Abstract
A total of 128 faecal samples/rectal swabs were collected from dogs showing signs of diarrhea/enteritis in and around Puducherry, South India. Eighteen clinical samples, showing high HA titre of 1:512 and above and positivity by polymerase chain reaction (PCR) with CPV-2ab primers, were subjected to virus isolation in CRFK cell line. Of the 18 samples processed, 3 samples (16.6%) were positive for CPV and were confirmed by haemagglutination, dot-ELISA, and IFAT. The three cell culture isolates were characterized as CPV-2b types by multiplex PCR as well as by monoclonal antibody typing.
Keywords: Cell culture, CPV, CRFK, HA, Dot-ELISA, PCR, IFAT, Monoclonal typing
Introduction
Canine parvovirus (CPV-2) is one of the most common pathogenic viruses causing diarrhoea in dogs [1]. After its emergence in the late 1970s [2] CPV-2 underwent a rapid evolution and, within few years, new antigenic types termed CPV-2a and CPV-2b, completely replaced the original CPV-2 [3]. Few years back an antigenic variant had been reported in Italy [4]. That variant has an amino acid substitution, Asp-426 → Glu, which occurs in a residue of the capsid protein that is considered important for the antigenic properties of CPV-2. This variant (CPV-2/Glu-426 mutant), currently named as CPV-2c, is distributed in Italy [5], Spain [6], United Kingdom [7], and very recently in Portugal [8]. The disease is reported to be prevalent among canine populations in Puducherry [9]. Isolation and subsequent typing of virus were considered important in epidemiological survey of the prevalent canine parvovirus (CPV) types and also in the effort to produce a vaccine incorporating local strains for control of CPV infection in this geographical location.
Materials and Methods
Processing of Clinical Samples
Faecal samples/rectal swabs from CPV suspected dogs were collected from Teaching Veterinary Hospital, RAGACOVAS, veterinary dispensaries and pet clinics situated in and around Puducherry, South India. The faecal samples/rectal swabs obtained from the suspected dogs were emulsified in 1 ml of 0.1 M PBS of pH 7.4 and centrifuged at 6000 rpm for 15 min at 4°C. The supernatant was collected and stored at −40°C until further use.
Screening of Clinical Samples by Haemagglutination and Haemagglutination Inhibition Test
Ninety microlitre of processed faecal sample was treated with 10 μl of chloroform and mixed well. The mixture was kept at 4°C for 10 min and centrifuged at 10000 rpm at 4°C for 10 min. The supernatant was collected and used for haemagglutination test [10]. The haemagglutination inhibition test was conducted using hyperimmune serum raised in rabbits and diluted 1:30 was used for specific inhibition of the HA activity of virus [10].
Screening of Clinical Samples by PCR Assay Using Cpv-2ab Primer Pair
The processed samples were screened by primer pair CPV-2ab (F)/2ab (R) that amplified a 681-bp fragment of the gene encoding capsid protein VP2 of both CPV-2a and CPV-2b types having the sequence as CPV-2ab (F) 5′-AAGAGTGGTTGTAAATAATT-3′ and CPV-2ab(R) 5′-CTATATAACCAAAGTTAGTAC-3′ [11].
Isolation of CPV
In the isolation studies Crandell Feline Kidney (CRFK) cell lines were used. Eighteen processed clinical samples, showing high HA titre of 1:512 and above and polymerase chain reaction (PCR) positivity, were filtered using 0.22 μm membrane filter (Millipore, Bangalore) and the filtrates were used in the isolation studies. For isolation of virus was each sample was subjected to five passages. The infected monolayers were harvested 4th day post-inoculation (with or without CPE) by three cycles of alternative freezing and thawing. The virus suspension was clarified at 6000 rpm for 15 min in a refrigerated centrifuge and supernatant was frozen at −40°C for further use [12].
Detection of the Presence of Virus in Cell Culture
The presence of the virus in the cell culture fluids up to third passage level was monitored by haemagglutination test. The presence of the virus in cell culture fluids at third passage levels was also detected by dot-ELISA [13] using CPV hyper immune serum (1:100) raised in rabbits. Anti rabbit IgG HRPO conjugates were used in 1:100 dilutions. Development of brown color spots after 5–10 min of substrate and chromogen administration indicated positive reactions. CPV vaccine (Megavac-P) and cell cultured fluids from uninfected controls were used as positive and negative controls, respectively [13]. Indirect fluorescent antibody technique for the detection of CPV don in the Leighton tubes containing monolayer of CRFK cell lines infected with CPV samples at third passage level were subjected to IFAT 4 days post-inoculation to detect the presence of virus infection. The cell cultured slides were washed thoroughly in 0.01 M PBS of pH 7.4, treated with CPV hyper immune serum (1:100) raised in rabbits and incubated at 37°C for 1 h. After through washing the slides were further treated with 1:100 dilution of anti rabbit IgG conjugated with FITC for 1 h. The slides examined under UV illumination in a fluorescent microscope [14].
Typing of CPV by Multiplex PCR
The isolates were characterized by a multiplex PCR utilizing primer pairs, CPV-2ab (F)/2ab (R) and CPV-2b (F)/CPV-2b(R) were used simultaneously, that amplified 681 and 427 bp fragment of the gene encoding capsid protein VP2, respectively. The sequence of the primer pair CPV-2ab was described earlier [11] and the sequence of the primer pair CPV-2b was of CPV-2b (F) 5′-CTTTAACCTTCCTGTAACAG-3′ and CPV-2b(R) 5′-ATAGTTAAATTGGTTATCTAC-3′ [15]. CPV 2b types would respond to both the primer pairs and will generate two specific products at 681 and 427 bp whereas CPV-2a would react with only CPV-2ab primer pair and generate only one specific product at 681 bp.
Typing of CPV by Monoclonal Antibodies
A panel of monoclonal antibodies (A4E3, C1D1, B4A2 and B4E1), supplied by Dr. C.R. Parrish of Cornell University, Ithaca, NY, USA, were used for the study. The CPV strains were characterized as CPV-2, CPV-2a or CPV-2b on the basis of monoclonal antibody reactivity. MAb A4E3, mouse IgG, which are specific for and react well with all CPV isolates. MAb C1D1, mouse IgG, which are very specific and react only with CPV type 2a and 2b isolates and not with CPV-2. MAb B4A2, a rat IgG, reacts with CPV type-2 and 2a isolates, but which reacts to a much lower level (100-fold less) with type 2b isolates. MAb B4E1, a rat IgG, which reacts with CPV-type 2 but which reacts too much lower level with CPV type 2a and 2b isolates [16].
Results and Discussion
Screening of Clinical Samples by Haemagglutination and Haemagglutination Inhibition Test
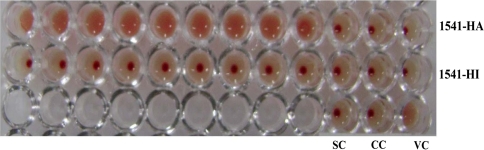
Out of the 128 samples screened, 47 (36.71%) samples had the HA titre of 1:32 and above and were considered positive for presence of CPV. Eighteen samples (14.06) showed high haemagglutination titre of 1:512 and above were taken up for virus isolation. The haemagglutination reaction of all the positive samples were specifically inhibited by performing haemagglutination inhibition test using CPV hyper immune serum raised in rabbits (Fig. 1). Mathys et al. [17] obtained 56.9% positivity for the detection of CPV in faecal samples by haemagglutination assay [17]. In other study the haemagglutination test performed with the 26 samples faecal samples showed 84.6% positively indicating the application of HA test in CPV diagnosis [18].
Fig. 1.
Haemagglutination and hamaggultination inhibition test of clinical sample 1541. VC virus control, CC cell control, SC serum control
Screening of Clinical Samples by PCR Assay
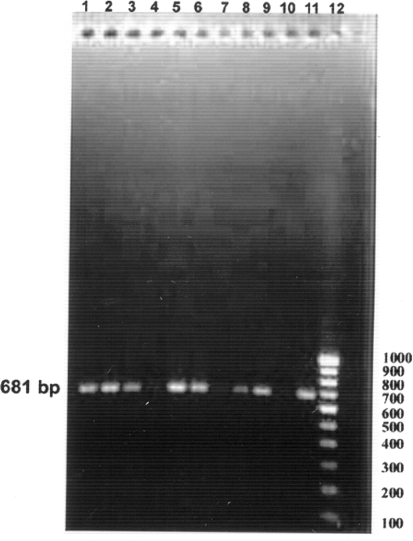
All the eighteen samples showing high haemagglutination titre of 1:512 and above also yielded a single DNA amplicon of 681 bp (Fig. 2) in PCR assay using CPV-2ab primer pair. Desario et al. [19] were utilized five laboratory tests for the diagnosis of CPV type 2 (CPV-2) infections in 89 faecal samples collected from dogs with diarrhoea. The tests employed were immunochromatography (IC), haemagglutination (HA), virus isolation (VI), conventional and real-time PCR the tests were able to detect CPV-2 antigen or nucleic acid in 41, 50, 54, 68 and 73 of the samples, respectively, indicating the higher sensitivity of PCR assay over other diagnostic techniques [19].
Fig. 2.
Screening of clinical samples by PCR assay using CPV-2ab primers. Lane 1 1544, lane 2 1353, lane 3 1272, lane 4 1145, lane 5 759, lane 6 1599, lane 7 4209, lane 8 105, lane 9 1379, lane 10 negative control, lane 11 positive control, lane 12 100 base pair ladder
Isolation and Identification of CPV
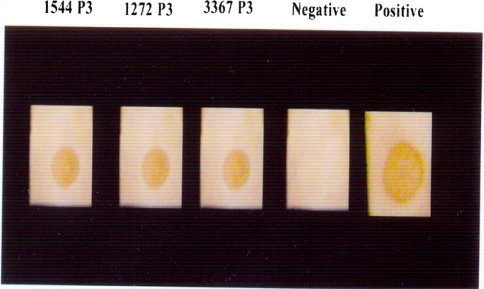
Three samples (1544, 1272 and 3367) (16.66%) out of 18 samples showed mild cytopathic effect in the form of rounding, increased granularity and detached cells 3–4 days post infection from third passage level onwards (Fig. 3). Rounding and degenerative changes are the cytopathic changes observed 72 h post infection after third passage is characteristic of CPV in CRFK cell lines [12, 19]. Of the 18 samples passaged in CRFK cells, 3 samples (1544, 1272 and 3367) which showed mild cytopathic effect also demonstrated high HA titres up to third passage levels. The HA titres at third passage level were 1:28, 1:211 and 1:26 for samples 1544, 1272 and 3367, respectively. Out of the 18 samples passaged in CRFK cells, cryolysates of the samples 1544, 1272 and 3367 showed positive reactions in the form of distinct brown spots by dot-ELISA at third passage level (Fig. 4). CPV isolates in cell culture were further confirmed by IFAT in the infected cell cultures at third passage level. Specific intracellular fluorescence observed in infected cell culture (1544, 1272 and 3367) demonstrated the presence of CPV in infected cell lines. The efficacy of CPV ELISA detection kit with HA and found that CPV-ELISA had a sensitivity of 87% and a specificity of 100% [20]. HA, HI, IFAT and PCR were further used for confirmation and antigenic analysis of the CPV isolates [21, 22].
Fig. 3.
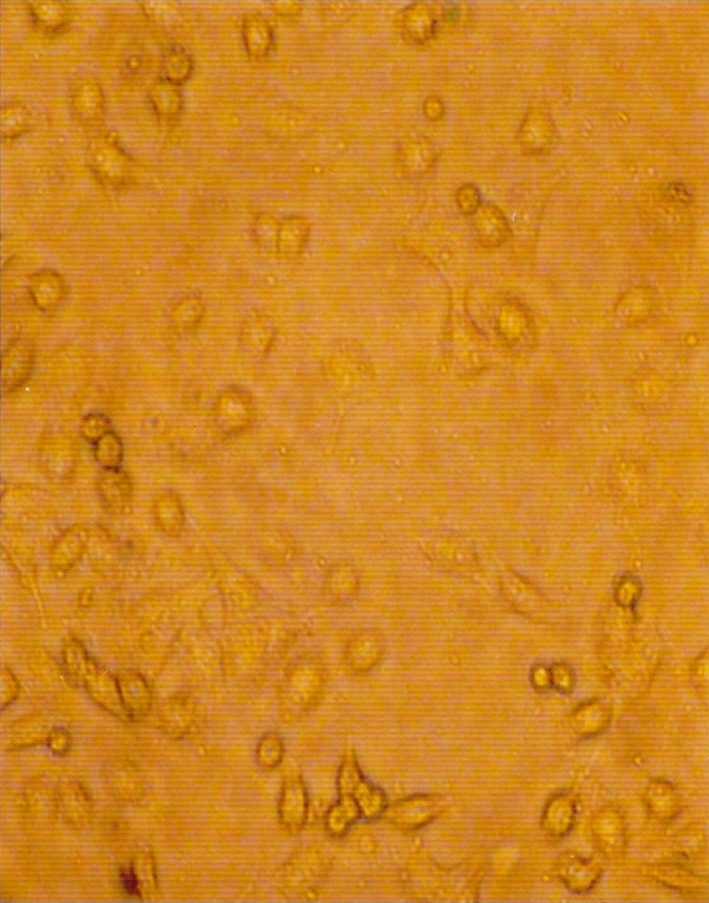
CPV infected CRFK cell line (4 days PI) showing rounding, increase granularity and detached cells (×100)
Fig. 4.
Dot-ELISA showing distinct brown spots by cell culture isolates (1544, 1272 and 3367) in third passage level along with positive and negative controls
Characterization of Cell Culture Isolates by Multiplex PCR Assay and Monoclonal Antibody Typing
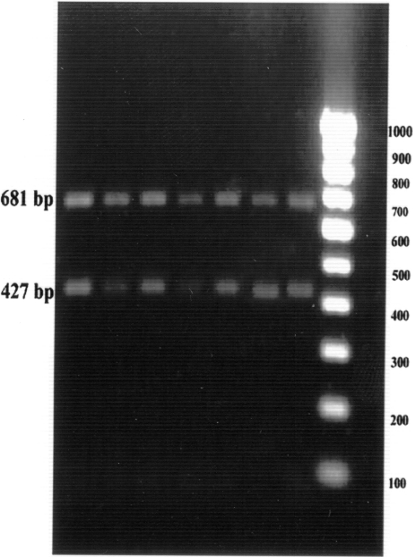
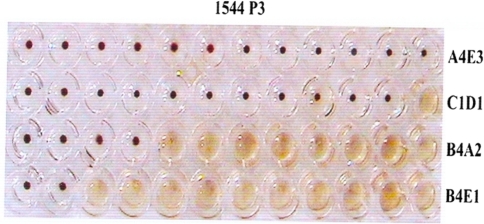
The cell culture fluids of the three samples (1544, 1272 and 3367 at third and fifth passage level were subjected to multiplex PCR. All the three isolates generated two specific amplicons of 681 and 427 bp with CPV-2ab and CPV-2b primers, respectively, and they were characterized as CPV-2b types (Fig. 5). The cell culture fluids of the three samples (1544, 1272 and 3367) at third passage level were also antigenically characterized by using a panel of four monoclonal antibodies. The cell culture fluids at third passage level were also found to be CPV-2b types (Fig. 6).
Fig. 5.
Characterization of cell culture isolates by multiplex PCR assay using CPV2ab and CPV-2b primers. Lane 1 1544 P3, lane 2 1544 P5, lane 3 1272 P3, lane 4 1272 P5, lane 5 3367 P3, lane 6 3367 P5, lane 7 positive control, lane 8 100 base pair ladder, lane 9 negative control
Fig. 6.
Antigenic typing of canine parvovirus from cell culture isolate (1544 P3) using HI test with a panel of four monoclonal antibodies
Three isolates of CPV could be obtained out of 18 samples processed in this study. Low isolation rate (16.6%) in this study may be attributable to lower sensitivity of the test as presence of antibodies in the intestinal lumen of the infected dog may bind the virions and prevent viral attachment to cell receptors and the isolation of CPV could be done only for few days post infection [19, 22]. All the three cell culture isolates also generated two specific amplicons of 681 and 427 base pairs confirming them as CPV-2b. Vieira et al. [8] examined 30 faecal samples from dogs with clinical signs of CPV infection for CPV diagnosis by ELISA, conventional real-time PCR, and by minor-groove binding Taqman PCR. Out of the 29 PCR positive samples, 15 were identified as CPV-2b and 14 as CPV-2a. No CPV-2 was detected [8]. The results of molecular typing by PCR assay in Brazil showed that out of the 38 isolates collected during the period from 1982 to 1995, showed that the relative prevalence of the antigenic types changed over time. The predominant strain found during 1980 was CPV-2a, which was substantially replaced by CPV-2b from 1990 to 1995 [15]. In the recent report from North Indian states Sanjukta et al. [23] showed that the CPV-2b was the prevalent strain in North India states. Battilani et al. [24] had typed two CPV isolates obtained from dogs and wolves using the haemagglutination inhibition test with specific monoclonal antibodies and found them as CPV-2b. In future the local isolates need to be incorporated in an attempt to produce a vaccine for successful control of parvovirus infection in canine population.
Acknowledgments
The authors are thankful to the Dean, Rajiv Gandhi College of Veterinary and Animal Sciences, Puducherry, for providing the facilities to carry out this work. Thanks are also due to Dr. C.R. Parrish, Cornell University, USA, for kind supply of monoclonal antibodies.
Contributor Information
S. Parthiban, Phone: +91-09618970321, FAX: +91-09894518244, Email: parthis17@gmail.com
Hirak Kumar Mukhopadhyay, Email: mhirak@rediffmail.com.
References
- 1.Carmichael LE, Binn LN. New enteric viruses in the dog. Adv Vet Sci Comp Med. 1981;25:37. [PubMed] [Google Scholar]
- 2.Kelly WR. An enteric disease of dogs resembling feline panleucopaenia. Aust Vet J. 1978;54:593. doi: 10.1111/j.1751-0813.1978.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 3.Parrish CR, O’ Connell PH, Evermann JF, Carmichael LE. Natural variation of canine parvovirus. Science. 1985;230:1046–1048. doi: 10.1126/science.4059921. [DOI] [PubMed] [Google Scholar]
- 4.Buonavoglia C, Martella A, Pratella M, Tempesta A, Cavalli D, Buonavoglia G, Bozzo G, Decaro N, Carmichael LE. Evidence for evolution of canine parvovirus type-2 in Italy. J Gen Virol. 2001;82:3021–3025. doi: 10.1099/0022-1317-82-12-3021. [DOI] [PubMed] [Google Scholar]
- 5.Martella V, Cavalli A, Pratelli A, Bozzo G, Camero M, Buonavoglia D, Narcisi D, Tempesta M, Buonavoglia C. A canine parvovirus mutant is spreading in Italy. J Clin Microbiol. 2004;42:1333–1336. doi: 10.1128/JCM.42.3.1333-1336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decaro N, Gabriella E, Martella V, Desario C, Sante R, Campolo M, Lorusso A, Cavalli A, Buonavoglia C. A minor groove binder probe real-time PCR assay for discrimination between type-2 based vaccines and field strains of canine parvovirus. J Virol Methods. 2006;136:65–70. doi: 10.1016/j.jviromet.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decaro N, Martella V, Gabriella E, Desario C, Campolo M, Lorusso E, Colaianni ML, Lorusso A, Buonavoglia C. Tissue disribution of the antigenic variants of canine parvovirus type 2 in dogs. Vet Microbiol. 2007;121:39–44. doi: 10.1016/j.vetmic.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira JM, Silva E, Oliveira v, Vieira LA, Decaro N, Desario C, Muller A, Carvalheira J, Buonavoglia C, Thompson G. Canine parvovirus 2c infection in central Portugal. J Vet Diagn Invest. 2008;20:488–491. doi: 10.1177/104063870802000412. [DOI] [PubMed] [Google Scholar]
- 9.Panneer D (2008) Development and evaluation of diagnostic test for detection of canine parvovirus. M.V.Sc Thesis, Pondicherry University
- 10.Carmichael LE. Haemagglutination (HI) and haemagglutination inhibition (HI) tests for canine parvovirus. Am J Vet Res. 1980;41:784–791. [PubMed] [Google Scholar]
- 11.Senda M, Parrish CR, Harasawa R, Gamoh K, Muramatsu M, Hirayama N, Itoh O. Detection by PCR of wild type canine parvovirus which contaminates dog vaccines. J Clin Microbiol. 1995;18:110–113. doi: 10.1128/jcm.33.1.110-113.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirayama K, Kano R, Kanai TH, Tuchiya K, Tsuyama S, Nakamura Y, Sasaki Y, Hasegawa A. VP2 gene of a canine parvovirus isolate from stool of a puppy. J Vet Med Sci. 2005;67:139–143. doi: 10.1292/jvms.67.139. [DOI] [PubMed] [Google Scholar]
- 13.Waner T, Mazar S, Nachmias E, Keren-Kornblatt E, Harrus S. Evaluation of a dot ELISA kit for measuring immunoglobulin M antibodies to canine parvovirus and distemper virus. Vet Rec. 2003;152:588–591. doi: 10.1136/vr.152.19.588. [DOI] [PubMed] [Google Scholar]
- 14.Cavalli A, Martella V, Desario C, Camero M, Bellacicco AL, Palo PD, Decaro N, Elia G, Buonavoglia C. Evaluation of the antigenic relationship among the canine parvovirus (CPV-2) variants. Clin Vaccine Immunol. 2008;15:534–539. doi: 10.1128/CVI.00444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira CA, Monezi TA, Mehnert DU, D’Anglo M, Durigon EL. Molecular characterization of canine parvo virus in Brazil by polymerase chain reaction assay. Vet Microbiol. 2000;75:127–133. doi: 10.1016/S0378-1135(00)00214-5. [DOI] [PubMed] [Google Scholar]
- 16.Parrish CR. Host range relationships and the evolution of canine parvovirus. Vet Microbiol. 1999;69:29–49. doi: 10.1016/S0378-1135(99)00084-X. [DOI] [PubMed] [Google Scholar]
- 17.Mathys R, Muller NC, Pederson NC, Theilen GH. Haemagglutination with formalin fixed erythrocytes for the detection of canine parvovirus. Am J Vet Res. 1983;44:171–175. [PubMed] [Google Scholar]
- 18.Rai A, Nauriyal DC, Mohan R. Faecal examination for diagnosis of canine parvovirus haemorrhagic gastroenteritis. Int J Anim Sci. 1994;9:195–196. [Google Scholar]
- 19.Desario C, Decaro N, Campolo M, Cavalli A, Cirone F, Gabriella E, Martella V, Lorusso E, Camero M, Buonavoglia C. Canine parvovirus infection: which diagnostic test for the virus? J Virol Methods. 2005;126:179–185. doi: 10.1016/j.jviromet.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Drane DP, Hamilton RC, Cox JC. Evaluation of novel diagnostic test for canine parvovirus. Vet Microbiol. 1994;41:293–302. doi: 10.1016/0378-1135(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Sakamoto M, Ikeda Y, Sato E, Kawakami K, Miyazawa T, Tohya Y, Takahashi E, Mikami T, Mochizuki M. Pathogenic potential of canine parvovirus types 2a and 2c in domestic cats. Clin Diagn Lab Immunol. 2001;8:663–668. doi: 10.1128/CDLI.8.3.663-668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decaro N, Elia G, Campolo M, Desario C, Lucente MS, Bellacicco AL, Buonavoglia C. New approaches for the molecular characterization of canine parvovirus type 2 strains. J Vet Med. 2005;52:316–319. doi: 10.1111/j.1439-0450.2005.00869.x. [DOI] [PubMed] [Google Scholar]
- 23.Sanjukta R, Maheshkumar L, Nagappa B, Joken B, Karuna I, Mandakini (2008) Molecular epidemiological study of canine parvovirus in some parts of India. In: International conference on emerging infectious diseases of animals and biotechnological applications, July 28th, 29th, 2008, TANUVAS, Compendium and Souvenir, p 65
- 24.Battilani M, Scagliarini A, Tisato E, Turilli C, Jacoboni I, Casadio R, Prosperi S. Analysis of canine parvovirus sequences from wolves and dogs isolated in Italy. J Gen Virol. 2001;82:1555–1560. doi: 10.1099/0022-1317-82-7-1555. [DOI] [PubMed] [Google Scholar]