Abstract
In the present study the haemolytic and proteolytic activity of extracellular products (ECP) secreted from Aeromonas hydrophila (CAHH14 strain) were studied with respect to temperature and different time of incubation as well as its lethal toxicity on rohu, Labeo rohita. The strain was isolated from Catla catla (showing abdominal dropsy symptom) collected from the pond of Central Institute of Freshwater Aquaculture (CIFA), Bhubaneswar, India and was characterized on the basis of biochemical tests. The highest production of haemolysin was achieved when the bacteria was grown at 35°C for 30 h. The proteolytic activity was found to be highest when the bacterium was grown at 30°C for 36 h. The haemolytic and proteolytic toxin produced by Aeromonas hydrophila was found to be lethal to rohu (LD50 1.7 × 104 cfu/ml). The lethality of ECP was decreased by heating and completely inactivated by boiling at 100°C for 10 min. This indicates that protease activity and haemolytic activity of A. hydrophila ECP was temperature dependant.
Keywords: Aeromonas hydrophila, Extracellular products, Lethal toxicity
Introduction
Aeromonas hydrophila is recognized as one of the most important potential freshwater fish pathogens responsible for causing gill and skin diseases among the fishes and leading to their high mortality [1]. It is consider as the etiological agent of fatal hemorrhagic septicemia and epizootic ulcerative syndrome that are manifested by external symptoms like local hemorrhages in the gills and anal area, dropsy, blisters, abscesses, scale protrusion, exophthalmia, tail rot, fin rot, abdominal swelling and internal symptoms like anemia, accumulation of ascetic fluid and damage to the organs, notably kidney and liver [2–5]. A. hydrophila is also suspected to be the principal causative agent of ulcerative disease noticed in cultured fish in Indo-Pacific region [6], Thailand and Malaysia [7]. Besides there was also report of isolation of A. hydrophila from dropsy infected common crap (Cyprinus carpio) from Meghalaya, India [8] that has caused massive mortality.
The pathogenesis of A. hydrophila is multifactorial [9, 10]. The extracellular product (ECP) secreted by A. hydrophila is regarded as an essential virulent factor as it contains a wide variety of enzymes and haemolysin [11–13] that exhibit cytotoxic, cytolytic, and haemolytic and enterotoxic properties [14, 15] leading to A. hydrophila infection in fishes. Allan and Stevenson [14] and Subashkumar et al. [16] have reported that the toxic fraction of ECP is associated with the haemolytic activity, whereas Kanai and Wakabayashi [17] and Sakai [18] have reported the protease as the major virulent factors in fish toxicity. The present investigation deals with the biochemical characterization of A. hydrophila (CAHH14 strain) collected from the infected Catla catla (showing abdominal dropsy symptoms) from the pond of Central Institute of Fresh water Aquaculture and to determine the potential hemolytic activity and proteolytic activity of crude ECPs secreted by A. hydrophila (CAHH14 strain) with respect to different incubation time. The lethal toxicity of ECP in Indian major crap, rohu, Labeo rohita was also evaluated using live and heat-killed A. hydrophila.
Materials and Methods
Collection and Identification of Bacteria
Aeromonas hydrophila strain was collected aseptically from the abdominal dropsy conditions of Catla catla obtained from the experimental ponds of Central Institute of Fresh Water Aquaculture. Single strain was found from 10 isolates basing on biochemical characterization [19]. The strain was coded as CAHH14 for all research purposes based on the virulence pattern. Presumptive identification of bacteria and colony morphology of bacteria was studied by observing the growth on the selective RS-media. The biochemical profile of CAHH14 strain with respect to ATCC-49140 strain of A. hydrophila was given in Table 1.
Table 1.
Biochemical properties of Aeromonas hydrophila
| Sl. No | Biochemical tests parameter | CAHH14 strain (10 isolates) | ATCC strain (reference strain) |
|---|---|---|---|
| 1. | Gram staining | − | − |
| 2. | Oxidation fermentation test | + | + |
| 3. | Oxidase | + | + |
| 4. | Catalase | + | + |
| 5. | Motility | + | + |
| 6. | Urease | + | + |
| 7. | RS growth | Y | Y |
| 8. | Indole | D | − |
| 9. | Methyl red | + | + |
| 10. | VP test | − | − |
| 11. | Citrate | + | + |
| 12. | Gas | + | + |
| 13. | Arginine dihydrolase | D | + |
| 14. | Lysine decarboxylase | + | + |
| 15. | Ornithine decarboxylase | − | − |
| 16. | 0/129 disc | R | R |
| 17. | Acid from | ||
| Lactose | + | D | |
| Galactose | + | D | |
| Maltose | + | + | |
| Fructose | + | + | |
| Mannose | + | − | |
| Rhamnose | + | + | |
| Dulcitol | + | + | |
| Inositol | + | + | |
| Sorbitol | + | − | |
| Adonitol | + | + | |
Y Yellow, D partial, + positive; − negative
Bacterial Strain and Growth Conditions
The CAHH14 strain was sub cultured on nutrient agar at 25°C for 48 h. Culture from slant was used to prepared nutrient broth cultures which were incubated at three different temperatures (25, 30 and 35°C) for 36 h with shaking. Samples were removed at specific time intervals (0, 6, 12, 18, 24, 30 and 36 h) for the observation of bacterial growth rate by determining the OD value at 610 nm [20].
Extracellular Products Collection
The ECP was collected as per the protocol given by Sirirat et al. [21] with modification. The bacterial cells were removed from the culture broth samples by centrifuging at 2,800 rpm for 45 min. The supernatant fluid collected was used as source of crude ECP for determination of hemolytic and proteolytic activity.
Haemolytic Activity of ECP
Haemolytic activity of ECP was determined by method given by Kwapinski [22] with slight modification. Blood was drawn aseptically from sheep and was mixed with equal volume of Alsever’s solution (citric acid 2 mM, dextrose 110 mM, sodium citrate 20 mM, NaCl 71 mM). It was then subjected for centrifugation at 2,500 rpm for 15 min. Supernatant was removed and RBC was collected, which was then washed in PBS (pH 7.4) and further centrifuged at 4,000 rpm for 5 min. A standard 2% erythrocytes suspension was prepared so that 0.5 ml of completely lysed cells in a total assay volume of 5 ml would give an OD value of 0.4 at 540 nm. The assay system contained 0.5 ml of standard erythrocytes suspension and 4.5 ml of appropriately diluted extracts of crude ECP and was incubated for 1 h at ambient temperature. The reaction mixtures were then centrifuged at 4,000 rpm for 5 min and the OD value of supernatant fluid were measured at 540 nm. One unit of hemolytic activity was defined as the amount of toxin that gave an OD value of 0.4 at 540 nm.
Proteolytic Activity of ECP
Proteolytic activity of ECP was determined as per the method described by Sakai [18]. A substrate solution was prepared by dissolving 1% (w/v) casein in 0.1 mol/l glycine–sodium hydroxide buffer (pH 9.6). Equal volume of ECP (1 ml each) was added to 1 ml of substrate solution and incubated at 30°C for 10 min. The reaction was then stopped by adding 0.1 ml of 1 mol/l trichloroacetic acid and the precipitate was removed by centrifuging at 6,000 rpm for 10 min. The OD value of the supernatant fluid was measured at 280 nm. As a control trichloroacetic acid was added immediately to the reaction mixture of ECP and substrate solution and 10 min incubation period was avoided. One unit of proteolytic activity was expressed as an increase of 0.01 in the OD value at 280 nm with casein as a substrate.
Determination of LD50 Value and Lethal Toxicity of ECP
For determining the LD50 value, groups of rohu fingerling weighing 12–15 g were used. Each group consisted of 10 fish and was kept in fiberglass reinforced plastic (FRP) tank with water of 30 l capacity. The fish of each group were injected intraperitoneally with 0.1 ml of bacterial cell suspensions (CAHH14 strain) at concentrations of 1.7 × 103, 1.7 × 104, 1.7 × 105 and 1.7 × 106 cfu/ml respectively. Control fish were injected with 0.1 ml of PBS (pH 7.4). Fish were monitored for 5 days and mortality was recorded. To verify the Koch’s postulates dead or moribund fish were sampled for the presence of challenge bacterium. LD50 value was calculated as per the method of Reed and Muench [23].
Lethal toxicity of ECP to rohu from of A. hydrophila culture grown at 30 and 35°C was estimated as per the method given by Khalil and Mansour [20]. Culture supernatant fluid was filter sterilized by using a 0.45 μ membrane filter. A portion of this filtered ECP from both cultures was heated at 60, 80 and 100°C for 10 min and 0.1 ml of each preparation was injected intraperitoneally into rohu of average weight of 17 g. Simultaneously, the precipitated cells from A. hydrophila culture was obtained through centrifugation and further resuspended in PBS. A portion of these resuspended cells were heated at 100°C for 10 min. They were then injected into fish at a concentration of 2.5 × 105 cfu/ml. PBS was used as a control.
Results
Isolation and Identification of Bacteria
Colony morphology study of A. hydrophila (CAHH14 strain) over the RS-plate showed small, smooth, convex, round and yellowish colonies. The strain was positive towards biochemical tests like oxidase, catalase, motility, O/F test and are resistant to 0/129 disc and novobiocin. The biochemical tests results of CAHH14 strain was similar to that of reference strain ATCC-49140 except in utilization of certain sugar such as mannose, sorbitol (Table 1).
Bacterial Strain and Growth
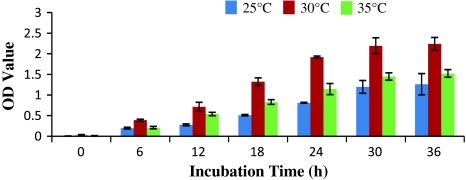
The growth rate of A. hydrophila at different incubation time period and temperature was shown in Fig. 1. Basing on OD value of the cultured nutrient broth medium, the optimum temperature for bacterial growth was found to be 30°C. At this temperature, the bacterial growth rate increases rapidly. Bacterial growth was found to be comparatively less when culture was incubated at 25°C, where as moderate growth was found at 35°C.
Fig. 1.
Effect of incubation time and temperature on A. hydrophila growth
Hemolytic Activity
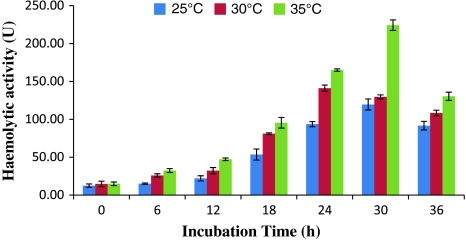
The highest hemolytic activity of ECP was found from the culture grown at 35°C for 30 h. After 30 h onwards hemolytic activity was decreased. Hemolytic activity was found to be less in the ECP obtained from the culture grown at 25°C (Fig. 2). The haemolysins product was secreted into the medium during the early exponential phase of bacterial growth and tends to increase throughout the remaining exponential phase and started degrading during stationary phase.
Fig. 2.
Effect of incubation time and temperature on the haemolytic activity of ECP
Proteolytic Activity
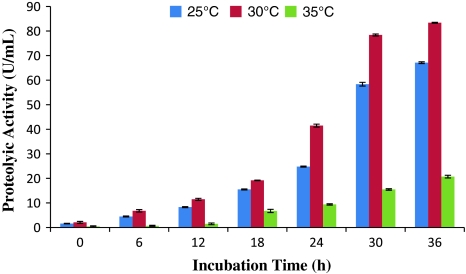
The proteolytic activity of ECP collected from the bacterium cultured at 30°C was found to be highest. The proteolytic activity was less till 18 h of incubation period and increases sharply from 24 h onwards. The ECP from the culture grown at 35°C showed very less proteolytic activity where as moderate proteolytic activity was found when the ECP was collected from the culture grown at 25°C (Fig. 3).
Fig. 3.
Effect of incubation time and temperature on the proteolytic activity of ECP
LD50 Value and Lethal Toxicity of ECP of A. hydrophila
The LD50 value obtained after 5 days of observation period for the viable cells of A. hydrophila (CAHH14 strain) injected to rohu at different concentration was found to be 1.7 × 104 cfu/ml.
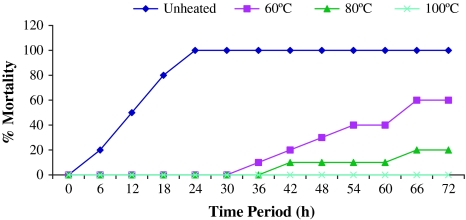
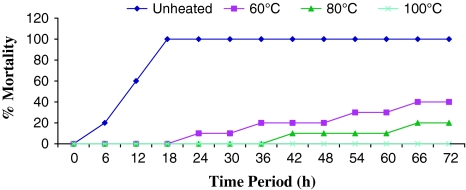
The lethal toxicity of different preparations of culture supernatant fluid as well as live and killed cells of A. hydrophila (CAHH14 strain) was represented in Table 2. The live cells as well as the filter sterilized supernatant fluid from cultures grown at 35 and 30°C killed all the rohu injected (100% mortality). On the other hand the ECP subjected to heat treatment at 60 and 80°C for 10 min showed less mortality than that of untreated ECP. ECP heated at 60°C from the bacteria grown at 35 and 30°C injected to fish caused 40 and 60% mortality respectively and ECP heated at 80°C resulted 20% mortality within 72 h (Figs. 4, 5). No mortality was recorded from 72 h of post injection. ECP heated at 100°C obtained from CAHH14 strain grown at 35 and 30°C resulted no mortality among the fish.
Table 2.
Lethal toxicity of A. hydrophila (CAHH14 strain) to rohu
| Treatment | Dose (μl) | Percentage of death | Death time | |
|---|---|---|---|---|
| ECP (35°C) | 100 | Unheated | 100 | 12 |
| ECP (35°C) | 100 | Heated 60°C | 40 | 72 |
| 100 | Heated 80°C | 20 | 72 | |
| 100 | Heated 100°C | 0 | 0 | |
| ECP (30°C) | 100 | Unheated | 100 | 24 |
| ECP (30°C) | 100 | Heated 60°C | 60 | 72 |
| 100 | Heated 80°C | 20 | 72 | |
| 100 | Heated 100°C | 0 | 0 | |
| Live cells | 2.5 × 105 cfu/ml | 100 | 30 | |
| Heat killed cells | 2.5 × 105 cfu/ml | 0 | 0 | |
| PBS | 100 | 0 | 0 |
Fig. 4.
Lethal toxicity of crude ECP of A. hydrophila (CAHH14) grown at 30°C challenged intraperitoneally to rohu (n = 10 fish per group)
Fig. 5.
Lethal toxicity of crude ECP of A. hydrophila (CAHH14) grown at 35°C challenged intraperitoneally to rohu (n = 10 fish per group)
Discussion
Aeromonas hydrophila was recognized as a significant constraint to aquaculture production and trade [1] leading to great economic losses due to outbreak of diseases among the fish. The present study was aimed for biochemical characterization of A. hydrophila (CAHH14 strain) and determination of toxicity of its crude ECP in rohu.
In our study presumptive identification of A. hydrophila (CAHH14 strain) was carried out from their growth over the RS-plate as recommended by Shotts and Rimler [24]. Further identification of strain was carried out through biochemical tests and were compared with the ATCC-49140 reference strains of A. hydrophila [19]. The strain was positive towards oxidase, catalase, and motility test and are resistant to 0/129 disc and novobiocin and differs from the reference strain only in utilization of certain sugar. These results show similarity with the finding of Nayak [25] who reported five strains of A. hydrophila from various organs of freshwater fish and Thankappan et al. [26] reported twelve strains of A. hydrophila from the fresh water fish of Eastern India basing on their biochemical properties.
The results obtained from present hemolytic activity revealed highest hemolytic activity of ECP obtained from the culture grown at 35°C for 30 h. It is interesting to note that bacterial growth rate is moderate in 35°C but it had a high hemolytic activity against sheep erythrocytes at this cultured condition. From this result, it can be concluded that the potential toxicity of the ECP might vary due to culturing at different incubation temperatures [27]. Similar results were reported by Sakai [18] who found that the supernatant fluid from A. salmonicida hemolysed the erythrocytes of sockeye salmon. Bernheimer [28] estimated the quantitative hemolytic activity of ECP of A. hydrophila on rabbit erythrocytes. Gonzalez-Serrano et al. [29] studied on the hemolytic assay of ECP obtained from A. hydrophila and A. biovar sobria at 28°C and reported that rabbit erythrocytes were much more sensitive to Aeromonas hemolysin. In our study we have also found sheep erythrocytes are equally potent to it.
Proteases secreted by aeromonads are thought to contribute to virulence for fish and other hosts [11]. Present study on proteolytic activity of ECP of A. hydrophila indicated highest proteolytic activity of the culture grown at 30°C where as the ECP from the culture grown at 35°C showed very less proteolytic activity. However, Gonzalez-Serrano et al. [29] also studied on the proteolytic effect and suggested that certain strains of A. hydrophila show lowest proteolytic effect at 28°C. This result was only because of ECP collected from the culture grown at different incubation temperature [30]. Cahill and Santosh et al. [31, 32] reported considerable differences between the number, types and quantities of proteases produced by aeromonads by using different growth media and incubation temperature.
On the basis of LD50 value obtained 1.7 × 104 cfu/ml, the present strain was included in the high virulent category (LD50 104–105) as reported by Mittal et al. [33]. Khalil and Mansour [20] reported the LD50 value at 5 days for viable cells of A. hydrophila in tilapia was 2.1 × 104 cells.
The results of lethal toxicity of different preparations of culture supernatant fluid as well as live and killed cells of A. hydrophila (CAHH14 strain) showed that when fish injected with untreated ECP, the heat labile potential virulent factor of ECP (protease and hemolysin) plays an important role in fish mortality. But the culture obtained from different growth condition i.e. at 35°C (hemolytic activity is more) causes 100% mortality in 12 h whereas culture grown at 30°C (proteolytic activity is more) in 24 h. This indicates that virulence of ECP closely associated with production of haemolysin compound as compared to protease activity. Since the morality rate was reduced in both cases when ECP heated at 60°C it indicated decrease in activity of hemolysin and protease compounds. Increasing the temperature up to 80°C for 10 min showed 20% mortality, this might be due to some other heat-stable virulent factors in ECP. Complete inactivation of haemolysins, protease and other unknown virulent factor was achieved by heating the ECP to 100°C that causes no mortality in fish. Similar mortality results were obtained by Inamura et al. [34] for Vibrio anguillarum against gold fish.
This study provide an evidence of production of hemolytic and proteolytic exotoxin as well as unknown heat stable virulent factors in ECP by A. hydrophila that are responsible for causing mortality in fish. The lethality of ECP was decreased by heating due to inactivation of hemolytic and proteolytic activities and completely inactivated by boiling at 100°C for 10 min. This study generated basic informations of strain and their virulent enzymes that would helpful for further work in its pathogenicity and development of effective measures for protection against this pathogen.
References
- 1.Smith P. Break points for disc diffusion susceptibility testing of bacteria associated with fish diseases, a review of current practice. Aquaculture. 2006;261:1113–1121. doi: 10.1016/j.aquaculture.2006.05.027. [DOI] [Google Scholar]
- 2.Rahman HM, Kawai K, Kusuda R. Virulence of starved Aeromonas hydrophila to cyprinid fish. Fish Pathol. 1997;32:163–168. doi: 10.3147/jsfp.32.163. [DOI] [Google Scholar]
- 3.Nayak KK, Mukherjee SC, Das BK. Observation on different strains of Aeromonas hydrophila from various diseased fishes. Indian J Fish. 1999;46:245–250. [Google Scholar]
- 4.Rahman MH, Somsiri T, Tajima K, Ezura Y. Distribution of Aeromonas sp. emphasizing on a newly identified species of Aeromonas sp. T8 isolated from fish and aquatic animals in Southeast Asia. Pak J Biol Sci. 2004;7:258–268. doi: 10.3923/pjbs.2004.258.268. [DOI] [Google Scholar]
- 5.Rahman MH, Huys G, John AM, Kuhn I, Mollby R. Persistence, transmission, and virulence characteristics of Aeromonas strains in a duckweed aquaculture-based hospital sewage water recycling plant in Bangladesh. Appl Environ Microbiol. 2007;73:1444–1451. doi: 10.1128/AEM.01901-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonguthai K (1985) Preliminary account of ulcerative fish diseases in the Indo-Pacific Region. FAO/TCP/RAS/4508 Project, pp 7–18
- 7.Torres JL, Shariff M, Law AT (1989) Identification and virulence screening of Aeromonas sp. isolated from healthy and epizootic ulcerative syndrome infected fish. In: Proceedings of the Society of Asian Fisheries Forum, Tokyo, Japan, pp 17–22
- 8.Shome R, Shome BR, Mazumder Y, Das A, Kumar A, Rahman H, Bujarbaruah KM. Abdominal dropsy disease in major carps of Meghalaya: isolation and characterization of Aeromonas hydrophila. Curr Sci. 2005;88:1897–1900. [Google Scholar]
- 9.Trower CJ, Abo S, Majeed KN, Itzstein M. Production of an enterotoxin by a gastro-enteritis-associated Aeromonas strain. J Med Microbiol. 2000;48:121–126. doi: 10.1099/0022-1317-49-2-121. [DOI] [PubMed] [Google Scholar]
- 10.Scoaris D, Colacite J, Nakamura CV, Ueda-Nakamura T, Aberu Filho BA, Filho Dias BP. Virulence and antibiotic susceptibility of Aeromonas spp. isolated from drinking water. Antonie van Leeuwenhoek. 2008;93:111–122. doi: 10.1007/s10482-007-9185-z. [DOI] [PubMed] [Google Scholar]
- 11.Pansare AC, Lewis NF, Venugopal V. Characterization of extracellular proteases of Aeromonas hydrophila. Agric Biol Chem. 1986;50:1743–1749. doi: 10.1271/bbb1961.50.1743. [DOI] [Google Scholar]
- 12.Esteve C, Birbeck TH. Secretion of haemolysins and proteases by Aeromonas hydrophila EO63: separation and characterization of the serine protease (caseinase) and the metalloprotease (elastase) J Appl Microbiol. 2004;96:994–1001. doi: 10.1111/j.1365-2672.2004.02227.x. [DOI] [PubMed] [Google Scholar]
- 13.Pembertona JM, Kidd SP, Schmidt R. Secreted enzymes of Aeromonas. FEMS Microbiol Lett. 1997;152:1–10. doi: 10.1111/j.1574-6968.1997.tb10401.x. [DOI] [PubMed] [Google Scholar]
- 14.Allan BJ, Stevenson RMW. Extracellular virulence factors of Aeromonas hydrophila in fish infection. Can J Microbiol. 1981;27:1114–1122. doi: 10.1139/m81-174. [DOI] [PubMed] [Google Scholar]
- 15.Yu HB, Kaur R, Lim S, Wang HX, Leung KY. Characterization of extracellular proteins of Aeromonas hydrophila-AH-1. Proteom Clin Appl. 2006;7:436–449. doi: 10.1002/pmic.200600396. [DOI] [PubMed] [Google Scholar]
- 16.Subashkumar R, Theyumanavan T, Vivekanandhan G, Perumalsamy L. Multiple antibiotic resistant, haemolytic and proteolytic activity of Aeromonas hydrophila isolated from acute gastroenteritis in children. Indian J Med Res. 2006;23:61–66. [PubMed] [Google Scholar]
- 17.Kanai K, Wakabayashi H. Purification and some properties of proteases from Aeromonas hydrophila. Bull Jpn Soc Sci Fish. 1984;50:1367–1374. doi: 10.2331/suisan.50.1367. [DOI] [Google Scholar]
- 18.Sakai DK. Electrostatic mechanism of survival of virulent strains in river water. Appl Environ Microbiol. 1985;51:1343–1349. doi: 10.1128/aem.51.6.1343-1349.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popoff M, Veron M. A taxonomic study of Aeromonas hydrophila and Aeromonas punctata group. J Gen Microbiol. 1976;94:11–22. doi: 10.1099/00221287-94-1-11. [DOI] [PubMed] [Google Scholar]
- 20.Khalil AH, Mansour EH. Toxicity of crude extracellular products of Aeromonas hydrophila in tilapia, Tilapia nilotica. Lett Appl Microbiol. 1997;25:269–273. doi: 10.1046/j.1472-765X.1997.00220.x. [DOI] [PubMed] [Google Scholar]
- 21.Sirirat T, Intuseth J, Chanphong J, Thompson K, Chinabut S, Adams A. Characterization of Aeromonas hydrophila extracellular products with reference to toxicity, virulence, protein profiles and antigenicity. Asian Fish Sci. 1999;12:371–379. [Google Scholar]
- 22.Kwapinski JB. Methods of serological research. New York: Wiley; 1965. [Google Scholar]
- 23.Reed LJ, Muench H. A simple method for estimating 50 percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 24.Shotts EB, Rimler R. Medium for the isolation Aeromonas hydrophila. J Appl Microbiol. 1973;26:550–553. doi: 10.1128/am.26.4.550-553.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nayak KK (1993) Studies on the role of various strains of Aeromonas hydrophila in fish diseases. Ph.D. thesis
- 26.Thankappan A, Das BK, Barman HK, Samal SK. Genetic fingerprinting of Aeromonas hydrophila isolated from diseased fresh water fishes of Eastern India. e-planet. 2008;6:1–6. [Google Scholar]
- 27.Venugopal V, Alur MD, Lewis NF. Extracellular protease from Pseudomonas morinoglutinosa: some properties and its action on fish actinomysin. J Food Sci. 1983;48:671–674. doi: 10.1111/j.1365-2621.1983.tb14872.x. [DOI] [Google Scholar]
- 28.Bernheimer AW. Assay of hemolytic toxins. In: Harshman S, editor. Microbial toxins: tools in enzymology. London: Academic Press; 1988. pp. 213–217. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Serrano CJ, Santosh JA, Garcia-Lopez ML, Otero A. Virulence marker in Aeromonas hydrophila and Aeromonas biovar sobria isolates from freshwater fish. J Appl Microbiol. 2002;93:414–419. doi: 10.1046/j.1365-2672.2002.01705.x. [DOI] [PubMed] [Google Scholar]
- 30.Mateos D, Anguita J, Naharro G, Paniagua C. Influence of growth temperature on the production of extracellular virulence factors and pathogenicity of environmental and human strains of Aeromonas hydrophila. J Appl Bacteriol. 1993;74:111–118. doi: 10.1111/j.1365-2672.1993.tb03003.x. [DOI] [PubMed] [Google Scholar]
- 31.Cahill MM. Virulence factors in Aeromonas species. A review. J Appl Bacteriol. 1990;69:1–16. doi: 10.1111/j.1365-2672.1990.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 32.Santosh JA, Gonzalez CJ, Lopez TM, Gracia-Lopez ML, Otero A. Extracellular protease production by dairy strains of Aeromonas hydrophila as affected by growth media and incubation temperature. Food Microbiol. 1996;13:47–51. doi: 10.1006/fmic.1996.0006. [DOI] [Google Scholar]
- 33.Mittal KR, Lalonde KK, Leblanc D, Olivier G, Lallier R. Aeromonas hydrophila in rainbow trout: relation between virulence and surface characteristic. Can J Microbiol. 1980;26:1501–1503. doi: 10.1139/m80-248. [DOI] [PubMed] [Google Scholar]
- 34.Inamura H, Muroga K, Nakai T. Toxicity of extracellular products of Vibrio anguillarum. Fish Pathol. 1984;19:89–96. doi: 10.3147/jsfp.19.89. [DOI] [Google Scholar]