Abstract
Wastewater particularly from electroplating, paint, leather, metal and tanning industries contain enormous amount of heavy metals. Microorganisms including fungi have been reported to exclude heavy metals from wastewater through bioaccumulation and biosorption at low cost and in eco-friendly way. An attempt was, therefore, made to isolate fungi from sites contaminated with heavy metals for higher tolerance and removal of heavy metals from wastewater. Seventy-six fungal isolates tolerant to heavy metals like Pb, Cd, Cr and Ni were isolated from sewage, sludge and industrial effluents containing heavy metals. Four fungi (Phanerochaete chrysosporium, Aspegillus awamori, Aspergillus flavus, Trichoderma viride) also were included in this study. The majority of the fungal isolates were able to tolerate up to 400 ppm concentration of Pb, Cd, Cr and Ni. The most heavy metal tolerant fungi were studied for removal of heavy metals from liquid media at 50 ppm concentration. Results indicated removal of substantial amount of heavy metals by some of the fungi. With respect to Pb, Cd, Cr and Ni, maximum uptake of 59.67, 16.25, 0.55, and 0.55 mg/g was observed by fungi Pb3 (Aspergillus terreus), Trichoderma viride, Cr8 (Trichoderma longibrachiatum), and isolate Ni27 (A. niger) respectively. This indicated the potential of these fungi as biosorbent for removal of heavy metals from wastewater and industrial effluents containing higher concentration of heavy metals.
Keywords: Industrial wastewater, Fungi, Biosorbent, Bioaccumulation, Heavy metals
Introduction
Use of wastewater in agriculture has increased in recent years due to inherent treatment capacity of soil and high contents of major and micronutrients in it. However, wastewater, particularly from industries contains high concentration of heavy metals which enter into human beings and animals through food chain. Therefore, it is desirable to remove these heavy metals from wastewater through low cost technology before its use in agriculture. Physico-chemical methods such as reverse osmosis, solvent extraction, lime coagulation ion exchange and chemical precipitation [1, 2]. for removal of heavy metals from wastewater are very expensive and these do not remove heavy metals from wastewater up to desired limits. Recently, microbes have been reported as biological adsorbents to remove heavy metals from wastewater at low cost and in eco-friendly way [3–6].
The bio-sorption of heavy metals using various live and heat treated fungi has been studied. These studies showed that the bio-sorption capacity of the heat treated cells might be greater, equivalent or less than that of their living counterparts [7–10].
Heavy metal resistant microbes might be present in heavy metal contaminated sites. The resistance and efficiency of microbes for removal of heavy metals vary greatly. Therefore, there is need to isolate and screen heavy metal tolerant fungi from heavy metals contaminated sites. The present study attempts to isolate and screen heavy metal tolerant fungi and to evaluate their efficiency to remove heavy metals from liquid media under laboratory conditions.
Materials and Methods
Collection of Samples
Samples of sewage, sludge and industrial effluents were collected in sterilized containers from sewage treatment plants at Karnal, Panipat and electroplating industry at Sonepat. These samples were brought to laboratory and kept in refrigerator at 4°C for further processing.
Isolation of Fungi
Fungal isolates were isolated from samples of sewage, sludge and industrial effluents by serial dilution method using potato dextrose agar (Hi-Media, Mumbai, India) containing 25 ppm of Pb, Ni, Cr, and Cd individually. The 1000 ppm stock solutions of Pb, Ni, Cr and Cd were made in double distilled water using Pb(NO3)2, NiCl2·6H2O, CdCl2, and K2Cr2O7 (sd fine-chem limited, Mumbai, India). The stock solution of heavy metals was sterilized separately through bacteriological filters and added to sterilized potato dextrose agar (PDA) medium to make the concentration at 25 ppm. A serial dilution of each sample was made up to 106 and one ml of dilution 104 and 106 was added in sterilized petri plates in duplicate. Twenty milliliter of PDA medium containing 25 ppm of one of these heavy metals was poured in these petri plates and incubated at 28°C for 48 h. The colonies of predominant genera of fungi were picked up and purified by pour plate method.
Three organic matter decomposing fungi namely Trichoderma viride, Aspergillus awamori, Phanerochaete chrysosporium were procured from Department of Microbiology, IARI, New Delhi for this study. Another fungus namely Aspergillus flavus isolated at CSSRI, Karnal was also included in this study.
Screening of Fungal Isolates for Tolerance to Heavy Metals
Heavy metal tolerant (25 ppm) fungal isolates were further screened for tolerance to Pb, Ni, Cr and Cd at 50, 100 and 400 ppm of heavy metals individually on PDA. All the fungal isolates were streaked on PDA medium containing 50, 100 and 400 ppm of each of the four heavy metals separately. Streaking of fungal isolates on normal PDA medium served as control (normal growth) for comparison of growth of fungal isolates on PDA medium containing different concentration of heavy metals. Observations on growth of fungal isolate were made after 72 h of incubation. The growth of fungal isolates was recorded as normal growth or absent growth in comparison to control.
Uptake of Heavy Metals by Fungal Isolates from Liquid Media
The highly tolerant fungal isolates to different heavy metals were evaluated for uptake of heavy metals in potato dextrose broth medium containing 50 ppm concentration of different heavy metals Pb, Ni, Cr and Cd individually in triplicate. Potato dextrose broth containing 50 ppm of one of the heavy metals was dispensed in 100 ml lots to 250 ml conical flasks and sterilized at 15 lbs/psi for 15 min. These flasks were inoculated with 1 ml of freshly prepared spore suspension (106–107 spores/ml) of each fungal isolate and put on shaker at 150 rpm at 28°C for 96 h. Un-inoculated flasks containing PD broth of 50 ppm concentration of different heavy metals served as control. Fungal growth was harvested after 96 h through filtration using Whatman filter No. 42. The harvested fungal biomass was rinsed with double distilled water 3–4 times and dried in hot air oven at 80°C for 18 h. The dried fungal biomass was weighed and heavy metal concentration in it was estimated by digestion with nitric acid and perchloric acid (3:1 ratio). The digested fungal biomass was filtered through Whatman filter No. 42. and made the volume of filtrate to 50 ml in volumetric flask. The heavy metals concentration in filtrate was estimated [11] by Atomic Absorption Spectrophotometer (GBC932, Semi-automatic). All the experiments were conducted in triplicate and data were analyzed statistically.
Tolerance and uptake of multiple heavy metals (Pb, Cd, Cr and Ni) by twelve fungi in PD broth containing 12.5 ppm each of Pb, Cd,Cr and Ni was studied by same method.
The uptake of heavy metal by fungal biomass was calculated using the following equation:
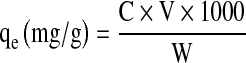 |
1 |
qe concentration of heavy metal accumulated by fungal biomass, (mg/g); C concentration of heavy metal (ppm); V (ml) the volume of the aqueous medium and W (g) is the dry weight of the fungal biomass.
Result and Discussion
Isolation of Heavy Metal Tolerant Fungi
Seventy-six fungal isolates tolerant to heavy metals were isolated from samples of sewage, sludge and industrial effluent contaminated with heavy metals such as Pb, Cd, Cr and Ni using standard methods [12]. This included 34 isolates tolerant to Pb, 9 each tolerant to Cd, Cr and 29 tolerant to Ni at 25 ppm. Some of the most heavy metal tolerant isolates were identified as Aspergillus niger (Pb2, 2CdF, 3CdF, Cr10, Ni19, Ni27, Ni30, Ni33) Trichoderma longibrachiatum (Cr8), Aspergillus flavus (Pb7, Pb8, Ni35, Ni36) respectively by the cultural and biochemical characteristic at Indian Culture Collection, Department of Pathology, Indian Agricultural Research Institute, New Delhi. Another lead tolerant fungal isolate Pb3 was identified from Institute of Microbial Technology, Chandigarh, India based on analysis of ITS1-5.8 rDNA-ITS2 and 28S rDNA as Aspergillus terreus.
Screening of Fungal Isolates for Tolerance to Heavy Metals
Thirty fungal isolates and four fungi (Phanerochaete chrysosporium, Aspergillus awamori, Aspergillus flavus, Trichoderma viride) tolerant to Pb were further screened for their tolerance to Pb at 25, 50, 100 and 400 ppm of Pb. Data indicated decrease in number of isolates tolerant to Pb at higher concentration of Pb. Out of 34 fungal isolates tolerant to Pb at 25 ppm, only 14 isolates could tolerate Pb at 400 ppm. Similar trend was observed for screening of fungal isolates for their tolerance to Cd, Cr and Ni. This indicated inhibition of some of the fungal isolates at higher concentration of heavy metals. Similar observations about toxic effect of higher concentration of heavy metals on growth of fungi have been reported [13, 14].
Growth and Uptake of Pb by Fungal Isolates
The maximum dry wt (0.48 g) was observed by Aspergillus niger followed by T. viride (0.34 g) in PD broth containing 50 ppm of Pb. The minimum dry wt (0.07 g) was observed by fungus Aspergillus terreus. The dry weight of A. niger and T. viride was statistically significant in comparison to all other fungi (Table 1) The maximum uptake (59.67 mg/g) of Pb was observed in Aspergillus terreus. Minimum uptake Pb (7.86 mg/g) found in A. niger (Table 1). Wherever there was less growth, there was higher uptake of Pb and vice versa. The uptake of Pb by A. terreus was most significant followed by P. chrysosporium in comparison to all the fungi (Table 1). The highest uptake of Pb (59.67 mg/g) by A. terreus indicated more binding sites on cell wall of this fungus and its potential as biosorbent to remove Pb from industrial wastewater containing higher concentration of Pb. Similar results with respect to differential Pb uptake by different fungi were reported by earlier workers [15–20].
Table 1.
Growth and uptake of Pb by different fungi from liquid medium containing 50 ppm lead
| Fungi | Dry weight (g) | Uptake (mg/g) |
|---|---|---|
| Pb2 (Aspergillus niger) | 0.48 | 7.86 |
| Pb3 (Aspergillus terreus) | 0.07 | 59.67 |
| Pb7 (A. flavus) | 0.21 | 12.45 |
| Pb8 (A. flavus) | 0.22 | 17.38 |
| Phanerochaete chrysosporium | 0.18 | 21.75 |
| Aspergillus awamori | 0.20 | 15.65 |
| Trichoderma viride | 0.34 | 10.34 |
| CD at 5% | 0.03 | 2.66 |
Growth and Uptake of Cd by Fungal Isolates
Dry wt of different fungi in the presence of Cd at 50 ppm was in the range from 0.02 to 0.5 g. The maximum dry wt (0.5 g) was observed by fungal isolate Phanerochaete chrysosporium, Aspergillus flavus, Aspergillus awamori in PD broth containing 50 ppm of Cd. The minimum dry wt (0.08 g) was observed in A. niger. The dry weight of P. chrysosporium, A. niger and A. awamori was significant in comparison to all other fungi (Table 2). The maximum uptake (16.25 mg/g) of Cd was observed in (Table 2) T. viride. Minimum uptake of Cd (0.38 mg/g) was observed in A. awamori. The uptake of Cd by T. viride was most significant in comparison to all other fungi (Table 2). The highest uptake of Cd by T. viride indicated its potential as biosorbent and efficacy to remove Cd from aqueous solution. Similar results with respect to biosorption of Cd and other heavy metals by fungi heave been reported earlier [21–23]. Higher fungi such as deuteromycetes Trichoderma viride have cell wells composed primarily of chitin and glucan polymers which help in binding of heavy metals like Cd [24].
Table 2.
Growth and uptake of Cd by different fungi from liquid medium containing 50 ppm cadmium
| Fungi | Dry weight (g) | Uptake (mg/g) |
|---|---|---|
| 2CdF (A. niger) | 0.02 | 6.20 |
| 3CdF (A. niger) | 0.02 | 6.46 |
| Aspergillus awamori | 0.50 | 0.38 |
| Phanerochaete chrysosporium | 0.50 | 0.71 |
| Aspergillus flavus | 0.50 | 0.50 |
| Trichoderma viride | 0.08 | 16.25 |
| CD at 5% | 0.05 | 2.27 |
Growth and Uptake of Cr by Fungal Isolates
The maximum dry wt (0.49 g) was observed in T. viride followed by A. niger (0.37 g). The minimum dry wt (0.18 g) was found in Trichoderma longibrachiatum. The dry weight of T. viride and A. niger was statistically significant in comparison to all other fungi. The maximum uptake (0.55 mg/g) and minimum uptake of Cr (0.03 mg/g) was observed in (Table 3) Trichoderma longibrachiatum and T. viride in PD broth containing 50 ppm of Cr respectively. The uptake of Cr by T. longibrachiatum was most significant in comparison to other fungi. The highest uptake of Cr (0.55 mg/g) by Trichoderma longibrachiatum indicated its efficiency to remove Cr from aqueous solution containing higher concentration of Cr. These results with respect to uptake of Cr by fungi are in agreement with those reported earlier [25–28].
Table 3.
Growth and uptake of Cr by different fungi from liquid medium containing 50 ppm chromium
| Fungi | Dry weight (g) | Uptake (mg/g) |
|---|---|---|
| Cr8 (Trichoderma longibrachiatum) | 0.18 | 0.55 |
| Cr10 (A. niger) | 0.32 | 0.05 |
| Aspergillus niger | 0.37 | 0.04 |
| Phanerochaete chrysosporium | 0.29 | 0.10 |
| Trichoderma viride | 0.49 | 0.03 |
| CD at 5% | 0.03 | 0.02 |
Growth and Uptake of Ni by Fungal Isolates
The maximum dry wt (1.66 g) was observed in (Table 4) fungal isolate Ni36 (A. flavus) in PD broth containing 50 ppm of Ni. The minimum dry wt (1.02 g) was found in Ni35 (Aspergillus flavus). The dry weight of Ni30 (A. niger), Ni33 (A. niger) and Ni36 (A. flavus) was statistically significant in comparison to all other fungi. The maximum uptake (0.55 mg/g) and minimum uptake of Ni (0.33) from PD broth containing 50 ppm of Ni was observed by isolates Ni27 (A. niger) and Ni36 (A. flavus) respectively. The uptake of Ni by A. niger was most significant in comparison to all other fungi. Similar results with respect to uptake of Ni by fungi have been reported earlier [7, 29].
Table 4.
Growth and uptake of Ni by different fungi from liquid medium containing 50 ppm nickel
| Fungi | Dry weight (g) | Uptake (mg/g) |
|---|---|---|
| Ni19 (A. niger) | 1.06 | 0.40 |
| Ni27 (A. niger) | 1.05 | 0.55 |
| Ni30 (A. niger) | 1.35 | 0.45 |
| Ni33 (A. niger) | 1.36 | 0.40 |
| Ni35 (A. flavus) | 1.02 | 0.47 |
| Ni36 (A. flavus) | 1.66 | 0.33 |
| CD at 5% | 0.03 | 0.02 |
Growth and Uptake of Multiple Metals by Fungi
The maximum growth in the presence of multiple metals was observed by Aspergillus niger. However, there was significant higher uptake of Pb (0.89 mg/g), Cd (0.16 mg/g) by Trichoderma viride, Cr (0.76 mg/g) by Aspergillus niger and Ni (0.66 mg/g) by fungal isolate Pb8 (A. flavus) (Table 5). The dry weight of A. niger was most significant in comparison to all other fungi. The uptake of Pb and Cd by T. viride, Cr by A. niger and Ni by Pb8 (A. flavus) was statistically significant in comparison to all other fungi. Fungi Phanerochaete chrysosporium, Trichoderma viride, Ni35 (A. flavus) were tolerant to all the four heavy metals and biosorbed substantial amount of the heavy metals from PD broth containing 12.5 ppm each of four heavy metals (Pb, Cd, Cr, Ni). There was lower uptake of all the four heavy metals (Pb, Cd, Cr, Ni) by these fungi in combination in comparison to individual heavy metal uptake. This was mainly due to competition of heavy metals for same and limited adsorption sites on fungal cell wall of these fungi. This indicated the potential of these fungi to remove heavy metals from liquids and industrial wastewater containing higher concentration of heavy metals. Similar observations regarding reduction of uptake of heavy metals in combination in comparison to individual heavy metal have been reported earlier [7, 9].
Table 5.
Tolerance and uptake of heavy metals by fungi from liquid medium containing 12.5 ppm each of Pb, Cd, Cr and Ni
| Fungi | Dry weight (g) | Pb uptake (mg/g) | Cd uptake (mg/g) | Cr uptake (mg/g) | Ni uptake (mg/g) |
|---|---|---|---|---|---|
| Pb2 (Aspergillus niger) | 0.51 | 0.46 | 0.08 | 0.76 | 0.64 |
| Ni35 (A. flavus) | 0.40 | 0.70 | 0.10 | 0.44 | 0.43 |
| Phanerochaete chrysosporium | 0.22 | 0.62 | 0.15 | 0.59 | 0.39 |
| Trichoderma viride | 0.01 | 0.89 | 0.16 | 0.55 | 0.38 |
| Aspergillus flavus | 0.39 | 0.51 | 0.11 | 0.66 | 0.23 |
| Aspergillus awamori | 0.34 | 0.21 | 0.10 | 0.53 | 0.23 |
| Pb7 (A. flavus) | 0.42 | 0.64 | 0.09 | 0.56 | 0.58 |
| Pb8 (A. flavus) | 0.41 | 0.56 | 0.09 | 0.54 | 0.66 |
| Ni27 (A. niger) | 0.45 | 0.67 | 0.08 | 0.49 | 0.33 |
| Ni30 (A. niger) | 0.48 | 0.61 | 0.08 | 0.49 | 0.31 |
| Ni33 (A. niger) | 0.48 | 0.58 | 0.09 | 0.62 | 0.45 |
| Ni36 (A. flavus) | 0.34 | 0.43 | 0.14 | 0.56 | 0.39 |
| CD at 5% | 0.04 | 0.03 | 0.02 | 0.03 | 0.03 |
The above data (Tables 2, 3, 4, 5) of dry weight of fungi and uptake of heavy metal by fungi indicated, in general, that where there is maximum dry wt of fungus, there is minimum uptake of heavy metal and vice versa. The higher uptake of heavy metal was due to less weight of fungi at a particular concentration of that heavy metal. Similar results have been reported by earlier workers [7, 29].
Conclusion
Seventy-six fungal isolates isolated from sewage, sludge, and industrial effluents and four identified fungi were screened for their tolerance to four heavy metals (Pb, Cd, Cr and Ni) in PDA medium containing heavy metal from 25 to 400 ppm. There was decrease in number of fungi for their tolerance to heavy metal with increase in concentration of heavy metal from 25 to 400 ppm. Majority of the fungal isolates were able to tolerate heavy metals up to 400 ppm. The most heavy metal tolerant (400 ppm) fungi were further screened for removal of heavy metals from PD broth containing 50 ppm of individual heavy metal. Data revealed that some of the fungi removed substantial amount of heavy metals particularly Pb (59.67 mg/g by Aspergillus terreus) and Cd (16.25 mg/g by Trichoderma viride). This indicated the potential biosorption capacity of these fungi to remove heavy metals from wastewater.
Acknowledgments
The authors are thankful to the Director, CSSRI, Karnal and Head, Division of Soils and Crop Management, CSSRI, Karnal for providing all the laboratory facilities for this work.
References
- 1.Donmez G, Aksu Z. Bioaccumulation of copper (II) and nickel (10 by the non-dapted and adapted growing Cundidu sp. J Water Res. 2001;35:1425–1434. doi: 10.1016/S0043-1354(00)00394-8. [DOI] [PubMed] [Google Scholar]
- 2.Peters RW, Young K, Bhattacharayan D. Evaluation of recent treatment technique for removal of heavy metals from industrial wastewater. AICHE Symp Ser. 1985;81:1695–1703. [Google Scholar]
- 3.Bai SR, Abrahim TE. Studies on chromium (VI) Adsorption–desorption using immobilized fungal biomass. Bioresour Technol. 2003;87:17–26. doi: 10.1016/S0960-8524(02)00222-5. [DOI] [PubMed] [Google Scholar]
- 4.Elizabeth KM, Anuradha TVR. Biosorption of haxavalant chromium by non-pathogenic bacterial cell preparations. Indian J Microbiol. 2000;40:263–265. [Google Scholar]
- 5.Gadd GM. Fungi and yeast for metal accumulation. In: Ehrlich HL, Brierley CL, editors. Microbial mineral recovery. New York: McGraw-Hill; 1990. pp. 249–276. [Google Scholar]
- 6.Veglio F, Beolcmi F. Removal of metals by biosorption: a review. J Hydrometall. 1997;74:301–316. doi: 10.1016/S0304-386X(96)00059-X. [DOI] [Google Scholar]
- 7.Ahmad I, Ansari MI. Biosorption of Ni, Cr and Cd by metal tolerant Aspergillus niger and Penicillium sp. using single and multimetal solution. Indian J Exp Biol. 2006;44:73–76. [PubMed] [Google Scholar]
- 8.Arıca MY, Arpa C, Ergene A, Bayramog˘lu G, Genc O. Ca-alginate as a support for Pb(II) and Zn(II) biosorption with immobilized Phanerochaete chrysosporium. Carbohydr Polym. 2003;52:167–174. doi: 10.1016/S0144-8617(02)00307-7. [DOI] [Google Scholar]
- 9.Kapoor A, Viraraghavan T, Cullimore DR. Removal of heavy metals using the fungus Aspergillus niger. Bioresour Technol. 1999;70:95–104. doi: 10.1016/S0960-8524(98)00192-8. [DOI] [Google Scholar]
- 10.Sag˘lam Y, Yacınkaya Y, Denizli A, Arıca MY, Genc O, Bektas S. Biosorption of mercury by carboxycellulose and immobilized Phanerochaete chrysosporium. Microchem J. 2002;71:73–81. doi: 10.1016/S0026-265X(01)00142-4. [DOI] [Google Scholar]
- 11.Greenberg AE, Trussell RR, Clesceri LS. Standard methods for the examination of water and wastewater. 16. Washington, DC: American Public Health Association; 1985. pp. 146–150. [Google Scholar]
- 12.Solarsk S, May T, Roddick FA, Lawrie AC. Isolation and screening of natural organic matter-degrading fungi. Chemosphere. 2009;75:751–758. doi: 10.1016/j.chemosphere.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Malik A. Metal bioremediation through growing cells. J Environ Int. 2004;30:271–278. doi: 10.1016/j.envint.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Rama Rao VSKV, Akhtar N, Maruthi MP. Isolation of a cadmium tolerant Curvularia sp. for polluted effluent. Curr Sci. 1997;73:453. [Google Scholar]
- 15.Li XM, Liao DX, Xu XQ, Yang Q, Zeng GM, Zheng W, Guo L. Kinetic studies for the biosorption of lead and copper ions by Penicillium simplicissimum immobilized within loofa sponge. J Hazard Mater. 2008;159:610–615. doi: 10.1016/j.jhazmat.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 16.Mittar D, Khanna PK, Marwaha SS, Kennedy JF. Biobleaching of pulp and paper mill effluents by Phanerochaete chrysosporium. J Chem Technol Biotechnol. 1992;53:81–92. doi: 10.1002/jctb.280530112. [DOI] [Google Scholar]
- 17.Say R, Denizli A, Arica MY. Biosorption of cadmium(II), lead(II) and copper(II) with the filamentous fungus Phanerochaete chrysosporium. Bioresour Technol. 2001;76:67–70. doi: 10.1016/S0960-8524(00)00071-7. [DOI] [PubMed] [Google Scholar]
- 18.Ahluwalia SS, Goyal D. Removal of lead from aqueous solution by filaments fungi. Indian J Micobiol. 2003;43:237–241. [Google Scholar]
- 19.Yetis U, Dolek A, Dilek FB, Qzcengiz G. The removal of Pb(II) by Phanerochaete chrysosporium. Water Res. 2000;34:4090–4100. doi: 10.1016/S0043-1354(00)00155-X. [DOI] [Google Scholar]
- 20.Melgar MJ, Alonso J, Garcia MA. Removal of toxic metals from aqueous solutions by fungal biomass of Agaricus macrosporus. Sci Total Environ. 2007;385:12–19. doi: 10.1016/j.scitotenv.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Bishnoi NR, Kumar R, Bishnoi K. Biosorption of Cr(VI) with Trichoderma viride immobilized fungal biomass and cell free Ca-alginate beads. Indian J Exp Biol. 2007;45:657–664. [PubMed] [Google Scholar]
- 22.Liu Y, Fan T, Zeng G, Li X, Tong Q, Ye F, Zhou Ming Xu W, Huang Y. Removal of cadmium and zinc ions from aqueous solution by living Aspergillus niger. Trans Nonferr Met Soc China. 2006;16:681–686. doi: 10.1016/S1003-6326(06)60121-0. [DOI] [Google Scholar]
- 23.Pogaku R, Kulkarni S. Biosorption of combined industrial effluents using Phanerochaete chrysosporium. Int J Chem React Eng. 2006;4:A16. [Google Scholar]
- 24.Morley GF, Gadd GM. Sorption of toxic metals by fungi and clay materials. Mycol Res. 1998;99:1428–1439. [Google Scholar]
- 25.Akar T, Tunali S. Biosorption characteristics of Aspergillus flavus biomass for removal of Pb(II) and Cu(II) ions from an aqueous solution. Bioresour Technol. 2006;97:1780–1787. doi: 10.1016/j.biortech.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Gopal M, Pakshirajan K, Swaminathan T. Heavy metal removal by biosorption using Phanerochaete chrysosporium. Appl Biochem Biotechnol. 2002;102:227–237. doi: 10.1385/ABAB:102-103:1-6:227. [DOI] [PubMed] [Google Scholar]
- 27.Preetha B, Viruthagiri T. Batch and continuous biosorption of chromium(VI) by Rhizopus arrhizus. Sep Purif Technol. 2007;57:126–133. doi: 10.1016/j.seppur.2007.03.015. [DOI] [Google Scholar]
- 28.Congeeraram S, Dhanarani S, Park J, Dexilin M, Thamaraiselvi K. Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J Hazard Mater. 2007;146:270–277. doi: 10.1016/j.jhazmat.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Mogollon L, Rodriguez R, Larrota W, Ramirez N, Torres R. Biosorption of nickel using filamentous fungi. Appl Biochem Biotechnol. 1998;70:593–601. doi: 10.1007/BF02920171. [DOI] [PubMed] [Google Scholar]


