Abstract
Yield and productivity are critical for the economics and viability of a bioprocess. In metabolic engineering the main objective is the increase of a target metabolite production through genetic engineering. Metabolic engineering is the practice of optimizing genetic and regulatory processes within cells to increase the production of a certain substance. In the last years, the development of recombinant DNA technology and other related technologies has provided new tools for approaching yields improvement by means of genetic manipulation of biosynthetic pathway. Industrial microorganisms like Escherichia coli, Actinomycetes, etc. have been developed as biocatalysts to provide new or to optimize existing processes for the biotechnological production of chemicals from renewable plant biomass. The factors like oxygenation, temperature and pH have been traditionally controlled and optimized in industrial fermentation in order to enhance metabolite production. Metabolic engineering of bacteria shows a great scope in industrial application as well as such technique may also have good potential to solve certain metabolic disease and environmental problems in near future.
Keywords: Recombinant DNA technology, Biosynthetic pathway, Metabolic network, Metabolic disease
Introduction
The term “metabolic engineering” is itself tells about the concept. It is made up of two different words metabolism and engineering. Metabolism is the entire set of enzyme catalyzed transformation of organic molecule that occurs in cell of an organism and engineering is to manipulating things to make it more fruitful. Metabolic engineering is generally referred to as the targeted and purposeful alteration of metabolic pathways found in an organism in order to better understand and utilize cellular pathways for chemical transformation, energy transduction, and supramolecular assembly [1]. It is applicable for the directed improvement of cellular properties through the modification of specific biochemical reactions or the introduction of new genes with the use of various techniques such as Recombinant DNA technology [2]. The goal of metabolic engineering is systematic analysis of metabolic and other pathways with molecular biological techniques to improve cellular properties for the product improvement by designing and implementing rational genetic modifications.
Today, there is a strong interest in the efficient and cost effective biotechnological production of bulk chemicals from renewable plant biomass. There is a long tradition of microbial fermentations in food and feed applications, for example for the production of organic acids, alcohols, amino acids and vitamins; but many microbial products can also be found in other segments, such as antibiotics and ergot alkaloids [3]. Strain development is of central importance for the optimization of biotechnological production processes [4]. Synthetic biology has also contributed and will continue to contribute to the field of metabolic engineering, particularly with next generation biofuel [5].
Metabolic engineering is well suited as a framework for the analysis of genome wide differential gene expression data, in combination with data on protein content and in vivo metabolic fluxes. This multidisciplinary field draws principles from chemical engineering, computational science, biochemistry, biotechnology and molecular biology. Metabolic engineering is the application of engineering principles of the design and analysis for metabolic pathway in order to achieve a particular goal [6]. Metabolism is a central process, common to all organisms, through which cells manage the free energy provided from organic or other sources, in order to build cellular materials and satisfy the energetic demands [7]. One of the main goals in metabolic engineering is to increase the productivity of a chemical through genetic modifications. Microorganisms are the source of many drugs including antibiotics, antitumor compounds, immunosuppressant, Antiviral, antiparasitic agents and enzyme inactivating compounds [8].
The interest in metabolic engineering is stimulated by potential commercial applications where improved methods for developing strains can increase production of useful metabolites [9]. Recent endeavors have focused on using biologically derived processes pursue goals related to “sustainable developments” and “green chemistry” as well as positioning companies to exploit advances in the biotechnology field [9]. The extension of metabolic engineering to production of desired compounds in plant tissues and to provide better understanding of genetically determined human metabolic disorders broadens the interest in this field beyond the fermentation industry and bodes well for its future importance [10].
Current methods to increase the productivity of industrial microorganisms go from the classical random mutagenesis performed in close association with optimization of large scale industrial fermentations, to the use of more rational method. One of this is metabolic engineering where, in order to maximize product yields, primary metabolic fluxes are redirected by the introduction of genetic modifications through recombinant DNA technology, in a manner that supports high secondary metabolite productivities. There are various methods that are useful in metabolic engineering like genetic circuit design and modeling of bacterial metabolism [11]. In order to capture the dynamics of the metabolic fluxes and the concentration of specific metabolites, an extension of the classical flux balance analysis formulation is used.
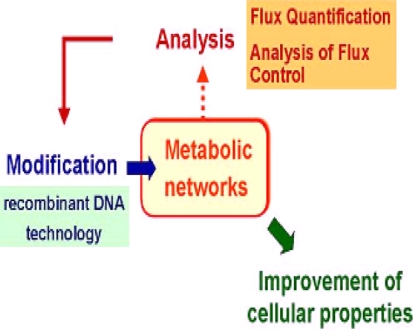
Cell’s metabolic network that evolved in nature is not optimized for practical application. In such cases, performance of metabolic pathways or bioprocesses can be modified by genetic manipulation of the cell so that the metabolite is overproduced (Fig. 1). The technology involved in biotechnology is described as metabolic engineering that is the improvement of cellular activities by various methods.
Fig. 1.
Overview of metabolic network
Although for metabolic overproduction, sometimes the strains of living organisms are improved by mutations and selection, the opportunity to introduce heterologous genes and regulatory elements made metabolic engineering, a very fascinating area of research [12]. In this area, cell function can be modified using targeted alterations in normal cellular activities [13]. This may involve not only biosynthesis of a metabolite but also manipulation of protein processing pathways. This review focuses on the use of metabolic engineering for strain development of bacterial species for the production of organic acids, ethanol, amino acids and some other metabolites.
Need of Metabolic Engineering
Microbes are used for the production of many valuable product in various industries such as pharmaceutical, agriculture, food, etc. due to wide spread use of microbes in industries. There is a need of metabolic engineering to achieve many goal such as (1) to increase product formation, (2) to speed up the process, (3) to save energy, (4) to stop production of byproduct, and (5) to develop strain with resistance to environmental stress. One way of grouping the different targets for metabolic engineering is as follows: extension of substrate range; improvements of productivity and yield; elimination of by-products; improvement of process performance; improvements of cellular properties; and extension of product range including heterologous protein production [13].
Steps of Metabolic Engineering
The process of metabolic engineering can be divided into three main steps. The first and important step in metabolic engineering is to study metabolic pathway which we want to engineer. For that we need to develop the analysis of dynamic changes occurring in a cell. A detailed biochemical study of particular pathway is required. Radiotracer technique is generally used if a pathway is unknown for biochemical studies [13].
The second step is a use of computational approach. On the basis of available information about particular metabolic pathway in silico models has been designed to engineered particular metabolic pathway. For example a computational approach known as “Knock” was developed for identifying the gene deletions needed to maximize the production of a desired chemical. Likewise it is developed for the up and down regulation of gene expression needed to engineered metabolic pathway.
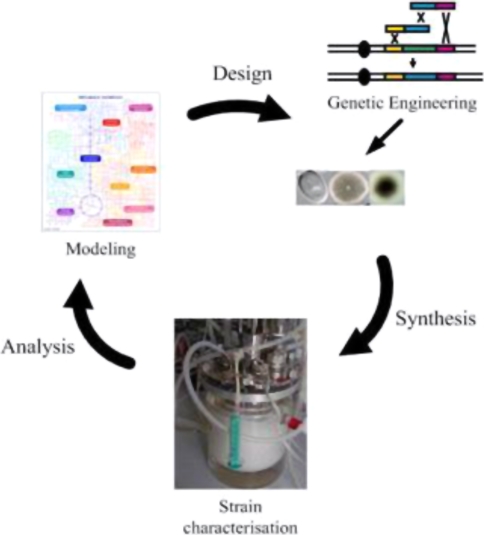
The third steps are to applied computational suggested design at experimental level using different genetic engineering approaches (Fig. 2). Traditionally, mutagenesis programs have been used for strain and production improvements. In the last few years, the development of technology such as recombinant DNA technology and gene shuffling has provided new tools for approaching yields improvement by means of genetic manipulation of biosynthetic pathways.
Fig. 2.
Stepwise process of metabolic
Approaches for Metabolic Engineering
Several approaches have been used for the improvement of secondary metabolites produced by certain bacteria in metabolic engineering. Some of these approaches are (1) heterologous expression of entire gene clusters, (2) engineering regulatory networks, (3) gene insertion and deletion, (4) redirecting metabolic pathway, (5) stimulation by precursors, (6) genetic knockout of loci, and (7) quorum sensing.
Heterologous Expression of Entire Gene Cluster
Advances in developing DNA manipulation tools and the improvement in genome sequencing technologies have proved fruitful for the isolation of many gene clusters involved in natural products biosynthesis. However, in some cases production titers of the encoded compound are low, and there are no genetic tools optimized for metabolic engineering in the particular producer strain. The solution of these problems is to transfer the whole metabolite pathway to new hosts where genetic tools are available or they have been previously engineered for the heterologous production of bioactive compounds. Cloning and heterologous expression of the complete gene cluster have been reported for the biosynthesis of secondary metabolites. Tetrangulol and tetrangomycin have been synthesized in S. rimosus NRRL 3016 [14].
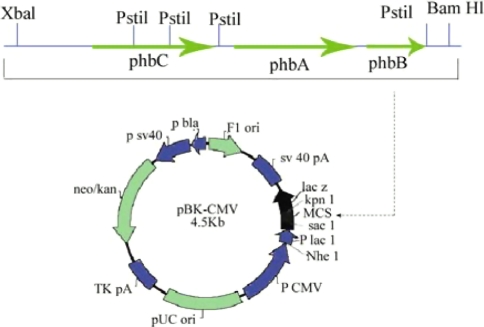
Another important example is the genes encoding the polyhydroxybutyrate (PHB) biosynthetic pathway of Ralstonia eutropha is transferred into industrially convenient organism Saccharomyces cerevisiae [15].
Yeast strains were equipped in their cytoplasm with the phaABCRe operon containing genes phbA, phbB and phbC of the PHA biosynthetic pathway of R. eutropha, which encode β-ketothiolase, NADPH linked acetoacetyl-CoA reductase and PHA synthase, respectively (Fig. 3). Resultant recombinant yeast was able to synthesize PHA and PHB in sufficient amount. This PHB is used to produce bioplastics which is biodegradable [16].
Fig. 3.
Diagram of the episomal vector pBKCMV, the linear map represents the PHB operon of R. eutropha derived from plasmid pBHR68 [14]
Engineering Regulatory Network
Genes for the biosynthesis of metabolic pathways are commonly grouped together in clusters on the chromosome including their pathway specific regulatory genes. Pathway specific regulators can have either positive (activators) or negative (repressors) effects on the expression of gene cluster elements. In microbes generally all the genes are not expressed at a time. Although microorganisms are extremely good in presenting us with an amazing array of valuable products, they usually produce them only in amounts that they need for their own benefit; thus, they tend not to overproduce their metabolites [16]. But in industries we want continuous synthesis of products. For that we engineered microbes by up and down regulation approach.
In up regulation approach we engineered the microbes in such a way that it synthesizes particular activator continuously so that microbes continuously synthesized our desired product even if it is not needed any more. For example continuous expression of SARP (Antibiotic Regulatory Protein) positive regulators has been reported to increase and continuous production of different secondary metabolites such as actinorhodin in Streptomyces coelicolor.
In down regulation approach we engineered microbes in such a way that it avoids the synthesis of particular inhibitor so that microbes continuously synthesize our desired product. For example inactivation of pathway specific repressors in Streptomycesgriseus leads to increase the production of chromomycin [17].
The importance of regulatory control in metabolic processes is widely acknowledged and several enquiries are being made in understanding regulation at various levels of the metabolic hierarchy [18]. Advancement in complementary aspects of regulation of the gene, protein, metabolite implementing their discoveries in practical applications in industries. Various functional genomics tools have helped to advance our understanding of stress signal perception and transduction and of the associated molecular regulatory network. These tools have revealed several stress-inducible genes and various transcription factors that regulate the stress inducible systems. More complex aspects of metabolism cannot be altered as desired by manipulating the metabolic gene(s), such as, increasing the pH tolerance or expanding the range of consumable substrates in microorganisms. This is because cells have evolved to comprise a complex network of regulatory mechanisms that counteract the genetic mutation by employing alternative pathways for continued robust performance [19]. Careful analysis of the regulatory control mechanisms governing the shift in metabolic processes facilitates the application of metabolic engineering with greater efficiency. The generation of high throughput global data which is used in the integrated methodologies proves to be an expensive venture and will undeniably require extensive knowledge about computer modeling, physiology, and metabolism as well as excellent technical skills in measuring gene and protein expression and metabolic flux analysis.
Gene Insertion and Deletion
Sometimes the expression of non native pathway may lead to metabolic imbalance. So it is desirable to seek the pathways that are compatible to the host and manipulating this pathway with addition of one or two gene, we might get new product. Likewise by deleting one or two genes we can restrict some pathways to get our desired product. For example in E. coli 2-keto acids are intermediates in amino acid biosynthesis pathways. These keto acids can be converted to higher alcohols, which have properties to be used as fuel. For that genes encoding 2-keto acid decarboxylase and alcohol dehydrogenase is inserted into E. coli from yeast S. cerevisiae.
Redirecting Metabolic Pathway
In this approach a multiple changes is made in a single pathway to direct it for the synthesis of particular product. For example E. coli produces succinic acid as a minor fermentation product. E. coli prefers to produce much more acetic acid, formic acid, lactic acid, and ethanol rather than succinic acid during anaerobic fermentation. Thus it is necessary to redirect metabolic fluxes for increasing succinic acid production as well as reducing formation of other metabolites.
New metabolic pathways are supported by integration of systems biology tools such as transcriptomics, proteomics, metabolomics, and fluxomics characterization of new mutants and for the production of the desired fuel-grade products [20]. Strain optimization process is improved by applying different models and computational analysis during fermentation process. The genome-scale metabolic modeling and analysis used to design culture media for enhancing the performance of P. pastoris. Functional genomics approach is one of the important tools used for overproduction of folate and genes are expressed in Lactobacillus plantarum WCFS1 [21].
Stimulation by Precursors
Precursors stimulate production of secondary metabolites either by increasing the amount of a limiting precursor or by inducing a biosynthetic enzyme. These are usually amino acids but other small molecules also function as inducers. Effectors are often precursors and one has to determine whether the effect is merely due to an increase in precursor supply and/or includes induction of one or more synthesis of the biosynthetic pathway. Stimulatory precursors that are also inducers include various amino acids viz. tryptophan for dimethylallyltryptophan synthetase in ergot alkaloid biosynthesis [22], leucine for bacitracin synthetase [23].
Genetic Knockout of Loci
Metabolic engineering has been used to introduce novel biochemical pathways and realign metabolic fluxes in microorganisms to improve product yields. But, concurrent host-specific physiological perturbations, such as altered specific growth rate and pleiotropic metabolic effects derived from the desired genotypic changes can limit overall productivity. Therefore, genetic knockout of loci approaches to “cell condition” or dynamically change intracellular architecture in response to environmental cues and hence affect heterologous product stability [24]. Transient down-regulation of a target gene using antisense technology was used to increase activity of a recombinant enzyme.
Quorum Sensing
Bacteria themselves can produce extracellular chemical signals for intercellular communication has evoked a new paradigm for gene regulation which is termed ‘quorum sensing’. Quorum sensing enables bacteria for bacterial cell-to-cell communication enables population density-based control of gene transcription via the production, release and sensing of low-molecular weight compounds [25]. Extracellular signaling provides a new basis for control over molecular and cellular processes as well as population behavior.
There are two kinds of quorum sensing: species-specific and interspecies. Species-specific quorum sensing in Gram-negative bacteria is mediated by acylhomoserine lactones (AHLs) with various moieties distinguishing signals among species. In Gram positive bacteria, species-specific quorum sensing is mostly facilitated through small peptides. Few reports on the use of quorum sensing have been introduced for improving recombinant protein yield in bacterial expression system.
Opportunities
Impact of new tool
In the last few years there have been significant advances in the genetic tools for metabolic engineering. A major difficulty in metabolically engineered microorganism for secondary metabolite production is that the biosynthetic pathway is often unknown. The lack of knowledge of biosynthetic pathway does not determined conventional strain improvement programs. For most industrially important antibiotics, a large number of strains have been isolated in the course of strain improvement “Reverse engineering” can possibly be combined with traditional strain improvement program leads to a more rational approach of metabolic engineering.
Integration of metabolic engineering and process engineering
Heterologous expression of proteins for secondary metabolite production, a function that is closer to reality, may enable microorganisms to produce an antibiotic they do not normally produce unaided. To date this application of this strategy is largely restricted to the production of single enzymes into the host microorganism. The possibility of expressing the entire pathway or a major portion of the pathway to a new host organism that exhibits more desirable characteristics has just begun to be exploited [26]. The original producer may not have the desired properties for large scale cultivation in industrial reactors, or may not have sufficient flux of the precursors. Heterologous expression enables a better host to be used for the production. It may also allow the combination of segment, from each of two different pathways. Each of the particular segments of the pathway may offer better reaction characteristics. The two segments may arise from the same or different species.
Future Prospects
Metabolic engineering approaches uses to design microbes for industrial application. It seems to be possible to obtain strains that would be able to function satisfactorily under unconventional environmental condition with high product producing ability [26].
The concept of green factories that is to engineer the green plant to produce desired product seems to be fulfill in near future with the advancement of this technologies.
With advance in experimental techniques, high throughput data from genomics, transcriptomics and proteomics will help in identify the elements of the biological networks and the interaction between them in great detail. And also development of new technologies like gene shuffling shown promising future of metabolic engineering.
It is clear that when the kinetic and biochemical information on the pathway leading to the product is available, a quantitative analysis to identify the rate limiting factors can drastically increase the probability of success in metabolic engineering.
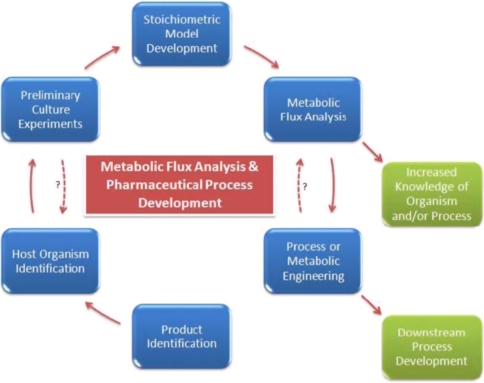
It is likely in the future that the metabolic engineering of secondary metabolism will involve not only the relaxation of the rate controlling steps in the structural genes, but also the deregulation and increased flux of the precursor biosynthesis. However, metabolic flux analysis remains the most widely used approach (Fig. 4). As more regulatory gene governing the biosynthesis of secondary metabolites is being unearthed, emphasis on the deregulation or restructuring of the regulatory network is likely to increase [28].
Fig. 4.
A schematic overview of low metabolic flux analysis fits into development of pharmaceutical production process [27]
Conclusion
The future success of the pharmaceutical industry depends on the identification or development of new compounds with novel activities or directed to more specific targets. The recent successes of metabolic engineering of some bacteria mark the beginning of a development towards sustainable biotechnological production of speciality fine and bulk chemicals [29]. It is expected that an increasing number of targets for strain development will be identified as the result of genome, transcriptome, proteome and other systems biology analyses. The current achievements and the observed trends bode well for an increasing impact of microbial biotechnology on chemical production.
Acknowledgments
We would like to thank P. P. Tyag Vallabh Swamiji to insist us in preparing the manuscript. This work was supported in part by Department of Biotechnology, Virani Science College, Rajkot.
References
- 1.Lessard P. Metabolic engineering, the concept coalesces. Nat Biotechnol. 1996;14:1654–1655. doi: 10.1038/nbt1296-1654. [DOI] [PubMed] [Google Scholar]
- 2.Stephanopoulos G. Metabolic fluxes and metabolic engineering. Metab Eng. 1999;1:1–11. doi: 10.1006/mben.1998.0101. [DOI] [PubMed] [Google Scholar]
- 3.Maris AJA, Abbott DA, Bellissimi E, Vanden BJ, Kuyper M, Luttik MAH, Wisselink HW, Scheffers WA, Dijken JP, Pronk JT. Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Antonie van Leeuwenhoek. 2006;90:391–418. doi: 10.1007/s10482-006-9085-7. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y-T, Bennet GN, San KY. Genetic and metabolic engineering. Electron J Biotechnol. 1998;1(3):134–141. doi: 10.2225/vol1-issue3-fulltext-3. [DOI] [Google Scholar]
- 5.Kell DB. Metabolomics and systems biology: making sense of the soup. Curr Opin Microbiol. 2004;7:296–307. doi: 10.1016/j.mib.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Farmer WR, Liao JC. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat Biotechnol. 2000;18:533–537. doi: 10.1038/75398. [DOI] [PubMed] [Google Scholar]
- 7.Yazdani SS, Gonzalez R. Engineering Escherichia Coli for the efficient conversion of glycerol to ethanol and coproducts. Metab Eng. 2008;10:340–351. doi: 10.1016/j.ymben.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Gupta PK (2007) Metabolic engineering for over production of metabolites. Elem Biotechnol 458–470
- 9.Lee FC, Rangaiph GP, Lee D. Modeling and optimization of multi-product biosynthesis factory for multiple objectives. Metab Eng. 2010;12:251–267. doi: 10.1016/j.ymben.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Adrio JL, Demain AL. Genetic improvement of process yielding microbial products. FEMS Microbiol. 2006;30(2):187–214. doi: 10.1111/j.1574-6976.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- 11.Schwender J, Ohlrogge JB, Shachar-Hill Y. A flux model of glycolysis and the oxidative pentose phosphate pathway in developing Brassica napus embryos. J Biol Chem. 2003;278:29442–29453. doi: 10.1074/jbc.M303432200. [DOI] [PubMed] [Google Scholar]
- 12.Ostergaard S, Olsson L, Nielsen J. Metabolic engineering of Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2000;64(1):34–50. doi: 10.1128/MMBR.64.1.34-50.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anesiadis N, Cluerr WR, Mahadevan R. Dynamic metabolic engineering for increasing bioprocess productivity. Metab Eng. 2008;10:255–266. doi: 10.1016/j.ymben.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Seong-Tshool H, et al. Cloning and heterologous expression of the entire gene clusters for PD 116740 from Streptomyces strain WP 4669 and tetrangulol and tetrangomycin from Streptomycesrimosus NRRL 3016. J Bacteriol. 1997;179(2):470–476. doi: 10.1128/jb.179.2.470-476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abd-el-Haleem D, Amara A, Zaki S, Abulhamad A, Abulreesh G. Biosynthesis of biodegradable polyhydroxyalkanoate biopolymers in genetically modifies yeast. Int J Environ Sci Technol. 2007;4(4):513–520. [Google Scholar]
- 16.Madison LL, Huisman GW. Metabolic engineering of poly (3-hydroxyalkanoates): from DNA to plastics. Microbiol Mol Biol Rev. 1999;63(1):21–63. doi: 10.1128/mmbr.63.1.21-53.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura CE, Whited GM. Metabolic engineering for the microbial production of 1,3-propanediol. Curr Opin Biotechnol. 2003;14:454–459. doi: 10.1016/j.copbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Castelo R, Roverato A. Reverse engineering molecular regulatory networks from microarray data with qp graphs. J Comput Biol. 2009;16(2):213–227. doi: 10.1089/cmb.2008.08TT. [DOI] [PubMed] [Google Scholar]
- 19.Valliyodan Babu, Nguyen HT. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr Opin Plant Biol. 2006;9:1–7. doi: 10.1016/j.pbi.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Wesolowski J. Antifungal compounds redirect metabolic pathways in yeasts: metabolites as indicators of modes of action. J Appl Microbiol. 2010;108:462–471. doi: 10.1111/j.1365-2672.2009.04443.x. [DOI] [PubMed] [Google Scholar]
- 21.Wegkamp A, et al. Physiological responses to folate overproduction in Lactobacillus plantarum WCFS1. Microb Cell Factor. 2010;9:100. doi: 10.1186/1475-2859-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krupinski VM, Robbers JE, Floss HG. Physiological study of ergot: induction of alkaloid synthesis by tryptophan at the enzymatic level. J Bacteriol. 1976;125:158–165. doi: 10.1128/jb.125.1.158-165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haavik HI, Froyshov O. On the role of l-leucine in the control of bacitracin formation by Bacillus licheniformis. In: Kleinkauf H, Dohren H, editors. Peptide antibiotics: biosynthesis and functions. Berlin: Walter de Gruyter & Co; 1982. pp. 155–159. [Google Scholar]
- 24.DeLisa MP, Bentley WE. Bacterial autoinduction: looking outside the cell for new metabolic engineering targets. Microb Cell Factor. 2002;1:5. doi: 10.1186/1475-2859-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.March JC, Bentley WE. Quorum sensing and bacterial cross-talk in biotechnology. Curr Opin Biotechnol. 2004;15:495–502. doi: 10.1016/j.copbio.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Falb M, Muller K, Konigsmaier L, Oberwinkler T, Horn P, Gronau S, Gonzalez O, Pfeiffer F, Bornberg-Bauer E, Oesterhelt D. Metabolism of halophilic archaea. Extremophiles. 2008;12:177–196. doi: 10.1007/s00792-008-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boghigian GA, Seth G, Kiss R, Pfeifer BA. Metabolic flux analysis and pharmaceutical production. Metab Eng. 2010;12:81–95. doi: 10.1016/j.ymben.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Olano C, Lombi F, Mendez C, Salas JA. Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab Eng. 2008;10:281–292. doi: 10.1016/j.ymben.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, et al. Expanding metabolism for biosynthesis of non natural alcohols. Proc Natl Acad Sci USA. 2008;105:20653–20658. doi: 10.1073/pnas.0807157106. [DOI] [PMC free article] [PubMed] [Google Scholar]