Abstract
Two fold increase in the yield of glucose and maltose containing exo-polysaccharide (EPS) by Rhizobium sp. was observed during its growth in modified YEMB. EPS production, plant growth promotion activity and root colonization of Rhizobium sp. studies showed enhanced EPS synthesis, more seed germination and over all improvement in plant growth over control and R. meliloti treatment. Groundnut seeds bacterized with Rhizobium sp. resulted in 69.75% more root length, 49.51% more shoot height, 13.75% more number of branches and 13.60% more number of pods over the control and R. meliloti treatment. Bacterization of wheat seeds increased the dry matter yield of roots (1.7-fold), and roots adhering soil (RAS) (1.5) and shoot mass (1.9-fold). Rhizobium sp. inoculation also increased the population density of EPS-producing bacteria on the rhizoplane. Roots of plants inoculated with Rhizobium sp. maintained a higher K+/Na+ ratio and K+–Na+ selectivity.
Keywords: Rhizobium sp, Rhizobium melioti, EPS, Optimization, Groundnut, Wheat
Introduction
A very large number of microorganisms including Rhizobium sp. [1–3] produce variety of exo-polysaccharide (EPS) possessing remarkably high moisture holding capacity and serve to maintain minimum moisture in their immediate environment. EPS protects the producing organism from desiccation and serves as a potential energy reserve as it can be catabolized under nutrient deficient conditions. Microbial EPS have been commercialized as possible future industrial commodities for food and in agriculture for the encapsulation of somatic embryoid, which offer a greater feasibility for precise delivery of plant growth regulators, fungicides and pesticides [4]. Influence of culture conditions on polysaccharide production are reported for various organisms [1–3, 5]. It has been reported that the use of sugar components e.g. sucrose, dextrose, mannitol etc. as a sole source of carbon yields more EPS than cell biomass [6]. Minerals and growth factors are also known to regulate EPS yield [4].
Role of EPS producing plant growth promoting rhizobacteria (PGPR) in providing moisture and thereby increasing water holding capacity of soil, chelating various metal ions and promoting the growth of plant is well established. The present study was aimed towards determining the influence of various physicochemical parameters on EPS production by Rhizobium sp. and its application for plant growth promotion.
Materials and Methods
Rhizobium sp. was isolated from root nodules of groundnut (Arachis hypogea) plant. For the isolation of Rhizobium sp., the groundnut plant was uprooted, roots having pink healthy nodules were selected, washed 4–5 times in physiological saline and surface sterilized with 0.1% HgCl2, aseptically crushed and grown in sterile yeast extract 3mannitol broth (YEMB) containing g l−1, mannitol, 10; CaCO3, 01; MgSO4, 0.0177; yeast extract, 01; K2HPO4, 0.1, pH; 7. Inoculated medium was incubated at 27°C at 120 rpm for 8–10 days. The enriched sample was grown on yeast extract mannitol agar (YEMA) and congo red (0.025 g l−1 of YEMA) yeast extract mannitol agar (CRYEMA) at 28°C for 24–48 h. The culture was routinely maintained on nutrient agar at 4°C.
Biochemical characterization, indole, methyl red, Voges Proskauer and citrate utilization (IMViC) tests, and enzyme profile was studied and antibiotic sensitivity of isolate towards different antibiotics was checked by disc diffusion method.
Screening and Production of EPS
Rhizobium sp. was screened for EPS production by growing it on CRYEMA [7] at 28°C for 48 h and observed for the formation of gummy/mucoid colonies of Rhizobium. EPS production in liquid media was carried out by separately growing the 6 × 106 cells ml−1 of Rhizobium sp. and R. meliloti (obtained from Indian Agriculture research Institute (IARI), New Delhi) in YEMB at 28°C for 8–10 days with constant shaking at 120 rpm. Following the incubation inoculated flasks were observed daily after third day of incubation up to 10 days for change in the rheology. Increase in viscosity of broth was taken as an indication of EPS production.
Extraction and Recovery of EPS
Cell free supernatant obtained from the centrifugation (10,000 rpm, 20 min) of fermentation broth was slowly added with equal volume of iso-propanol with constant stirring and EPS was separated by spooling. Spooled samples were oven dried at 50°C till the constant weight and weighed for the estimation of EPS [8].
Analytical Methods
Viscosity of fermented broth was measured with viscometer (Brookfield, DV III) at 29°C at 100 rpm with uninoculated medium as reference. Viscosity measured was expressed in terms of % and centipoises (cP). Residual sugar from fermented broth was estimated by DNSA method [9] and growth was measured by taking dry weight of cell mass. Amount of EPS was measured gravimetrically.
Determination of Biochemical Nature of EPS
Chemical composition like presence of monosaccharide, disaccharide or polysaccharide of EPS produced was determined by various qualitative tests namely, Fehling’s, Benedict’s, Molisch’s, Seliwanoff’s, Bial’s, Iodine, Anthron’s, Barfoed’s, Mucic Acid and Osazone tests [10].
Optimization Studies
For the optimization of incubation period, Rhizobium sp. (6 × 106 cells ml−1) was grown in YEMB at 28°C at 120 rpm for 8 days. Initially samples were withdrawn after 3 days of incubation and thereafter at the interval of 24 h. Estimation of viscosity, residual sugar, cell mass and EPS was done as described earlier.
For checking the influence of inoculum level, biomass of Rhizobium sp. in the range of 1–10% was separately grown in YEMB at 28°C at 120 rpm for 8 days followed by measuring the viscosity, cell mass, residual sugar and EPS. Influence of pH on growth and EPS production was studied by growing Rhizobium sp. (6 × 106 cells ml−1) in YEMB separately prepared with different pH (5.5 to 9.5) at 28°C at 120 rpm for 8 days. For studying the influence of temperature, five Erlenmeyer flask each with 100 ml YEMB separately inoculated with Rhizobium sp. (6×106 cells ml−1) and individually incubated at 15, 28, 37, 50, 55, and 60°C at 120 rpm for 6 days.
For determining the threshold level of Fe2+, Ca2+, K+, Mg2+ that regulate EPS production, Rhizobium sp. (6 × 106 cells ml−1) was grown in YEMB separately prepared with different concentrations of Fe2+ (1–10 μm), Ca2+(0.1–1.0 g l−1), K+(0.1–1.0 g l−1) and Mg2+ (0.1–1.0 g l−1) at 28°C at 120 rpm for 6 days.
For the optimization of carbon substrate, Rizobium sp. (6 × 106 cells ml−1) was grown in YEMB separately prepared with different sugars (10 g l−1) like mannitol, sucrose, dextrose, fructose, maltose, lactose, sorbitol, mesoinositol and starch at 28°C at 120 rpm for 6 days. For the optimization of nitrogen source, Rhizobium sp. (6 × 106 cells ml−1) was grown in YEMB medium containing different nitrogen sources (1 g l−1) like sodium nitrate, urea, casein, casein hydrolysate, yeast extract, ammonium sulphate, ammonium phosphate at 28°C at 120 rpm for 6 days. Viscosity, cell mass, residual sugar and EPS were estimated.
Plant Growth Promotion Activity of EPS Producing Rhizobium sp.
Plant growth promoting potential of Rhizobium sp. was checked at plate assay and pot assay level. Seeds of groundnut and wheat surface sterilized with 0.1% HgCl2 were bacterized with EPS rich YEMB containing Rhizobium sp. (108 CFU ml−1). Its bioefficaceous potential was measured by simultaneous bacterization of groundnut and wheat seeds with the standard culture of R. meliloti.
Plate Assay
Plate assay was performed by placing the bacterized seeds (5 seeds/plate each for wheat and 3 seeds/plate for groundnut seeds) in the sterile petri plate with blotting paper. These plates were incubated at 28°C for 10 days in light and daily observed for seed germination. Sterile distilled water was added on filter paper as and when required to maintain the moisture necessary for the germination of seed. Percent increase in germination of seeds and its comparison with control seeds and seeds bacterized with R. meliloti was calculated.
Pot Assay
Seeds of groundnut and wheat (5 seeds/pot) were separately soaked in bacterial slurry of Rhizobium sp. and R. meliloti for 15 min and transplanted into pots containing sterile soil. Seeds soaked in sterile distilled water were transplanted into the soil as a control. The soil moisture was measured gravimetrically [11] and it was maintained at 60% level throughout the experiment. After 60 days incubation, bioefficacy of EPS coated seeds was calculated as percent increase in seed germination, increase in root length, increase in shoot length, roots adhering soil (RAS), root tissue (RT) and RAS dry mass.
Estimation of Na, Cl and K from Plant Cells
Sodium, calcium and potassium concentrations in plant tissues (acid digests) and in RAS (1:1 soil/water extracts) were determined by flame- photometry. K–N ratio, Ca–Na ratio, K–Na and Ca–Na selectively (K or Ca–Na selectively equal K/Na or Ca/Na ratio divided by K/Na or Ca/Na ratio of soil used) were calculated. Water-insoluble saccharides in the RAS were determined in air dried residual soil after filtering the RAS-water suspension.
Soil and Root Colonization by Rhizobium
Most of PGPR perform well under plate assay conditions but when subjected to soil environment they fail to do so. Only those PGPR which are efficient in colonizing the roots of plant will perform well and will promote the plant growth. Therefore, root or seed colonization of PGPR should be considered as important parameter of plant growth promotion. For this purpose, the population densities of Rhizobium sp. on the rhizoplane of wheat and groundnut were determined by taking the plate count of soil (1 gm in 100 ml diluted to 10−6) on CRYEMA and the number of colonies (CFU g m−1 soil) was taken as means of root and seed colonization.
Result and Discussion
Biochemical Characterization of Isolate
Formation of mucoid colonies, growth on selective CRYEMA medium, fermentation of maltose, lactose, sorbitol, mesoinositol, dextrose, mannitol, sucrose with acid and gas formation, production of amylase and β-galactosidase, negative test of indole and methyl red and positive Voges Proskauer and citrate utilization test very well matched with the characteristics of Rhizobium sp. The antibiogram of Rhizobium sp. revealed the sensitivity of organism towards aminoglycosides antibiotics such as erythromycin, gentamicin, vancomycin, tetracycline, co-trimoxazole, amikacin, streptomycin and kanamycin.
Screening, Production and Extraction of EPS
Formation of mucoid gummy colonies on YEMA and increase in the viscosity of YEMB from 10 to 97 cp indicated the ability of Rhizobium sp. to produce EPS. The quantitative yield of EPS produced during submerged fermentation was 2.5 g l−1. Similar yield have been reported for A. faecalis var. Myxogenes [12]. Sayyed and Chincholkar [13] have obtained a gravimetric yield of 1.3 g l−1 with A. faecalis. When cell free supernatant was added to equal volume of iso-propanol (30%) the EPS was found spooling on constantly moving glass rod [8]. The spooled extract when dried at 50°C, a brown colored powder of EPS was obtained. Sayyed and Chincholkar [13] have reported the extraction of EPS produced by A. faecalis with 50% iso-propanol.
Determination of Biochemical Nature of EPS
Rhizobium sp. under study gave positive results for Molisch test, Barfoed’s test and Bials’s indicating the presence of glucose and maltose containing EPS. Sayyed and Chincholkar [13] have reported the production of glucose and maltose containing EPS from A. faecalis.
Optimization Studies
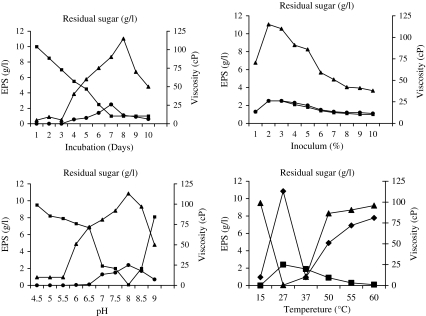
Accumulation of EPS started from 3rd day of incubation and was found increasing with increase in time course. Optimum viscosity (115 cP), maximum EPS (2.5 g l−1) minimum residual sugar (50 μg l−1) was recorded during 6th day of incubation. Further increase in incubation beyond 6 days (192 h) period resulted in decline in the amount of EPS, on 7th day the amount of residual sugar was 2.10 g l−1 and decreased thereafter (Fig. 1a). Therefore, fermentation was stopped after 8 days (192 h). The decline in the amount of EPS may be due to the mobilization of EPS by the organism itself probably under the influence of EPS hydrolase. It can also be correlated to the exhaustion of carbon source (sugar), the residual sugar at this stage was only 50 μg l−1. Decrease in the viscosity of fermented broth also supported this belief. It is well documented fact that increase in incubation period beyond the optimal period causes the EPS lyase mediated hydrolysis of EPS [14] and therefore reduction in the amount of EPS. Most of the EPS producing bacteria are known to accumulate EPS from third day of incubation [12].
Fig. 1.
Influence of physical factors on EPS yield by Rhizobium sp.
Amongst the different levels of inoculum added in YEMB, 2%, yielded optimum EPS, less biomass, more viscosity of fermented medium and rapid utilization of sugar from broth. With further increase in inoculum although the cell mass and sugar utilization increased noticeably but no further increase in EPS could occur (Fig. 1b). Optimum EPS (2.45 g l−1) production with maximum viscosity of broth and maximum utilization of sugar was reported at slight alkaline pH value of 8.0 (Fig. 1c). Observation from influence of temperature on biomass and EPS revealed that the organism grows well and produces optimum EPS (2.40 g l−1) with maximum viscosity in broth and maximum utilization of sugar at 28°C (Fig. 1d).
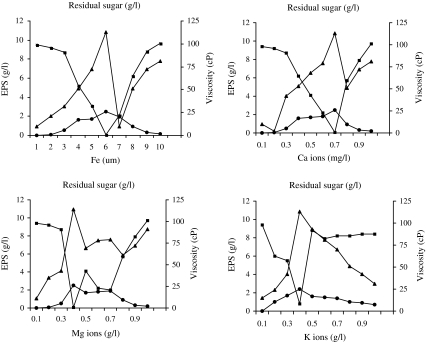
Increase in Fe2+ levels increased the viscosity as well as EPS yield (Fig. 2a). 6 μM of Fe2+ was threshold level for optimum (2.41 g l−1) yield. The concentration of 0.7 g l−1 of Ca2+ yielded maximum viscosity (113 cP), optimum EPS (2.43 g l−1) and rapid utilization of sugar (Fig. 2b). Among different levels of Mg2+ ions used, concentration of 0.4 g l−1 yielded maximum viscosity (114 cP), optimum EPS (2.44 g l−1) and rapid utilization of sugar (Fig. 2c). 0.4 g l−1 K+ ions resulted in maximum viscosity (114 cP), optimum EPS (2.44 g l−1) and rapid utilization of sugar (Fig. 2d).
Fig. 2.
Influence of physical metal ions on EPS yield by Rhizobium sp.
Sucrose resulted in maximum biomass while dextrose gave optimum EPS yield. Among the different nitrogen sources, ammonium sulphate and yeast extract gave optimum yield of EPS while urea inhibited both EPS production and growth of organism (Table 1). The C/N ratio have a role in EPS production, more EPS have been reported with high carbon and low nitrogen ratio.
Table 1.
Influence of carbon and nitrogen source on growth and EPS production by Rhizobium sp.
| Carbon/Nitrogen source | Growth (OD 610 nm) | Residual sugar (g l−1) | Viscosity (cP) |
EPS (g l−1) |
|---|---|---|---|---|
| Carbon source (10 g l−1) | ||||
| Mannitol | 0.10 | 8.47 | 32 | 1.20 |
| Sucrose | 0.65 | 0.70 | 114 | 2.47 |
| Dextrose | 1.80 | 7.76 | 45 | 1.50 |
| Fructose | 0.40 | 6.81 | 54 | 1.04 |
| Maltose | 0.20 | 7.14 | 69 | 1.10 |
| Lactose | 0.25 | 8.21 | 78 | 0.30 |
| Sorbitol | 0.45 | 8.51 | 79 | 0.40 |
| Meso-inositol | 0.30 | 8.75 | 48 | 0.20 |
| Nitrogen source (1 g l−1) | ||||
| Sodium nitrate | 0.10 | 9.21 | 25 | 0.01 |
| Urea | 0.10 | 8.86 | 38 | 0.00 |
| Casein | 0.30 | 7.10 | 47 | 0.50 |
| Casein hydrolysate | 0.43 | 6.51 | 71 | 1.10 |
| Ammonium sulphate | 0.90 | 0.44 | 114 | 2.47 |
| Yeast extract | 0.82 | 0.52 | 113 | 2.39 |
| Ammonium phosphate | 0.67 | 0.65 | 90 | 1.50 |
Under optimized conditions in modified YEMB containing g l−1, dextrose, 10; CaCO3, 0.7; 6 μM of Fe2+, MgSO4, 0.04; yeast extract, 01; pH; 8 inoculated with 2% inoculum and incubated at 28°C for 6 days at 120 rpm, yielded 2.80 g l−1 of EPS.
Plant Growth Promotion Activity of EPS Producing Rhizobium sp.
During plate assay studies, groundnut seeds showed 20% increase in germination while wheat seeds showed only 10% increase in germination. More increase in seed germination of groundnut seeds may be due to more specificity of Rhizobium sp. towards groundnut seeds. Control preparation did not show any increase in seed germination. The plant growth promotion activity of EPS producing Rhizobium sp. in soil under natural conditions demonstrated that the application of EPS producing Rhizobium sp. enhances the overall growth and vigor of groundnut plant (Table 2). Groundnut seeds bacterized with Rhizobium sp. resulted in 69.75% more root length, 49.51% more shoot height (cm), 13.75% more number of branches and 13.60% more number of pods which was 17, 10.35, 6.56, 17.10 and 13.60% more over the control treatment. Results of inoculation with wheat seeds substantially increased the dry matter yield of roots (80–280%), and RAS (166.7–383.3%). A much more pronounced effect, 153.8–453.8% increase over control of inoculation was evident on the mass of shoots (Table 3). Rhizobium sp. increased dry weight of RAS 1.5, root 1.7 and shoot 1.9 times as compared with control. Rhizobium inoculation also increased the population density of EPS-producing bacteria on the rhizoplane, followed by R. meliloti. The population density of EPS-producing bacteria on the rhizoplane was significantly correlated with the mass of RAS (r = 0.8069), and the dry matter yields of roots (r = 0.8204) and shoots (r = 0.8814). Moreover, the population density of EPS producing bacteria on the rhizoplane, and the dry weights of RAS, roots and shoots were significantly correlated with the content of water insoluble saccharides in the RAS being r = 8468, 9603, 9708 and 9711, respectively (Table 4).
Table 2.
Influence of EPS producing Rhizobium inoculation on seed germination
| Seeds | Germination | % increase in germination | |
|---|---|---|---|
| Test | Control | ||
| Ground nuts | 5 | 3 | 20 |
| Wheat | 5 | 4 | 10 |
Each value is an average of three replicates
Table 3.
Influence of EPS producing Rhizobium inoculation on root, shoot and RAS weight in wheat seeds
| Treatments | RAS dry weight (g/pot) | Root dry weight (mg/pot) | Shoot dry weight (mg/pot) | RAS Root Ratio | Saccharide in RAS (mg/pot) |
|---|---|---|---|---|---|
| Control | 0.42 | 0.05 | 0.13 | 8.4 | 0.15 |
| Rhizobium sp. | 2.03 | 0.19 | 0.72 | 10.68 | 7.75 |
| R. meliloti | 1.86 | 0.15 | 0.59 | 12.4 | 6.16 |
RAS Roots adhering soil, each value is an average of three replicates
Table 4.
Concentrations of K+, Na+, and Ca2+ in RAS, wheat roots and shoots due to inoculation with Rhizobium sp. and R. meliloti
| Treatments | K+ | Na+ | Ca2+ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RAS dry weight (μmol/g) | Root dry weight (μmol/g) | Shoot dry weight (μmol/g) | RAS dry weight (μmol/g) | Root dry weight (μmol/g) | Shoot dry weight (μmol/g) | RAS dry weight (μmol/g) | Root Dry weight (μmol/g) | Shoot dry weight (μmol/g) | |
| Control | 0.64 | 13.8 | 247.2 | 145 | 1152 | 1456 | 24.2 | 55.2 | 54.2 |
| Rhizobium sp. | 0.35 | 22.5 | 387.8 | 95 | 673 | 814 | 5.4 | 45.1 | 41.8 |
| R. meliloti | 1.32 | 14 | 264.7 | 82 | 726 | 856 | 5.2 | 38.2 | 33.9 |
Estimation of Na, Cl and K from Plant Cells
In all treatments, roots of inoculated plants compared with control plants maintained a higher K+/Na+ ratio and K+–Na+ selectivity (the K+/Na+ ratio of Rhizobium sp. the plant tissues divided by K+/Na+ ratio of the soil used), the most pronounced effect was archived by inculcation with slime culture of Rhizobium sp. However, in shoots, all the strains inoculation caused higher K+/Na+ ratio and K+–Na+ selectivity, again the slime culture of Rhizobium sp. showed the maximum effect (Table 5).
Table 5.
Effect of inoculating Rhizobium sp. and R. meliloti on K+/Na+ and Ca2+/Na+ ratio and selectivity of wheat roots and shoots
| Treatments | K+/Na+ ratio | K+–Na+ selectivity | Ca2+/Na+ ratio | Ca2+–Na+ selectivity | ||||
|---|---|---|---|---|---|---|---|---|
| Shoots | Roots | Shoots | Roots | Shoots | Roots | Shoots | Roots | |
| Control | 0.012 | 0.170 | 0.179 | 2.54 | 0.048 | 0.037 | 0.115 | 0.135 |
| Rhizobium sp. | 0.024 | 0.280 | 0.358 | 4.179 | 0.059 | 0.045 | 0.215 | 0.164 |
| 0.033 | 0.479 | 0.493 | 7.179 | 0.067 | 0.051 | 0.244 | 0.185 | |
| R. meliloti | 0.016 | 0.243 | 0.239 | 3.267 | 0.050 | 0.038 | 0.182 | 0.138 |
| 0.019 | 0.309 | 0.284 | 4.612 | 0.054 | 0.040 | 0.196 | 0.145 | |
Soil and Root Colonization by Rhizobium sp.
The population densities from rhizosphere and rhizoplane soil showed the abundance of Rhizobium sp., 6 × 107 and 7 × 107 cells ml−1 of Rhizobium sp. were obtained on CRYEMA from rhizosphere and rhizoplane soil of groundnut seeds and 4 × 107 and 5 × 107 cells ml−1 of Rhizobium sp. were obtained on CRYEMA from rhizosphere and rhizoplane soil of wheat seeds. More cell density with groundnut soil is attributed to the host specificity of Rhizobium sp.
Conclusion
Under optimized conditions Rhizobium sp. inoculated in modified YEMB and incubated at 28°C for 6 days at 120 rpm, yielded 2.60 g L−1 of EPS. Increase in seed germination and over all growth and vigor in groundnut and wheat and effective root colonization reflected the potential of Rhizobium sp. as efficient bioinoculant for sustainable agriculture.
References
- 1.Bergmaier D, Champagne CP, Lacroix C. Exopolysaccharide production during batch cultures with free and immobilized Lactobacillus rhamnosus RW-9595 M. J Appl Microbiol. 2003;95:1049–1057. doi: 10.1046/j.1365-2672.2003.02084.x. [DOI] [PubMed] [Google Scholar]
- 2.Xiao JH, Chen DX, Liu JW, Liu ZL, Wan WH, Fang N, Xiao Y, Qi Y, Liang ZQ. Optimization of submerged culture requirements for the production of mycelial growth and exopolysaccharide by Cordyceps jiangxiensis JXPJ 0109. J Appl Microbiol. 2004;96:1105–1116. doi: 10.1111/j.1365-2672.2004.02235.x. [DOI] [PubMed] [Google Scholar]
- 3.El-Tayeb TS, Khodair TA. Enhanced production of some microbial exo-polysaccharides by various stimulating agents in batch culture. Res J Agric Biol Sci. 2006;2(6):483–492. [Google Scholar]
- 4.Mathur NK, Mathur V. Microbial polysaccharides: emerging new industrial products. Chem Week. 2001;46:151–159. [Google Scholar]
- 5.Yeh JY, Chen J. Production of slime polysaccharide by EHEC and STEC as well as the influence of culture conditions on slime production in Escherichia coli O157:H7. Lett Appl Microbiol. 2004;38:488–492. doi: 10.1111/j.1472-765X.2004.01523.x. [DOI] [PubMed] [Google Scholar]
- 6.Pace GW, Righelta RC. Production of extracellular microbial polysaccharides. Adv Biochem Eng. 1980;15:41–70. [Google Scholar]
- 7.Khandelwal SR (2002). Studies on the siderphores of Rhizobium sp. and its applicability in plant growth promotion and disease suppression. Ph.D. thesis, North Maharashtra University
- 8.Sutherland IW. Extracellular polysaccharide. In: Rehm HJ, Reed G, editors. Biotechnology vol. 3. Weinheim: Verlag Chemie; 1983. pp. 531–568. [Google Scholar]
- 9.Miller GL. Use of dinitrosalicylic acid for determination of reducing sugars. Anal Chem. 1972;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 10.Pattabiraman TN (1998) Tests for monosaccharides, disaccharides and polysaccharides. Laboratory manual in biochemistry. All India publisher and Distributor, Chennai, pp 21–27
- 11.Black CA. Methods of soil analysis: part I physical and microbiological properties. Madison: American Society of Agronomy; 1965. [Google Scholar]
- 12.Harada TC. Production properties and application of Curdlan. ACS Sym Series. 1965;45:265–283. doi: 10.1021/bk-1977-0045.ch020. [DOI] [Google Scholar]
- 13.Sayyed RZ, Chincholkar SB. Production of exopolysaccharide (EPS): a biopolymer from A. feacalis. J Food Sci Technol. 2008;45(6):531–533. [Google Scholar]
- 14.Pharm PL, Dupont I, Roy D, Lapointe G, Cerning J. Production of exopolysaccharide by Lactobacillus rhamnsus R and analysis of its enzymatic degradation during prolonged fermentation. Appl Environ Microbiol. 2000;66:2302–2310. doi: 10.1128/AEM.66.6.2302-2310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]