Abstract
The present study deals with the occurrence of vesicular arbuscular mycorrhizal fungi in three cultivars of rice in Barak valley. Three cultivars of rice were Pankaj, Malati and Ranjit. The results revealed the association of VAM fungi in all the cultivars of rice. The association was maximum in Pankaj cultivar followed by Malati, and Ranjit, respectively, in all the three sampling phases. All the three cultivars of rice crop showed maximum soil spore population and number of VAM fungal species at the harvesting phase (135 DAS) and minimum at the phase of maturation (90 DAS). Glomus species were found dominating followed by Acaulospora species. Glomus microcarpum, Glomus claroideum, Glomus mosseae and Acaulospora scrobiculata were found in all the three fields.
Keywords: Colonization, Spore population, Vesicular arbuscular mycorrhizal fungi, Barak valley
Introduction
Vesicular arbuscular mycorrhizal fungi forms symbiotic association with roots of most terrestrial plants including many agricultural crops [1]. These are known to occur globally in a broad range of dissimilar environments from artic to tropic and occupy a wide range of ecological niches [2]. The role of VAM fungi in the improvement of crop plant is well documented [3, 4, 5]. VAM fungi are known to improve the nutrient status of the plants, increase growth and development, protects plant against pathogen and confer resistance to drought and salinity [6].
Colonization by native AMF in rice plant has been reported earlier [7]. Partial dependency of upland rice on native AMF for phosphorus acquisition has also been reported by earlier worker [8]. The occurrence of VAM fungi at varying stages of growth of rice plants has been studied by Dubey et al. [9].
In recent years, the application of artificially produced inoculum of VAM fungi has increased its significance in the field of agriculture, horticulture and forestry. Application of mycorrhizal inoculum increased the soil nutrients and root colonization in rice plants as reported by earlier worker [10].
During last two decades, different aspects of mycorrhizal association on crop plants have been studied extensively in different geographical and agricultural conditions. However, in North East India, particularly in Barak valley, very little or almost no attention has been paid on the study of VAM fungi. Rice is one of the most important crops and staple food of people in North East India. Therefore, an attempt has been made in the present investigation to study the occurrence and distribution of VAM fungi in three rice cultivars of Barak valley.
Materials and Methods
Three crop fields with rice cultivars of Pankaj, Malati and Ranjit, respectively, were selected from Barak valley which is located in Southern part of Assam, India and between 23 and 24°N latitude and between 92 and 93°E longitude. The sowing time of all the three rice crops ranged from May to June and harvesting time ranged from October to November. Sampling was done in three growth phases of the rice plants namely seedling phase (45 DAS), phase of maturation (90 DAS) and harvesting phase (135 DAS). Samples consisting of roots and soils were collected from the rhizospheric region of all the plants mentioned above. Five plants were selected from each site. Fine roots were collected from each plant. Soil samples were collected from 0–10 cm depth in the adjoining regions of the roots as most spores were reported to be concentrated in this zone. Soils and roots were collected in sterile polythene bags. VAM spores were isolated from each soil sample using wet sieving and decanting technique [11]. Fifty grams of soil sample was dissolved in 500 ml distilled water and allowed to stand. The suspension was passed through a series of sieves of sizes ranging from 200 to 45 μm. Sieved spores were counted under steriozoom dissecting microscope. Spores were separated from soil debris by sucrose centrifugation [12]. The sieved spores were suspended in 40% sucrose solution and centrifuged at 2,000 rpm for 1 min. The supernatant containing spores were pored through the sieve of 400 mesh and rinsed with distilled water to remove sucrose. Sieved spores were mounted in polyvinyl lacto phenol (PVL) on glass slides for microscopic observation. Spores were identified using the taxonomic keys of http://invam.caf.wvu.edu. The roots were washed several times in tap water and cut into pieces of 1 cm length. The roots were processed as per Philips and Hayman [13]. Roots were heated in 5% KOH at 90°C for 15 min. Roots were rinsed in tap water followed by treatment with 1% HCl for 10 min. Roots were stained in Trypan blue for 15 min at 90°C. Roots were stored in glycerol. Evaluation of root colonization was done following Grid line intersect method [14]. A Grid made of 1.2 cm2 was etched on plastic petri dish lid. Stained roots were deposited in the lid, flattened with the petri dish bottom and examined under microscope at 100×. Colonized root sections intersecting the lines of grid were counted and compared with the total number of roots intersecting the grid lines. The percent root colonization was calculated by the following formula:
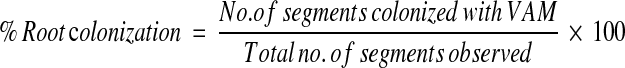 |
Soil Samples were Analyzed for Different Physico Chemical Properties
Soil pH
For determination of soil pH, 20 g of sample soil was taken in a clean beaker and mixed with 100 ml of distilled water. The mixture was stirred for 10 min and allowed to stand for 30 min. An electronic digital pH meter was used for measuring pH.
Soil Moisture
Soil moisture content was determined by oven dry technique [15]. 10 g of freshly collected soil sample was dried in a hot air oven at 105°C for 24 h. The percentage of moisture content was calculated by the following formula:
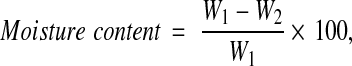 |
where W1 = wt. of soil before drying (g) and W2 = wt. of soil after drying (g).
Soil Texture
The soil texture was determined by Bouyoueos Hydrometer method [16]. Fifty grams air-dried and sieved soil was taken in a beaker and 25 ml of 5% calgon and 400 ml distilled water were added to it. The suspension was mixed thoroughly for 15 min with the help of a magnetic stirrer and the volume was made up to 1 l. The initial reading was taken with the help of soil Hydrometer. The system was allowed to stand for 4 h and final reading was taken. Percentage of sand, silt and clay were estimated by the following formula:
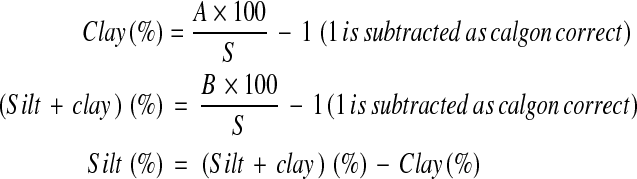 |
where A = Final hydrometer (g l−1) reading, B = Initial hydrometer (g l−1), S = wt. of the airdried soil (g).
Soil Nitrogen
Soil nitrogen was estimated by alkaline permanganate method [17]. 20 g of soil was taken in an 800 ml kjeldahl flask and 20 ml water was added and swirled. To it, 1 ml of liquid paraffin and a few glass beads were added to prevent frothing and bumping during distillation. 100 ml of 0.32% KMnO4 solution was added to it. Twenty millilitre of Boric acid containing mixed indicator (methyl red plus Bromocresol green) was taken in a 250 ml conical flask and the end of delivery tube was fitted to it. One hundred millilitres of 2.5% NaOH solution was added to the distillation flask and immediately fitted with the distillation apparatus. Liberated ammonia turned pinkish colour of Boric acid with mixed indicator solution to green. The distillate was titrated with 0.02 N H2SO4 to the original pink. Blank titration was carried for final calculation. Available nitrogen (kg ha−1) was calculated by the following formula:
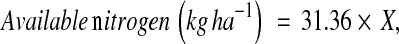 |
where X = actual volume of H2SO4 used in titration.
Soil Phosphorus
Spectrophotometric method was followed for estimation of available Phosphorus [18]. Five grams of air-dried soil was mixed with 50 ml of Bray and Kurtz extracting solution (0.03 N NH4F in 0.025 N HCl solution) was added. The mixture was filtered through Whatman No. 42 filter paper. Five millilitres of aliquot of the extract was taken, the volume was made 20 ml with distilled water. To it 4 ml Murphy-Riley colour developing solution (a solution containing 250 ml of 2.5 M H2SO4, 75 ml ammonium molybdate solution, 50 ml ascorbic acid solution, 25 ml of antimony potassium tartrate solution and 100 ml of distilled water) was added. Blank was prepared without soil. After 15 min, the intensity of blue colour was measured with the help of spectrophotometer at 730 nm. With the help of standard curve, the quantity of available phosphorus in the soil was calculated by the following formula:
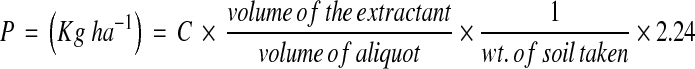 |
where C = μg P in the aliquot (obtained form the standard curve).
Soil Potassium
Available potassium in the soil was estimated using flame photometer. Five grams of air-dried soil was taken in a centrifuge tube and 25 ml of neutral normal ammonium acetate solution was added, shaken and centrifuged at 2,000 rpm for 10 min. The supernatant was collected and fed in the flame photometer. Potassium content in the sample was determined with the help of standard curve. Potassium was calculated by the following formula:
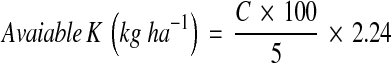 |
where C = μg ml−1
Soil Organic Carbon
Organic carbon content of the soil was determined by colorimetric method [19]. One gram soil sample was mixed with 10 ml of 1 N Potassium dichromate and 20 ml conc. sulphuric acid, swirled and kept for 30 min on an asbestos sheet. The content was centrifuged to a clear state. The green colour of the supernatant was read on the colorimeter at 660 nm. For preparation of Standard curve, 1–10 mg anhydrous sucrose were taken in conical flasks and 10 ml of 1 N Potassium dichromate and 20 ml of conc. sulphuric acid were added to each flask. After 30 min, readings were taken in colorimeter at 660 nm.
A graph of absorbance against the known standards was plotted. The percent organic carbon was calculated from the standard curve with the help of colorimetric reading of the test sample.
Statistical Analysis
Correlation coefficient was calculated for VAM spore population and different soil physico-chemical parameters by computational method using SPSS 7.5 compiler in windows mode.
Results and Discussion
The data on the soil physico- chemical parameters, spore population and root colonization of VAM fungi associated with three rice cultivars Pankaj, Malati and Ranjit are presented in the Table 1. The result revealed that all the three rice cultivars harboured mycorrhizal association. The maximum spore population was found in Pankaj cultivar followed by Malati and Ranjit cultivar. The data presented in the Table 1 also showed that spore population in the three rice cultivars decreased in the phase of maturation (90 DAS) and then again increased during harvesting phase (135 DAS). The spore population in Pankaj variety was 475 per 50 g soil in the seedling phase (45 DAS) which came down to 290 per 50 g soil in the phase of maturation (90 DAS) and then again increased to 587 per 50 g soil in the harvesting phase (135 DAS). Similar trend was also observed in other two cultivars of rice crops.
Table 1.
Soil physico chemical parameters, root colonization and spore population per 50 g soil in three (3) rice fields
| Rice cultivars | Soil physico-chemical parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sampling Phase | pH | Moisture Content (%) | Texture | Organic carbon (%) | N (kg ha−1) | P (kg ha−1) | K (kg ha−1) | % Root colonization (mean of 5 plants) |
Spore population per 50 g Soil |
|
| PANKAJ | Seedling (45 DAS) | 5.2 | 24 | CLAY LOAM | 1.22 | 288 | 20.5 | 124 | 68 | 475 |
| Maturation (90 DAS) | 5.1 | 27.5 | 1.18 | 275 | 18 | 115.3 | 45 | 290 | ||
| Harvesting (135 DAS) | 4.9 | 22 | 1.2 | 298 | 17.5 | 112.5 | 80 | 587 | ||
| MALATI | Seedling (45 DAS) | 5.4 | 23 | 0.99 | 275 | 29.6 | 130 | 54 | 457 | |
| Maturation (90 DAS) | 5 | 28 | 0.98 | 273 | 25.7 | 122 | 45 | 399 | ||
| Harvesting (135 DAS) | 5.2 | 21.7 | 0.98 | 278 | 24 | 118 | 70 | 560 | ||
| RANJIT | Seedling (45 DAS) | 5.1 | 23.5 | 1.15 | 265 | 39.5 | 153 | 55 | 370 | |
| Maturation (90 DAS) | 5 | 28 | 1.13 | 259 | 37.5 | 151 | 36 | 203 | ||
| Harvesting (135 DAS) | 4.9 | 21 | 1.13 | 267 | 35 | 145 | 64 | 435 | ||
The data presented in the Table 1 also showed that highest root colonization was observed in Pankaj cultivar followed by Ranjit and Malati cultivar. All the three rice cultivars showed maximum root colonization at the harvesting phase and minimum at the phase of maturation.
Soil physico-chemical parameters viz. pH, moisture content, soil texture, organic carbon, available N, P and K of all the three crop fields are presented in the Table 1 and values of co-efficient of correlation of VAM spore population with different physico-chemical parameters are presented in the Table 2. In the present investigation, pH and organic carbon showed no significant correlation with spore population. Available N showed significant positive correlation with VAM spore population in all the three rice fields, i.e., increase in nitrogen concentration of soil was followed by increase in VAM spore population. Concentration of nitrogen was recorded higher in the rhizospheric soil of Pankaj cultivar than those of other two cultivars which might have contributed to higher population of VAM fungi in Pankaj rice cultivar as it has been reported by the earlier worker [20] that higher concentration of nitrogen increased the incidence and population of VAM fungi. The increase in the soil nitrogen contents during the harvesting phase of all the rice cultivars may be attributed to nitrogenase activity of soil diazotrophs. Deb Roy et al. [21] isolated six species of soil diazotrophs from the rice agroecosystems of Barak velley. Earlier worker [22] also reported that soil diazotrophs contributes from 10 to 80 kg N ha−1 per cropping season.
Table 2.
Rice: coefficient of correlation of VAM spore population with different soil physico-chemical parameters in three rice cultivars
| Soil physico-chemical parameters | Coefficient of correlation | ||
|---|---|---|---|
| Pankaj | Malati | Ranjit | |
| pH | −0.542 | 0.356 | −0.272 |
| Moisture content % | −1.000* | 0.884 | −0.996 |
| Organic Carbon % | −0.617 | 0.159 | 0.246 |
| Nitrogen (kg ha−1) | 0.998** | 0.999** | 0.999** |
| Phosphorous (kg ha−1) | 0.015 | −0.444 | −0.333 |
| Potassium (kg ha−1) | −0.095 | 0.474 | −0.528 |
* Significant at P < 0.01
** Significant at P < 0.05
In the present investigation, available P and K content of soil showed no positive correlation with the VAM spore population in the rhizospheric soil of three rice cultivars.
Distribution of VAM fungal species at the three different rice fields are presented in the Table 3. All together 17 VAM fungal species were found in three rice field during investigation. The data revealed uneven distribution of VAM fungal species in three rice crops field. Not only spore population but the number of VAM fungal species was also maximum in Pankaj variety followed by Ranjit and Malati cultivars of rice. The total number of associated VAM fungal species were 14 in Pankaj variety, 11 in Malati and 9 in Ranjit cultivars. Variation in the distribution of VAM fungal species was observed at the three sampling phases in all the three rice crops fields. Highest number of VAM fungal species was found in the harvesting phase and minimum in the phase of maturation. The data presented in the Table 3 also indicated that Glomus microcarpum, Glomus claroideum, Glomus mosseae and Acaulospora scrobiculata were common in all the three fields. Glomus facundisporum was found in Pankaj rice field only. Glomus leptoitichum was found in Ranjit rice field and Acaulospora laevis was found in Malati rice crop field only. The presence of Acaulospora scrobiculata, Glomus microcarpum, Glomus claroideum and Glomus mosseae in all the rice fields may depict that these microsymbionts were favoured by the host.
Table 3.
VAM fungal species isolated from the rhizosphere of three rice cultivars
| VAM fungal species | Pankaj | Malati | Ranjit | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S 1 | S 2 | S 3 | S 1 | S 2 | S 3 | S 1 | S 2 | S 3 | |
| 1 | − | − | − | + | − | + | − | − | − |
| 2 | + | + | + | + | + | + | + | + | + |
| 3 | + | + | + | − | − | − | + | − | + |
| 4 | + | + | + | + | + | + | − | − | − |
| 5 | + | + | + | + | + | + | − | − | − |
| 6 | + | + | + | − | − | − | − | − | − |
| 7 | + | + | + | + | + | + | + | + | + |
| 8 | + | − | + | − | − | − | − | − | + |
| 9 | − | − | + | − | − | − | − | − | − |
| 10 | + | + | + | + | − | + | − | − | − |
| 11 | − | − | + | + | − | + | − | − | − |
| 12 | − | − | − | − | + | + | − | − | + |
| 13 | − | − | + | + | + | − | − | − | |
| 14 | − | − | − | − | − | − | + | + | + |
| 15 | + | + | + | + | + | + | + | + | + |
| 16 | + | + | + | + | + | + | + | + | + |
| 17 | + | − | + | − | − | − | + | + | + |
S 1 Seedling phase (45 DAS), S 2 Phase of maturation (90 DAS), S 3 Harvesting phase (135 DAS)
+ Presence, − Absence
1. Acaulospora laevis Gerdemann & Trappe
2. Acaulospora scrobiculata Trappe
3. Acaulospora spinosa Walker & Trappe
4. Gigaspora gilmorei Trappe & Gerdemann
5. Gigaspora margarita Becker & Hall
6. Glomus aggregatum Schenck & Smith
7. Glomus claroideum Schenck & Smith
8. Glomus coronatum Giovannetti
9. Glomus facundisporum Schenck & Smith
10. Glomus fasciculatum (Thaxter) Gerd. & Trappe emend. Walker & Koske
11. Glomus fulvum (Berk. & Broome) Trappe & Gerd.
12. Glomus intradices Schenck & Smith sp. nov.
13. Glomus invermaium Hall
14. Glomus leptoitichum Schenck & Smith
15. Glomus microcarpum Tulasne & Tulasne
16. Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe
17. Scutellospora biornata Spain, Sieverding & Toro
The result indicated that three rice cultivars developed higher mycorrhizal association at the time of harvest than those of seedling phase and maturation phase. VAM spore population as well as number of VAM fungal species were more in the harvesting phase than the other two phases i.e. seedling phase and phase of maturation. Minimum spore population and number of VAM fungal species in the phase of maturation might be due to the presence of mycelial stage of VAM fungi in the plant roots and also due to the fact that plants were submerged in water at that phase. The reports of earlier workers [23, 24] revealed that spore population and number of VAM fungal species decreased during submerged condition of crop plants. The present findings are similar to the earlier reports.
It is well known fact that VAM fungi are not host specific [25, 26]. But there are reports of dissimilar colonization patterns of the same fungus in different host plants [27]. Colonization of a root system by VAM fungi is a dynamic process which is markedly influenced by climatic and edaphic factors such as season, soil fertility, pH, temperature, soil moisture, soil organic matter and host cultivar susceptibility to root infection. Quality and type of VAM propagules also influenced the dynamics root colonization [28]. The rate at which the root system becomes colonized is influenced not only by the formation of infective units and their rate of growth but also by the rate of growth of the root system [29, 30, 31].
Among the total 17 VAM fungal species reported from the rhizosphere soil of three rice cultivars, 11 species belonged to genus Glomus, three species belonged to genus Acaulospora, two species belonged to genus Gigaspora and only one species belonged to genus Scutellospora. The number of Glomus species was higher than other VAM fungal species associated with all the three rice cultivars. The result revealed that Glomus was the dominant genus in the rhizospheric soil of all the three rice cultivars namely Pankaj, Malati and Ranjit. The report of earlier worker [9] revealed that Glomus was the dominant genus in rhizosphere of rice plants followed by Acaulospora. The reports of earlier workers also revealed that Glomus is the dominant genus occurring in Indian soil [32, 33]. Monoharachary et al. [34] reviewed the diversity of VAM fungi in India and stated that genus Glomus is ubiquitous in various ecosystems in India. The observations made by the earlier workers support our present findings.
Acknowledgments
Authors are thankful to HOD, Department of Life Science, Assam University for extending all sorts of support to undertake the investigation etc.
References
- 1.Mohadevan A, Rahman N, Natarajan K. Mycorrhizae for green Asia. Madras: University of Madras; 1988. [Google Scholar]
- 2.Shrivastava D, Kapoor R, Shrivastava SK, Mukherji KA (1996) Vesicular arbuscular mycorrhiza—an overview. In: Mukherji KG (ed) Kluwer Academic Publishers, Netherlands. p.1–39
- 3.Krishna KR, Bagyaraj DJ. Influence of vesicular-arbuscular mycorrhiza on growth and nutrition of Arachis hypogea. Legume Res. 1982;5:18–22. [Google Scholar]
- 4.Katiyar RS, Das PK, Chowdhury PC, Ghosh A, Singh GB, Datta RK. Response of irrigated mulberry (Morus alba L.) to VA mycorrhizal inoculation under graded doses of phosphorus. Plant Soil. 1994;5:369–373. [Google Scholar]
- 5.Rao VP, Pawar SE, Singh SN (1995) Production and application of vesicular-arbuscular mycorrhizal inocula for sustainable agriculture. In: Adholaya A, Singh S (eds) Mycorrhizae Biofertilizer for the Future. TERI Publication, New Delhi, pp 424–428
- 6.Bagyaraj DJ, Varma V (1995) Interaction between arbuscular mycorrhizal fungi and plants. Their importance in sustainable agriculture and in arid and semiarid tropics. In: Advances in microbial ecology, Academic Press, London, pp 119–142
- 7.Maiti D, Variar M, Saha J (1995) Colonization of upland rice by native VAM under rainfed monocropped ecosystem. In: Roy AK, Sinha KK (eds) Recent advances in phytopathological research, MD Publication, New Delhi, pp 45–51
- 8.Saha R, Saha J, Bhattacharya PM, Maiti D, Chowdhury S (1999) Arbuscular mycorrhizal responsiveness of two varieties in nutrient deficient laterite soil (abstr.). In: Proc Nat Conf on Mycorrhiza, Barkhatullah University, Bhopal, pp 28, 5–7 March 1999
- 9.Dubey A, Mishra MK, Singh PK, Vyas D. Occurrence of AM fungi at varying stages of growth of rice plants. Proc Nat Acad Sci Ind. 2008;78(1):51–55. [Google Scholar]
- 10.Yeasmin T, Zaman P, Rahman A, Absar N, Khanum NS. Arbuscular mycorrhizal fungus inoculum production in rice plants. African J Agri Res. 2008;2:463–467. [Google Scholar]
- 11.Gerdemann JW, Nicolson TH. Spores of mycorrhizal endogone extracted from soil by wet sieving and decanting. Trans Br Mycol Soc. 1963;46:235–244. doi: 10.1016/S0007-1536(63)80079-0. [DOI] [Google Scholar]
- 12.Daniels BA, Skipper HD. Methods for the recovery and quantitative estimation of propagules from soil. In: Schenck NC, editor. Methods and principles of mycorrhizal research. St. Paul: The American Phytopathological Society; 1982. pp. 20–40. [Google Scholar]
- 13.Phillips JM, Hayman DJ. Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 1970;55:158–161. doi: 10.1016/S0007-1536(70)80110-3. [DOI] [Google Scholar]
- 14.Giovannetti M, Mosse An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980;84:489–500. doi: 10.1111/j.1469-8137.1980.tb04556.x. [DOI] [Google Scholar]
- 15.Allen SE. Chemical analysis of ecological materials. New Delhi: Blackwell Scientific Publications; 1974. [Google Scholar]
- 16.Piper CS. Soil and plant analysis (Asian Edition) Bombay: Hans Publishers; 1966. pp. 189–245. [Google Scholar]
- 17.Subbiah BV, Asija GL. A rapid procedure for the determination of available nitrogen in soils. Curr Sci. 1956;25:259–260. [Google Scholar]
- 18.Bray RH, Kurtz LT. Determination of total organic and available forms of phosphorus in soils. Soil Sci. 1945;59:39–45. doi: 10.1097/00010694-194501000-00006. [DOI] [Google Scholar]
- 19.Datta ND, Khera MS, Saini TR. A rapid colorimetric procedure for the determination of the organic carbon in soils. J Indian Soc Soil Sci. 1962;10:67–74. [Google Scholar]
- 20.Mosse B. Advances in the study of vesicular arbuscular mycorrhiza. Annu Rev Phytopathol. 1973;11:171–196. doi: 10.1146/annurev.py.11.090173.001131. [DOI] [Google Scholar]
- 21.Deb Roy B, Deb B, Sharma GD. Diversity of soil diazotrophs in acid stress rice agroecosystems of Barak valley of Assam, India. J Pure Appl Microbiol. 2009;3:117–124. [Google Scholar]
- 22.Rao VR, Ramakrishnan B, Adhya TK, Kanungo PK, Nayak DN. Review: current status and future prospects for associative nitrogen fixation in rice. World J Microbiol Biotechnol. 1998;14:621–633. doi: 10.1023/A:1008831914095. [DOI] [Google Scholar]
- 23.Ilag LL, Rasales AN, Elazegui FA, Mew TW. Changes in the population of infective endomycorrhizal fungi in a rice based cropping system. Plant Soil. 1987;103:67–73. doi: 10.1007/BF02370669. [DOI] [Google Scholar]
- 24.Vyas D, Dubey A, Singh PK, Mishra MK, Gupta RK. Distribution of VAM Fungi in two different rice crop fields. J Bot Soc Univ Sagar. 2006;41:160–170. [Google Scholar]
- 25.Mosse B. Specificity in vesicular-arbuscular mycorrhizae. In: Sanders FE, Mosse B, Tinker PB, editors. Endmycorrhizas. London: Academic Press; 1975. pp. 469–484. [Google Scholar]
- 26.Hayman DS. The physiology of vesicular arbuscular endo-mycorrhizal symbiosis. Can J Bot. 1983;61:944–963. doi: 10.1139/b83-105. [DOI] [Google Scholar]
- 27.Hepper C M (1985) Vesicular arbuscular mycorrhiza. In: Powell CL, Bagyaraj DJ (eds) CRC Press, Florida, p 5–112
- 28.Chandra KK, Jamaluddin Distribution of vascular arbuscular mycorrhizal fungi in coal mine overburden dumps. Indian Phytopathol. 1999;52(3):254–258. [Google Scholar]
- 29.Sutton JC. Development of vesicular-arbuscular mycorrhizae in crop plants. Can J Bot. 1973;50:1909–1914. doi: 10.1139/b72-241. [DOI] [Google Scholar]
- 30.Smith SE, Walker NA. A quantitative study of mycorrhizal infection in Trifolium: separate determination of the rates of infection and of mycelial growth. New Phytol. 1981;89:225–240. doi: 10.1111/j.1469-8137.1981.tb07485.x. [DOI] [Google Scholar]
- 31.Sanders FE, Sheikh NA. The development of vesicular-arbuscular mycorrhizal infection in plant root systems. Plant Soil. 1983;71:223–246. doi: 10.1007/BF02182658. [DOI] [Google Scholar]
- 32.Muthukumar T, Udaiyan K. Arbuscular mycorrhiza of plants growing in the Western Ghats region, Southern India. Mycorrhiza. 2000;9:297–313. doi: 10.1007/s005720050274. [DOI] [Google Scholar]
- 33.Bhattacharya S, Bagyaraj DJ. Arbuscular mycorrhizal fungi associated with Arabica coffea. Geobios. 2002;29:93–96. [Google Scholar]
- 34.Monoharachary C, Sridhar K, Singh R, Adholeya A, Suryanarayanam TS, Rawat S, Johri BN. Fungal biodiversity, distribution, conservation and prospecting of fungi from India. Curr Sci. 2005;89:1. [Google Scholar]


