 and the other was an 11-year old boy with corneal involvement [3]. Again, Bretagne et al. [22] reported microsporidia in stool samples of 8 out of 980 African children with no clinical signs. Tumwine et al. [7] performed PCR in stool samples from children who suffered from diarrhea more than 72 h and identified E. bieneusi in 17.4% of the children. In a study from Thailand by Termmathurapoj et al. [8], the rate of 1.3% microsporidia infection was detected by calcofluor and Gram-chromotrope staining. These suggest that presence of microsporidia does not always correlate with immunocompromised children and typical symptomatic disease. Therefore, this condition should be considered in children admitting with different complaints.
Many studies investigating the prevalence of intestinal parasites have been carried out in Turkey. In these studies, high percentages were obtained. (15, 23–25). Studies of intestinal parasites other than microsporidia were performed involving 0–18 years of age group from other regions of Turkey. Durmaz et al. [23] reported intestinal parasites other than microsporidia rate as 89.4%. The prevalence rates of intestinal parasitic other than microsporidia infections may vary from one area to other. The rate of infection with more than one species of parasite was reported in children 6–12 years of age as 48.8, 56 and 65.9% by Gunes et al. [24], Zeyrek et al. [25] and Celiksoz et al. [26], respectively. Ercevik and Idil [27] reported that when both of the schools were evaluated with respect to prevalence or intestinal parasites in Ankara, the prevalence of intestinal parasites was 10.6% in Ertuğrulgazi Primary Education School and 27.9% in Şahinbey Primary Education School. Also, average prevalence of intestinal parasites in all of the participant students was 18.4%. In the present study the rates of infection with microsporidia and other intestinal parasites were 7.8 and 27.8%, respectively. A significant association was found between the infections with microsporidia and other intestinal parasites. Concerning to our study, the infection rates of other intestinal parasites in the previous mentioned studies were slightly higher.
The obtained lower rates of infection compared to other studies may be due to the reasons: (a) screening for intestinal parasites was performed in schools in the region; (b) the implementation of treatments for children with parasite infection; (c) the effectiveness of public health education given to students and employees in the school. In this study, since the children applying to the hospital with digestive system problems were evaluated, the carriers found in public health screening remained outside the research.
There has been no large scale epidemiological screening of the microsporidiosis in children from Turkey. However, other intestinal parasites are similar transmission route to microsporidia. The available literature indicates the routes for the transmission of microsporidiosis through fecal-oral contamination and inhalation by water, soil and contaminated food [28, 29]. Therefore, microsporidia may be in children. In addition, studies in adults are supporting this result [30]. The cellophane tape method, native-Lugol and sedimentation methods are routinely performed in patients with digestive system diseases. However, the staining methods of MTS, acid-fast-trichrome and calcofluor used for the diagnosis of parasites are not routinely carried out in every hospital. But since these methods can be easily done with chemicals used in laboratory, these procedures do not bring additional costs.
We also assessed the intestinal parasites other than microsporidia in children with gastrointestinal complaints in Malatya. It was also concluded that public health education towards prevention of transmission routes of intestinal parasites should be performed. A more comprehensive study is needed for covering larger areas of Turkey with species differentiation. Phylogenetic analysis of microsporidia may increase the knowledge of these organisms and help greatly in evaluating the threat to health of children in Turkey.
Acknowledgments
We thank to Prof. Dr. Rainer Weber, Prof. Dr. Lynne S. Garcia, Prof. Dr. Nilgun Daldal and Assoc. Prof. Dr. Mustafa Yaman for the contributions of this study.
References
- 1.Shadduck JA. Human microsporidiosis and AIDS. Rev Infect Dis. 1989;11:203. doi: 10.1093/clinids/11.2.203. [DOI] [PubMed] [Google Scholar]
- 2.Shadduck JA, Greeley E. Microsporidia and human infections. Clin Microbiol Rev. 1989;2:158–165. doi: 10.1128/cmr.2.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franzen C, Müller A. Molecular techniques for detection, species differentiation, and phylogenetic analysis of microsporidia. Clin Microbiol Rev. 1999;12:243–285. doi: 10.1128/cmr.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eeftinck Schattenkerk JKMT, Gool T. Clinical and microbiological aspects of microsporidiosis. Trop Geogr Med. 1992;44:287. [PubMed] [Google Scholar]
- 5.Franzen C, Müller A, Salzberger B, Fätkenheuer G, Eidt S, Mahrle G, Diehl V, Schrappe M. Tissue diagnosis of intestinal microsporidiosis using a fluorescent stain with Uvitex 2B. J Clin Pathol. 1995;48:1009–1010. doi: 10.1136/jcp.48.11.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caramello P, Mazzucco G, Romeo M, Ullio A, DeRosa G, Lucchini A, Forno B, Brancale T, Macor A, Preziosi C, et al. Clinical and microscopical features of small-intestinal microsporidiosis in patients with AIDS. Infection. 1995;23:362–368. doi: 10.1007/BF01713566. [DOI] [PubMed] [Google Scholar]
- 7.Tumwine JK, Kekitiinwa A, Nabukeera N, Akiyoshi DE, Buckholt MA, Tzipori S. Enterocytozoon bieneusi among children with diarrhea attending Mulago Hospital in Uganda. Am J Med Hyg. 2002;67(3):299–303. doi: 10.4269/ajtmh.2002.67.299. [DOI] [PubMed] [Google Scholar]
- 8.Termmathurapoj S, Engkanun K, Naaglor T, Taamsri P, Wirote A, Leelayoova S, Mungthin M. Cross-sectional study of intestinal protozoan infections in orphans and childcare workers at the Phayathai babies’ home, Bangkok, Thailand. J Trop Med Parasitol. 2000;23:21–27. [Google Scholar]
- 9.Yazar S, Eser B, Yalcin S, Sahin I, Koc AN. A case of pulmonary microsporidiasis in an acute myeloblastic leukemia (AML)—M3 patient. Yonsei Med J. 2003;44(1):146–149. doi: 10.3349/ymj.2003.44.1.146. [DOI] [PubMed] [Google Scholar]
- 10.Buget E, Buyukbaba-Boral O, Kirkoyun-Uysal H, Nazlican O, Ogut T, Sengur G. First case report in Turkey: microsporidiosis and pulmonary cryptosporidiosis in an AIDS patient. T J Micro Soc. 2000;30(3–4):166–170. [Google Scholar]
- 11.Ozkirim A, Keskin N. A survey of Nosema apis of honey bees (Apis mellifera L.) producing the famous Anzer honey in Turkey. Z Naturforsch [C] 2001;56(9-10):918–919. doi: 10.1515/znc-2001-9-1042. [DOI] [PubMed] [Google Scholar]
- 12.Eroksüz H, Eroksüz Y, Metin N, Ozer H. Bir tavşan kolonisindeki doğal encephalitozoonosisolgları üzrine morfolojik incelemeler. Turk J Vet Anim Sci. 1999;23:191–195. [Google Scholar]
- 13.Yaman M, Radek R. Nosema chaetocnemae sp.n. (Microspora:Nosematidae), a microsporidian parasite of Chaetocnema tibialis (Coleoptera:Chrysomelidae) Acta Portozool. 2003;42:231–237. [Google Scholar]
- 14.Jerrold HZ (1999) Biostatistical analysis, 4th edn. Prentice-Hall Inc, New Jersey. ISBN-10: 013081542X, ISBN-13: 9780130815422
- 15.Yilmaz H, Göz Y, Bozkurt H. Distribution of intestinal parasites in children from the 23 Nisan Primary School in Hakkari. Acta Parazitol Turcica. 1999;23(1):28–31. [PubMed] [Google Scholar]
- 16.Kokoskin E, Gyorkos TW, Camus A, Cedilotte L, Purtill T, Ward B. Modified technique for efficient detection of microsporidia. J Clin Microbiol. 1994;32(4):1974–1975. doi: 10.1128/jcm.32.4.1074-1075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeGirolami PC, Ezratty CR, Desai G, McCullough A, Asmuth D, Wanke C, Federman M. Diagnosis of intestinal microsporidiosis by examination of stool and duodenal aspirate with Weber’s modified trichrome and Uvitex 2B strains. J Clin Microbiol. 1995;33(4):805–810. doi: 10.1128/jcm.33.4.805-810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raynaud L, Delbac F, Broussolle V, Rabodonirina M, Girault V, Wallon M, Cozon G, Vivares CP, Peyron F. Identification of Encephalitozoon intestinalis in travelers with chronic diarrhea by specific PCR amplification. J Clin Microbiol. 1998;36(1):37–40. doi: 10.1128/jcm.36.1.37-40.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter PL, MacPherso DW, McKenzie RA. Modified technique to recover microsporidian spores in sodium acetate-acetic acid-formalin-fixed fecal samples by light microscopy and correlation with transmission electron microscopy. J Clin Microbiol. 1996;34(11):2670–2673. doi: 10.1128/jcm.34.11.2670-2673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanyüksel M, Gün H. Mikrosporidia. Acta Parazitol Turcica. 1995;19(2):200–209. [Google Scholar]
- 21.Leelayoova S, Vithayasai N, Watanaveeradej V, Chotpitayasunondh T, Therapong V, Naaglor T, Mungthin M. Intestinal microsporidiosis in HIV-infected children with acute and chronic diarrhea. Southeast Asian J Trop Med Public Health. 2001;32(1):33–37. [PubMed] [Google Scholar]
- 22.Bretagne S, Foulet F, Alkassoum W, Fleury-Feith J, Develoux M. Prevalence of Enterocytozoon bieneusi spores in the stool of AIDS patients and African children not infected by HIV. Bull Soc Pathol Exot. 1993;86(5):351–357. [PubMed] [Google Scholar]
- 23.Durmaz B, Yakinci C, Rafiq M, Durmaz R. The prevalence of intestinal parasites among orphans and primary school children in Malatya. Acta Parazitol Turcica. 1997;21:391–394. [Google Scholar]
- 24.Günes G, Celik T, Regiq M, Kaya M, Pehlivan E, Daldal N. Parasitological investigations of children and staff in orphanages in Malatya. Acta Parazitol Turcica. 2000;24:290–293. [Google Scholar]
- 25.Zeyrek FY, Ozbilge H, Zeyrek CD. Prevalence of intestinal parasitic infections among nursery school and orphanage. Acta Parazitol Turcica. 2003;27(2):133–135. [Google Scholar]
- 26.Celiksöz A, Demirtas S, Sümer Z, Özcelik S, Saygı G. A survey of intestinal parasites in orphan of orphanage in Sivas. Acta Parazitol Turcica. 1997;21:45–47. [Google Scholar]
- 27.Ercevik HE, Idil A. Prevalence of intestinal parasites and associated factors of this into primary school which have different socioeconomic level. T Klin J Med Sci. 2002;22:113–118. [Google Scholar]
- 28.Didier ES, Stovall ME, Green LC, Brindley PJ, Sestak K, Didier PJ. Epidemiology of microsporidiosis: sources and modes of transmission. Vet Parasitol. 2004;126:145–166. doi: 10.1016/j.vetpar.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 29.John DE, Haas CN, Nwachuku N, Gerba CP. Chlorine and ozone disinfection of Encephalitozoon intestinalis spores. Water Res. 2005;39(11):2369–2375. doi: 10.1016/j.watres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Karaman U, Daldal N, Atambay M, Colak C. The epidemiology of microsporidias in human (Malatya sample) T J Med Sci. 2009;39(2):281–288. [Google Scholar]