Abstract
Forty two Streptomycetes isolates from soils of Kodachadri region in Western ghats were recovered by soil dilution technique. Cross streak method was followed for primary screening of antifungal activity. Positive isolates were subjected to secondary screening by cold extraction of fermentation broth in butanol solvent. Six isolates exhibited broad spectrum antifungal activity against all the tested yeast pathogens like Candida albicans, Candida lipolytica, Cryptococcus neoformens and Saccharomyces cerevisiae. One isolate showed excellent antifungal activity against all test organisms with maximum zone of inhibition 60 mm each incase of C. neoformens and C. albicans. Partial characterization of antifungal metabolite by TLC resulted in a purple spot with an Rf value 0.50. The UV absorption spectra at 218 nm indicated possible chemical nature of the active metabolite as polyene group and purity was assessed by analytical HPLC.
Keywords: Antifungal activity, Cross streak, Secondary screening, HPLC, UV absorption
Introduction
The need for new, safe and effective antifungal antibiotics is a major challenge to the Pharmaceutical industry today, especially with the increased opportunistic infections in immunocompromised host and also lack of non toxic antifungal antibiotics [1]. Actinomycetes are one of the most attractive sources of antibiotics, of all the antibiotics 66% are produced by Actinomycetes [2–4]. Antibiotics predominate in therapeutic and commercial importance. One of the modern approaches is isolation and screening of Actinomycetes from relatively unknown areas. In this meaning Western ghats are of significant interest, as they are proven as eminent systems enriched with unraveled biological diversity. Aim of the present study was screening of antifungal metabolites from Actinomycetes against human yeast pathogens.
Materials and Methods
Sampling
72 soil samples were collected (from May 2005 to October 2006) from Kodachadri forest areas of Western Ghats. Samples were collected from 20 cm depth into sterile polythene bags, air dried at room temperature and stored under aseptic condition until processing [5].
Isolation of Actinomycetes
Isolation and enumeration of actinomycetes was performed by soil dilution plate technique [6]. One gram of dried soil was serially diluted (up to 10−5 dilutions). Different aqueous solutions from 10−4 and 10−5 of the suspension were pour inoculated on plates containing selective media like Starch casein agar [7], Modified albumin agar, Actinomycetes isolation agar and Chitin agar [8, 9]. The incubation was carried out at 30 ± 2°C for 7–10 days [10, 11].
Characterization of Actinomycetes
Morphological observations of selected isolates were made with light microscope by using method of Shirling and Gottlieb [12]. The isolates were identified up to genus level as described in Bergy’s manual and by cover slip method. In cover slip method spore suspension of the actinomycetes were placed on the starch casein agar blocks covered with sterile cover slips and incubated in moist chamber for 2–3 days [13]. Biochemical characterization was done by performing starch hydrolysis, gelatin hydrolysis, casein hydrolysis, sugar fermentation and H2S production [2, 14].
Bioassay
Pathogenic yeasts tested for in vitro antifungal activity are C. albicans NCIM-3100, C. lipolytica NCIM-3472, C. neoformens NCIM-3541 and S. cerevisiae NCIM-3095 procured from National Collection of Industrial Microorganisms Pune, India.
The promising isolates identified in the present study were subjected to primary screening by cross streak method [15]. Actinomycetes were swab inoculated on half of the Petri plates containing Sabouraud’s dextrose agar and incubated at 30°C for 72 h. Effectivity of the isolates was assessed by growth inhibition of pathogenic yeasts [16].
Isolates possessing antifungal activity were subjected to secondary screening by inoculating the culture to starch casein broth and incubated at 30°C for 8–10 days. After the 10th day the broth was centrifuged at 10,000 rpm for 20 min to separate mycelial biomass. For extraction of antibiotic, the supernatant was mixed with Butanol solvent, in 1:1 proportion (v/v), the solvent supernatant mixture was agitated for 45 min in homogenizer; the solvent was then separated by using separating funnel. Obtained extracts were assayed for antifungal activity by agar well diffusion method [16] using respective solvents as control.
Separation of Antibiotic
The solvent was evaporated by subjecting the crude extract to hot air at 40°C in an oven for 96 h. The concentrated residue obtained was dissolved in sterile water following which crude antibiotic obtained was subjected for purification [17]. The crude antibiotic was tested for its components by using precoated TLC plates using Ethanol:Chloroform:Water (40:40:20) [17] and Butanol:Acetic acid:water solvent systems [5]. Chromatograms were developed in Iodine chamber [17] and sprayed with Ninhydrin to know the possible chemical nature of the active component.
The UV–Visible absorption spectra of the bioactive component in solvent extracts were determined with a SHIMADZU UV-2550 spectrophotometer at 200–400 nm to determine the λmaximum of the band [6, 18].
High Performance Liquid Chromatography (HPLC)
The purity of the bioactive component from KSRO 04 was tested using HPLC analysis by the method employed by Chakravarthi et al. [19] with minor modifications. HPLC (Shimadzu) separation was performed using a C18-column (250 × 4.6 mm) at a flow rate of 1 ml/min and pressure 142 kgf by injecting 20 μl of sample. The mobile phase used was methanol:water (70:30, v/v). The absorbance was measured at 203 nm.
Results
Isolation of Actinomycetes
Forty two isolates were recovered from Kodachadri soils, among which six showed broad spectrum antifungal activity against test organisms.
Characterization of Actinomycetes
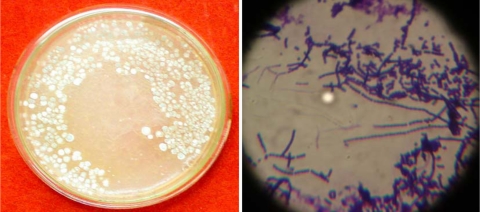
Six promising isolates were characterized by morphological and biochemical methods (Table 1). Microscopic characterization of these isolates by Cover slip method revealed them to be of the genus Streptomyces. Isolate KSRO4 showed straight arrangement of twelve to sixteen spores (Fig. 1).
Table 1.
Biochemical activities of Streptomycetes isolates
| Isolate no | KSRO1 | KSRO2 | KSRO3 | KSRO4 | KSRO5 | KSRO6 |
|---|---|---|---|---|---|---|
| Starch hydrolysis | + | − | + | + | + | + |
| Gelatin hydrolysis | + | − | − | − | + | a |
| Casein hydrolysis | + | − | − | − | − | a |
| Sugar fermentation | ||||||
| S | − | + | − | − | − | a |
| M | − | + | − | − | − | a |
| G | + | + | − | − | − | a |
| H2S production | − | − | + | + | − | a |
aResults not clear
Fig. 1.
Morphology and microscopic view of isolate KSRO4 (magnification 2000×)
Bioassay
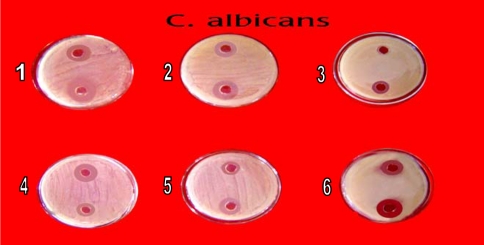
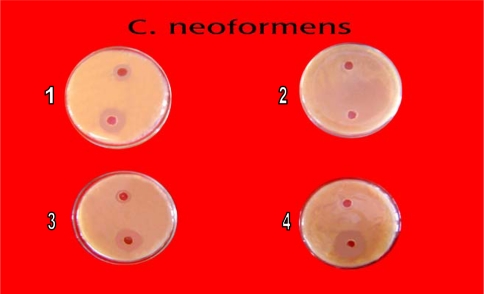
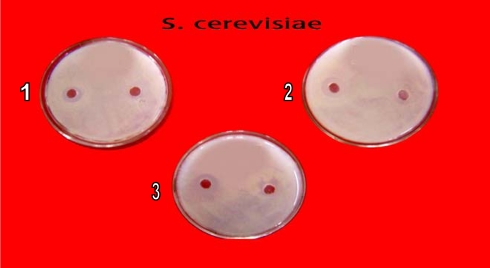
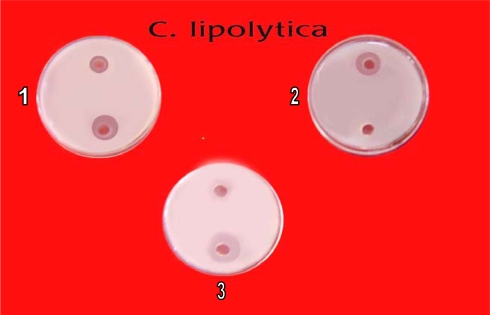
In primary screening 6 of the 42 isolates showed antagonistic activity against test fungi. Solvent extracts of culture filtrate tested in secondary screening showed inhibition zone varying from 10 mm to 60 mm by well in agar method. Two isolates showed potent antifungal activity against tested fungi (Table 2). All the six isolates inhibited C. albicans (Fig. 2), C. neoformens was inhibited by 4 isolates (Fig. 4) and three were effective on S. cerevisiae and C. lipolytica (Figs. 3 and 5). One isolate showed excellent antifungal activity in all tested fungi (Isolate KSRO4). The results are represented in bar diagram as follows.
Table 2.
Antifungal activities of Streptomycetes isolates
| Sl no | Isolate no | C. albicans | C. neoformens | C. lipolytica | S. cerevisiae |
|---|---|---|---|---|---|
| 1 | KSRO1 | 18 | 10 | – | 20 |
| 2 | KSRO2 | 10 | 16 | – | – |
| 3 | KSRO3 | 50 | 20 | – | 80 |
| 4 | KSRO4 | 60 | 60 | 50 | 40 |
| 5 | KSRO5 | 11 | – | 30 | – |
| 6 | KSRO6 | 10 | – | 80 | – |
Fig. 2.
Antifungal activity of Streptomyces isolates against C. albicans by well in agar method (1) KSRO-01, (2) KSRO-02, (3) KSRO-03, (4) KSRO-04, (5) KSRO-06, (6) KSRO-05
Fig. 4.
Antifungal activity of Streptomyces isolates against C. neoformens by well in agar method (1) KSRO-01, (2) KSRO-02, (3) KSRO-04, (4) KSRO-03
Fig. 3.
Antifungal activity of Streptomyces isolates against S. cerevisiae by well in agar method (1) KSRO-01, (2) KSRO-04, (3) KSRO-03
Fig. 5.
Antifungal activity of Streptomyces isolates against C. lipolytica by well in agar method (1) KSRO-06, (2) KSRO-04, (3) KSRO-05
Partial Characterization of Antibiotic
Concentrated solvent extract of isolate four subjected to TLC using solvent system [Butanol:Acetic acid:Water] showed spot having Rf value 0.50. The chromatogram developed in Iodine chamber, showed purple band indicating unsaturated fatty acid nature of active components (Fig. 6).
Fig. 6.
Thin layer chromatogram of isolate no KSRO-04
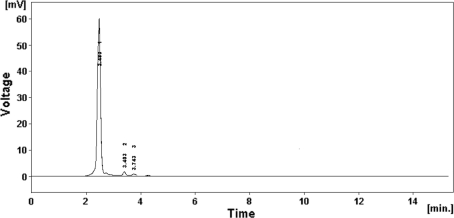
UV absorption range of the isolate KSRO-04 was 218 nm suggesting polyene nature of the component [5]. HPLC analysis was performed to check purity of extract obtained. Solvent system was standardized with different ratio of Methanol and Water. The solvent extract of isolate KSRO-04 revealed a prominent peak along with short peaks revealing traces of additional compounds which are indicated by additional peaks in the graph (Fig. 7).
Fig. 7.
High performance liquid chromatography (HPLC) analysis of the solvent extract of KSRO-04 isolate
Discussion
The present study emphasized on screening of actinomycetes from Western Ghats an unexplored biodiversity hot spot and partial characterization of antifungal metabolite effective against opportunistic yeast pathogens causing infections in immunocompromised hosts [20, 21]. Characterization of the antifungal metabolite indicated polyene nature of the active principle, similar activities have been earlier conducted and polyene nature has been confirmed by Sahin et al. [5]. Characterization of a non polyene antifungal antibiotic from Streptomycetes effective against yeast pathogen like C. albicans has been carried out by Augustine et al. [2]. The present investigation in comparison with other works focused on characterization of the antifungal metabolite, as in majority of the earlier studies Candida albicans has only been considered as a test pathogen but characterization of the metabolite has not been carried out. In this regard the metabolite is of high importance as polyene group of non toxic antifungal metabolites like Nystastin and Amphotericin B are very less in number.
Conclusion
With the above obtained results we conclude that polyene group of antibiotics are well suited for the control of opportunistic yeast pathogens and Western Ghats soils are a promising source for new and potent actinomycetes.
Future Prospect
In the past few decades emergence of new and resistant pathogens has increased the requirement of effective antibiotics. In this regards, further investigations such as the taxonomic categorization of isolates by 16S rDNA analysis, electron microscopic studies, identification of purified active components by NMR and IR techniques and metabolites have to be subjected for assessment of their toxicological profiles, as a mandatory process suggested by all regulatory authorities are to be under taken.
Acknowledgments
The authors greatly acknowledge Prof. T.S. Hoovaiah Gowda, Principal, Sahyadri Science College (autonomous), Shimoga for providing facilities and moral support.
References
- 1.Suzuki K, Sako T, Morioka M, Nagai K, Yamaguchi H, Saito T. Tetrazomine, a new antibiotic produced by an actinomycete strain, taxonomy, fermentation, isolation and characterization. J Antibiot. 1991;44:479–483. doi: 10.7164/antibiotics.44.479. [DOI] [PubMed] [Google Scholar]
- 2.Augustine SK, Bhavsar SP, Kapadnis BP. A nonpolyene antifungal antibiotic from Streptomyces albidoflavus PU23. J Biosci. 2005;30(2):201–211. doi: 10.1007/BF02703700. [DOI] [PubMed] [Google Scholar]
- 3.naidenova Mariana, vladimiva Denista. Isolation and taxonomic investigation of actinomycetes from specific biotypes in Bulgaria. J Cult Collect. 2002;3:15–24. [Google Scholar]
- 4.Wu X, Chen W, Qian C, Li O, Li P, Wen Y. Isolation and identification of newly isolated antagonistic Streptomyces sp. strain AP19–2 producing chromomycins. J Microbiol. 2007;45(6):499–504. [PubMed] [Google Scholar]
- 5.sahin Nurettin. Investigation of the antimicrobial activity of some streptomycetes isolates. Turk J Biol. 2003;27:79–84. [Google Scholar]
- 6.Ellaih P, Ramana T, Bapi Raju KVVSN, Sujatha P, Uma Sankar A. Investigation on marine actinomycetes from Bay of Bengal near Kakinada coast of Andhra Pradesh. Asian J Microbiol Biotechnol Environ Sci. 2004;6(1):53–56. [Google Scholar]
- 7.Bergey’s . Bergey’s manual of determinative bacteriology. 9. Baltimore: Williams & Wilkins; 1994. p. 787. [Google Scholar]
- 8.Haque SF, Sen SK, Pal SC. Screening and identification of antibiotic producing strains of actinomycetes. Hindustan Antibiot Bull. 1992;34(3):76–84. [PubMed] [Google Scholar]
- 9.Hayakawa M, Ishizawa K, Yamazaki T, Nonomura H. Distribution of antibiotic producing Microbiospora strains in soils with different pH s. Actinomycetes. 1995;6(3):12–16. [Google Scholar]
- 10.Ouhdouch Y, Barakate M, Finance C. Actinomycetes of Moroccan habitats: isolation and screening for antifungal activities. Eur J Soil Biol. 2001;37(2):69–74. doi: 10.1016/S1164-5563(01)01069-X. [DOI] [Google Scholar]
- 11.Hsu SC, Lockwood JL. Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl Microbiol. 1975;29:422–426. doi: 10.1128/am.29.3.422-426.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirling JL, Gottlieb D. Methods for characterization of streptomycetes species. Int J Syst Bacteriol. 1966;16:313. doi: 10.1099/00207713-16-3-313. [DOI] [Google Scholar]
- 13.Sauden I, Gharailbh R. The Streptomycetes flora of Badia region of Jordan & its potential as a source of antibiotics active against antibiotic resistant bacteria. J Arid Environ. 2003;53:365–371. doi: 10.1006/jare.2002.1043. [DOI] [Google Scholar]
- 14.Labeda DP, Shearer MC (1990) Isolation of actinomycetes for biotechnological applications. In: Labeda DP (ed) Isolation of biotechnological organisms from nature. McGraw-Hill, New York, pp 1–19
- 15.Ilic SB, Konstantinovic SS, Todorovic ZB. UV/VIS analysis and antimicrobial activity of Streptomyces isolates. Med Biol. 2005;12(1):44–46. [Google Scholar]
- 16.Bevan P, Ryder H, Shaw I. Identifying small-molecule lead compounds: the screening approach to drug discovery. Trends Biotechnol. 1995;113:115–121. doi: 10.1016/S0167-7799(00)88916-7. [DOI] [PubMed] [Google Scholar]
- 17.Augustine SK, Bhavsar SP, Baserisalehi M, Kapadnis Isolation characterization and optimization of antifungal activity of actinomycetes of soil origin. Indian J Experiment Biol. 2004;42:928–932. [PubMed] [Google Scholar]
- 18.Dhanasekaran D, Rajakumar G, Sivamani P, Selvamani A, Pannerselvam N. Screening of saltpan actinomycetes for antibacterial agents. Internet J Microbiol. 2005;1(2):1–8. [Google Scholar]
- 19.Chakravarthi BVSK, Das P, Surendranath K, Karande AA, Jayabaskaran C. Production of paclitaxel by Fusarium solani isolated from Taxus celebica. J Biosci. 2008;33(2):1–9. doi: 10.1007/s12038-008-0043-6. [DOI] [PubMed] [Google Scholar]
- 20.Shadomy S (1987) Preclinical evaluation of antifungal agents. In: Fromtling RA (ed) Recent trends in the discovery development and evaluation of antifungal agents. Prous Science, New Jersy, pp 8–14
- 21.Lemonick MD. The killers all around world. Time. 1994;37:40–47. [Google Scholar]