Abstract
Thermostable alkaline α-amylase producing bacterium Bacilluscereus strain isolated from Cuddalore harbour waters grew maximally in both shake flask and fermentor, and produced α-amylase at 35°C, pH 7.5 and 1.0% of substrate concentrations. α-Amylase activity was maximum at 65°C, pH 8.0, 89% of its activity was sustained even at pH 11.0. Added with MnCl2, α-amylase activity showed 4% increase but it was inhibited by EDTA. The molecular weight of the purified α-amylase is 42 kDa.
Keywords: Cuddalore harbour waters, Bacillusα-amylase, Thermostable, Alkaline tolerant
Introduction
Starch degrading enzymes like amylases have received great deal of attention because of their perceived technological significance and economic benefits. Micro organisms that produce α-amylase have commercial application in industries such as food and beverages, textiles and detergents, drugs and pharmaceuticals, brewing and fine chemicals. Although raw starch granules degrading amylase from moulds and bacteria have been frequently reported, reports on thermostable amylase capable of degrading raw starch granules are limited [1, 2]. The starch producing industries require high temperature (60°C) resistant amylolytic enzymes. As thermostability is an important future of the enzymes sold for bulk industrial usage, the thermophilic organisms are of special interest as a source of novel thermostable enzymes [3]. α-Amylases (1,4-a-d-glucan-4 glucanohydrolase; EC 3.2.1.1) are a wide spread group of enzymes that catalyse the hydrolysis of the α-1,4 glucosidic linkages of raw and soluble starch and thereby generate smaller dextrins and oligosacchrides. They are classified into family 13 in the sequence-based classification of glucoside hydrolases (GH-13) [4]. This communication describes the optimal temperature pH and substrate concentration, at which B. cereus releases α-amylase maximally.
Materials and Methods
Bacterial Strain
The strain B. cereus [5] used in this study was isolated from Cuddalore Fishing Harbour waters, India (Lat. 11°42′N; Lon. 79°46′E) for biodegradation of crude oil. Interestingly, the strain showed promising results, while screening for amylase.
Optimization of Culture Conditions
Bacillus cereus was subjected to different culture conditions to derive the optimum conditions for amylase production. Growth and amylase production were estimated at selected temperatures (30, 35, 40, 45, and 50°C), substrate (starch) concentrations (0.5, 1.0, 1.5, 2.0, 2.5, and 3.0%) and pH (5.0, 6.0, 7.0, 8.0, and 9.0). All the experiments were carried out in 500 ml Erlenmeyer flask containing 100 ml of starch medium (0.5% peptone, 0.3% yeast extract, 1% soluble starch, 0.3% NaCl, 0.1% K2HPO4 and 0.02% MgSo4·7H2O). Sterile medium was inoculated with 2.0% of inoculum containing 2.15 × 106 cells ml−1. Inoculated flasks were maintained in water bath shaker at 150 rpm for 48 h.
Enzyme Production in Mini Fermentor
Amylase production was performed in 3 l laboratory fermentor (Scigenics, Chennai) with a working volume of 2.1 l. The culture conditions were as follows: pH 7.5, temperature 35°C, salinity (NaCl) 30 ‰ and 1.0% starch, 400 rpm of agitation and 6.0 mg l−1 of dissolved oxygen concentration. Culture medium was seeded with 2% inoculum (2.15 × 106 CFU ml−1) and maintained at above culture conditions for 48 h. Growth and enzyme activity determined from the aliquots (5 ml) collected at every 6 h. Growth was estimated turbidimetrically and the optical density of the culture broth was measured at 660 nm in spectrophotometer (Varian, Cary Eclipse, Spectrophotometer, Australia).
Extraction and Purification of Amylase Enzyme
Culture broth was centrifuged at 6,000 rpm for 30 min and enzyme in the culture broth was precipitated with 80% ammonium sulphate saturation. The precipitate was dialysed against 20 mM potassium phosphate buffer for 12 h at 4°C. Further purification was carried out in ion exchange chromatography (DEAE-Sephadex). The dialysed protein was applied to a DEAE-Sephadex A-50 column (20 mm diameter × 60 mm long), pre-equilibrated with 20 mM potassium phosphate buffer (pH 7.0). After washing the column with three volume of equilibration buffer, bound proteins were eluted stepwise using phosphate buffers of increasing molarity and decreasing pH values at room temperature (approx. 25°C). The flow rate was adjusted to 24 ml h−1 and fractions (1 ml each) were collected. The fractions showing high α-amylase activity were pooled, concentrated in lyophilizer. Protein content of the amylase was estimated during different levels of purification [6].
Estimation of Substrate Utilization
Substrate utilization was estimated by the method described by Astolfi-Filfo [7]. 5 ml of iodine solution (KI-5, I2-1.5 and distilled water g/l) was added with 0.5 ml of culture broth and mixed well. The volume of the above mixture was made up to 15 ml by adding distilled water and OD was measured at 550 nm. OD values were plotted on a standard graph prepared with different concentrations of starch and the amount of substrate utilization was calculated.
Enzyme Assay
Amylase enzyme activity was estimated by reducing sugar method [8] using 3,5-dinitrosalicylic acid (DNS). The assay mixture containing 250 μl of 50 mM Tris/HCl buffer (pH 7.5), 250 μl of 1% soluble starch (substrate), and 500 μl of appropriately diluted enzyme solution and the mixture was incubated at 50°C for 10 min. The reaction was stopped by adding 3 ml of DNS reagent and maintained in boiling water for 3 min and 1 ml of Rochelle salt solution was added finally. OD of the reaction mixture was measured at 540 nm. OD values were plotted in a standard graph prepared with different concentration of d-glucose. One unit of enzymatic activity was defined as the amount of enzyme required to produce 1 μM of glucose/min under the assay condition.
Thin-layer Chromatographic Analysis
The products liberated by the action of amylase on starch were identified by spotting the starch digest and standard sugars (glucose, maltose and maltotriose) on a silica gel plate activated at 110°C for 2 h. The plates were developed in butanol:ethanol:water solvent (5:3:2) and dried overnight at room temperature. The individual sugars were visualized by spraying with aniline-diphenylamine reagent [9].
Effect of Temperature and Metals on Enzyme Activity
The effect of temperature on enzyme activity was measured by incubating the enzyme at different temperatures for 10 min and the remaining activity was assayed at 50°C for 10 min in 50 mM Tris/HCl buffer (pH 7.5) [10].
Effect of metals on enzyme activity was measured with different metals namely CuCl2, CaCl2, HgCl2, FeCl2, MnCl2, NiCl2, MgCl2, PbCl2 and EDTA at 1 mM concentration. The activity of enzyme assayed without any metal was considered as control and the activity was taken as 100%.
Molecular Weight Determination in SDS-PAGE
Molecular weight of the amylase was determined by performing SDS-PAGE with 10% polyacrylamide gel following the method described by Laemmli [11]. The purified enzyme was loaded into the wells parallel to standard protein markers containing myosin (205 kDa), phosphorylase (97 kDa), bovine serum albumin (66 kDa), ovalbumin (43 kDa) and carbonic anhydrase (29 kDa). The protein bands were detected by coomassie brilliant blue staining and the molecular weight was calculated using Total Lab software (version 10).
Results
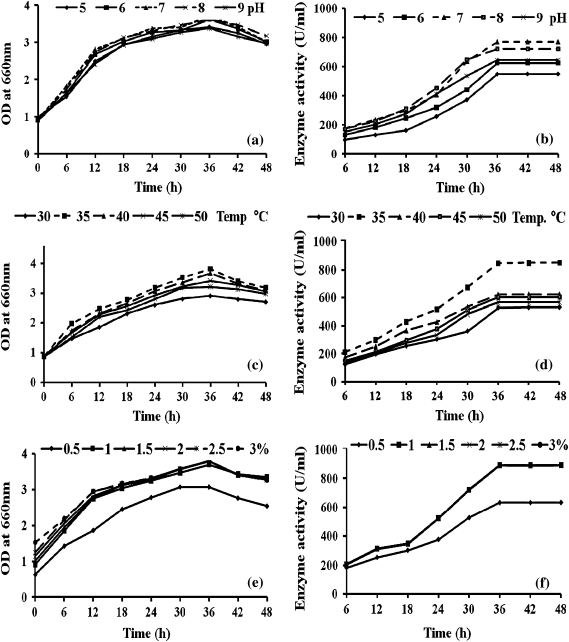
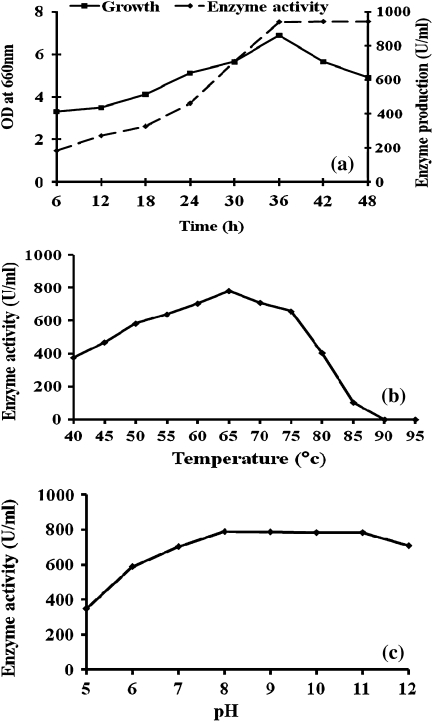
Maximum growth of B. cereus (Table 1) and α-amylase production occurred at 35°C, pH 7.0 and 1% of substrate concentration (Fig. 1). The amylase production was high during the stationary phase (42 h) of growth of the organism (Fig. 1b), whereas maximum bacterial growth occurred at 36 h (Fig. 1a). When the bacteria were cultured in fermentor, there was a slight increase in bacterial growth up to 36th hour, but the enzyme production continued to remain high up to 48th hour (Fig. 2a).
Table 1.
Physiological and biochemical characteristics of B. cereus
| Name of the test | Observation |
|---|---|
| Gram reaction | + |
| Shape of the cell | Rod |
| Spore formation | + |
| Motility | + |
| Utilization of carbohydrates | |
| Adonitol | − |
| Arabinose | − |
| Mannitol | + |
| Xylose | − |
| Hydrolysis | |
| Starch | + |
| Gelatin | + |
| Fat | + |
| Casein | + |
| Catalase | − |
| Nitrate reduction | + |
| Citrate utilization | + |
+, positive; −, negative
Fig. 1.
Growth (left panels) and enzyme production (right panels) by B. cereus a selected pH (a, b), temperature (c, d) and substrate (starch) concentration (e, f)
Fig. 2.
a Growth and enzyme production by B. cereus in fermentor, b effect of temperature on enzyme activity, and c effect of pH on enzyme activity
Substrate utilization revealed that during the period 0–6 h incubation, 0.2% of the substrate was utilized but the maximum substrate utilization (0.3%) occurred at 36 h followed by complete utilization of substrate at 48 h. Using thin-layer chromatographic analysis, it was ascertained that the amylase produced by the bacterial culture was α-amylase owing to the presence of maltose and maltotrioses with very low amounts of glucose and other oligosaccharides as the main end-products of starch hydrolysis.
When the enzyme was treated with MnCl2 enzyme activity increased to 977 U/ml which is 4% higher than control (940 U/ml). However, treatment with HgCl2 or EDTA there was less (19 U/ml) or no enzyme activity (Table 2). The activity was at its maximum of 780.2 U/ml at 65°C (Fig. 2b). Subsequently the enzyme activity progressively decreased up to 85°C, and no enzyme activity was found beyond 90°C. Amylase activity was maximal (791 U/ml) at pH 8.0, and maintained at level of about 720 U/ml even at pH 11 (Fig. 2c).
Table 2.
Effect metals on enzyme activity of B. cereus
| Metals | Enzyme activity | |
|---|---|---|
| (U/ml) | (%) | |
| Controla | 940 | 100 |
| CuCl2 | 498 | 53 |
| CaCl2 | 846 | 90 |
| HgCl2 | 19 | 2 |
| FeCl2 | 893 | 95 |
| MnCl2 | 977 | 104 |
| NiCl2 | 808 | 86 |
| MgCl2 | 836 | 89 |
| EDTA | 0 | 0 |
| PbCl2 | 225 | 24 |
aWithout addition of metals
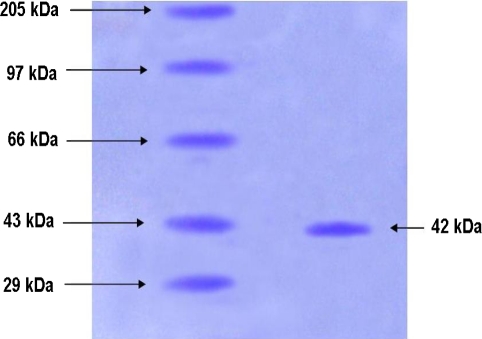
With the purified enzyme a high specific activity of 43.9 U/mg was found (Table 3). Molecular weight determination of the purified amylase on SDS-PAGE showed a single band with molecular weight of 42 kDa, indicating the purity of the amylase (Fig. 3).
Table 3.
Summary of the purification steps of α-amylase from culture supernatant of B. cereus
| Purification step | Total activity (U) | Total protein (mg) | Specific activity (U/mg) | Purification (fold) |
|---|---|---|---|---|
| Culture supernatant | 940 | 350 | 2.68 | 1.0 |
| (NH4)2SO4 precipitation (80% saturation) and dialysis | 465 | 161 | 2.88 | 1.0 |
| DEAE-cellulase chromatography | 180 | 8.1 | 43.9 | 16.3 |
Purification was commenced with 1.5 l of culture supernatant collected after cells had been cultivated for 48 h in a fermentor
Fig. 3.
SDS-PAGE analysis of purified B. cereus α-amylase. Lane 1 molecular mass markers: myosin (205 kDa), phosphorylase (97 kDa), bovine serum albumin (66 kDa), ovalbumin (43 kDa) and carbonic anhydrase (29 kDa) and lane 2 amylase purified from B. cereus
Discussion
Many micro-organisms especially several species belonging to Bacillus are known to produce variety of extracellular enzymes and they have a wide range of industrial applications. Of these enzymes, amylases are of particular significance to industry. The present study has reported the optimization of culture conditions for amylase production by B. cereus, which shows optimum growth and enzyme production at pH 7.5, 35°C and 1.0% of substrate concentrations. The ability of B. cereus to grow maximally and to secrete a significant amount of α-amylase at pH 7.5 and 35°C clearly indicates that the optimum temperature and pH required for the growth and production of the enzyme from this strain are the same. Amylase production by this strain was growth independent as maximum enzyme production was achieved during the stationary phase (42 h) of growth of the organism. Further, soluble starch serves as the best carbon source for maximum growth and enzyme production, a phenomenon that has been described for a number of α-amylase-secreting Bacillus strains [10, 12, 13]. Enzyme activity found in the present study (940 U/ml) is higher than the results obtained by Najafi et al. [14], who have recorded 590 U/ml at 36 h from marine Vibrio sp. maximum enzyme activity found at 36 h.
That the α-amylase activity of our strain of B. cereus is sustained even at such high temperature as 65°C and pH 8.0 and retained up to pH 11.0 is new and has immediate relevance to industrial application. Earlier studies have shown that amylases are active up to the pH of 5–10 and temperature 30–60 and are stable between 50 and 60°C (Table 4). It is comparatively less and the enzyme of our B. cereus strain has still wider ranges of tolerance than the previously reported for amylases [3, 10, 15, 18]. Many of the amylases reported were thermostable but not alkaline active whereas some of them were alkaline active but not thermostable. But the amylase from present study was thermostable (65°C) and also alkaline active (pH 11.0) which is significant in industries dealing with amylases.
Table 4.
Biochemical properties of some of the previously reported amylases
| Strains | References | Optimum temperature (°C) | Optimum pH | Stability (°C) | Molecular weight (kDa) |
|---|---|---|---|---|---|
| Bacillus flavothermus | Balton et al. [3] | 60 | 5.5–6 | 60 | ND |
| Vibrio sp. | Najafi et al. [14] | 60 | 6.5 | 50 | 52.4 |
| Bacillus subtilis DM-03 | Das et al. [18] | 55 | 9.0 | 60 | 42.8 |
| Thermus sp. | Shaw et al. [19] | 50 | 5.5–6.5 | 60 | 59 |
| Bacillus sp. | Carbajal and Soto [20] | 50 | 6–7 | 50 | ND |
| B. cereusa | Present study | 65 | 8–11 | 65 | 42 |
ND not determined
Lin et al., Vihinen et al. and Farez-vidal [10, 16, 17] reported that metals like Hg2+, Pb2+and Cu2+ strongly inhibit the amylases of microbial origin and these findings support our results, in which EDTA, Hg2+, and Pb2+ strongly inhibited the amylase activity. Inhibitory activity found with Hg2+, Pb2+, and Cu2+ ions on amylase activity may be due to competition between the exogenous cations and the protein-associated cations, resulting in decreased metalloenzyme activity [10]. Mn2+ and Ca2+ ions catalyzed the enzyme activity as well as stability. These metals may act as co-factor which is required to increase the enzyme activity.
Molecular weight of the amylase enzyme found in the present study as 42 kDa was analogous to the results obtained by B. subtilis [18]. Many investigators reported different molecular mass of α-amylase isolated from Bacillus sp. Lin et al. [10] reported an amylase with 42.8 kDa molecular mass from Bacillus sp. and opined that Bacillus sp. can produce five forms of amylase, but in present study only one protein band was detected.
The extracellular α-amylase of our strain B. cereus resembles all other known α-amylases but has unique properties for immediate industrial application. For large scale production of the enzyme with alternate carbon sources and its cost effectiveness are in progress.
References
- 1.Hyun HH, Zeikus JG. General biochemical characterization of thermostable extracellular ß-amylase from Clostridium thermosulfurogens. Appl Environ Microbiol. 1985;49:1162–1167. doi: 10.1128/aem.49.5.1162-1167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitamato N, Yamagata H, Kato T, Tsukagoshi N, Udka S. Cloning and sequencing of the gene encoding thermophilic β-amylase of Clostridium thermosulfurogens. J Bacteriol. 1988;170:5848–5854. doi: 10.1128/jb.170.12.5848-5854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balton DJ, Kelly CT, Fogarty WM. Purification and characterization of the α-amylase of Bacillus flavothermus. Enzyme Microb Technol. 1997;20:340–343. doi: 10.1016/S0141-0229(96)00147-0. [DOI] [Google Scholar]
- 4.Henrissat B, Bairoch A. Updating the sequence-based classification of glycosyl hydrolases. Biochem J. 1996;316:695–696. doi: 10.1042/bj3160695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thavasi R, Jayalakshmi S. Bioremediation potential of hydrocarbonoclastic bacteria in Cuddalore harbour waters (India) Res J Chem Environ. 2003;7:17–22. [Google Scholar]
- 6.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 7.Astolfi-Filfo S, Galembeck EV, Faria JB, Franscino ACS. Stable yeast transformants that secrete functional α-amylase encoded mouse pancreatic DNA. Biotechnology. 1986;4:311–315. doi: 10.1038/nbt0486-311. [DOI] [Google Scholar]
- 8.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–429. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 9.Hansen SA. The thin-layer chromatographic method for the identification of mono-, di-, and trisaccharides. J Chromatogr. 1975;107:224–226. doi: 10.1016/S0021-9673(00)82770-3. [DOI] [Google Scholar]
- 10.Lin LL, Chyau CC, Hsu WH. Production and properties of a raw-starch-degrading amylase from the thermophilic and alkaliphilic Bacillus sp. TS-23. Biotechnol Appl Biochem. 1998;28:61–68. [PubMed] [Google Scholar]
- 11.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Takase K, Mizuno H, Yamane K. NH2-terminal processing of Bacillus subtilis alpha-amylase. J Biol Chem. 1988;263:11548–11553. [PubMed] [Google Scholar]
- 13.Igrashi K, Hatada Y, Hagihara H, Saeki K, Takaiwa M, Uemura T, Ara K, Ozaki K, Kawai S, Kobayashi T, Ito S. Enzymatic properties of a novel liquefying α-amylase from an alkaliphilic Bacillus isolate and entire nucleotide and amino acid sequences. Appl Environ Microbiol. 1998;64:3282–3289. doi: 10.1128/aem.64.9.3282-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Najafi MF, Kembavi A. One step purification and characterization of an extra cellular α-amylase from marine Vibrio sp. Enzyme Microb Technol. 2004;36:535–539. doi: 10.1016/j.enzmictec.2004.11.014. [DOI] [Google Scholar]
- 15.Stamford TLM, Stamford NP, Coelho JM, Araujo JM. Production and characterization of a thermostable α-amylase from Nocardiopsis sp. endophyte of yam bean. Bioresour Technol. 2001;76:137–141. doi: 10.1016/S0960-8524(00)00089-4. [DOI] [PubMed] [Google Scholar]
- 16.Vihinen M, Ollika P, Niskenen J, Meyer P, Suominen I, Karp M, Holm L, Knowles J, Mantsala P. Site directed mutagenesis of a thermostable α-amylase from Bacillus stearothermophilus: putative role of three conserved residues. J Biochem. 1990;107:267–291. doi: 10.1093/oxfordjournals.jbchem.a123037. [DOI] [PubMed] [Google Scholar]
- 17.Farez-Vidal ME, Fernandez-Vivas A, Gonzalez F, Arias JM. Properties and significance of an alpha amylase produced by Myxococcus coralloides D. J Appl Bacteriol. 1995;78:14–19. doi: 10.1111/j.1365-2672.1995.tb01667.x. [DOI] [Google Scholar]
- 18.Das K, Doley R, Mukherjee AK. Purification and biochemical characterization of a thermostable, alkaliphilic, extracellular α-amylase from Bacillus subtilis DM-03, a strain isolated from the traditional fermented food of India. Biotechnol Appl Biochem. 2004;40:291–298. doi: 10.1042/BA20040034. [DOI] [PubMed] [Google Scholar]
- 19.Shaw JE, Lin FP, Chen SC, Chen CC. Purification and properties of an extracellular α-amylase from Thermus sp. Bot Bull Acad Sin. 1995;36:195–200. [Google Scholar]
- 20.Carbajal AF, Soto JO. Thermostable α-1,4- and α-1,6-glucosidase enzymes from Bacillus sp. isolated from a marine environment. World J Microbiol Biotechnol. 2002;18:791–795. doi: 10.1023/A:1020433210432. [DOI] [Google Scholar]