Abstract
Thermophilic bacteria are actively prevalent in hot water springs. Their potential to grow and sustain at higher temperatures makes them exceptional compare to other microorganism. The present study was initiated to isolate, identify and determine the feasibility of extraction of copper using thermophilic heterotrophic bacterial strain. Bacillus stearothermophilus is a thermophilic heterotrophic bacterium isolated from hot water spring, Atri, Orissa, India. This bacterium was adapted to low-grade chalcopyrite ore and its efficiency to solubilize copper from Malanjkhand low-grade ore was determined. The low-grade copper ore contains 0.27% Cu, in which the major copper-bearing mineral is chalcopyrite associated with other minerals present as minor phase. Variation in parameters such as pulp-density and temperatures were studied. After 30 days of incubation, it was found that Bacillus stearothermophilus solubilize copper up to 81.25% at pH 6.8 at 60°C.
Keywords: Malanjkhand low-grade ore, Bioleaching, Thermophilic bacteria, Copper
Introduction
Thermophilic microorganisms prefer living at temperatures not commonly found in nature, but in hot springs and water heaters [1]. They have the potential to grow and sustain above 70–80°C or even more. Their preference for hot temperatures has given rise to some speculation about their evolution. This ability makes them exceptional compare to other mesophilic microorganism, which normally grow around 25–37°C. Thermophilic microorganisms have the capability to produces heat stable extra cellular enzymes and proteins might be consequence of various changes, which contribute to the whole stability of the protein in additive manner [2]. Thermal stability and massive enzyme production are two key factors that make them a suitable candidate for the industrial applications [3].
Recovery of metal values through bioleaching process is very common technique and gaining importance in the field of waste treatment. Both chemolithotrophic and heterotrophic microorganisms play an active role in the solubilization of metal ions [4–8]. Heterotrophic bacteria and fungi are known to produce organic acids which can dissolve metals by forming salts or complexes like chelates [6, 9]. On other hand, limitations due to low extractions and low kinetics with mesophiles have limited its implementation on commercial scale. Therefore search for those microorganisms that can sustain at high temperatures and efficiently solubilize metal from low-grade ores is important. Thermopiles turned out to be an extraordinarily useful group of bugs because of their enzymes works in high temperatures, where chemical reactions occur more quickly.
The most important copper-bearing sulphidic mineral is chalcopyrite (CuFeS2). Unlike many other ores it is known to be recalcitrant to hydrometallurgical processing [10]. Due to its abundance in nature, it is encouraging to find some microorganisms that could do it efficiently. As large quantities of the metals are embodied in these low-grade ores and mining residues, this can be efficiently recovered by applying thermophilic microorganisms. Therefore, an attempt was made to isolate, identify and determine the feasibility of extraction of copper using thermophilic heterotrophic bacterium Bacillus stearothermophilus.
Materials and Methods
Sampling Site and Sample Collection
Atri in Orissa is well known for its hot sulphur springs located at a convenient distance of 42 km from Bhubaneswar, the capital of Orissa. The hot sulphur springs maintain a temperature between 58 and 60°C throughout the day. The peculiarity of the site is that there is constant release of bubbles from the core of the well with vigorous exothermic reaction. The temperature of the water measured at the site was found to be 60°C. The water gives an odor of sulphur. Water samples were collected with the help of sampler bottle from a depth of 7 m for isolation of the microorganisms prevalent in the hot spring. Water sample collected was taken for detailed study such as measurement of pH, Eh, ambient temperatures etc., were noted and given in Table 1.
Table 1.
Physico-chemical characteristics of Atri Water Sample
| Sampling site | Sample | pH | Eh (mV) | Temperature (°C) |
|---|---|---|---|---|
| Atri, Hot sulphur spring | Water | 6.8 | 108 | 60 |
Mineral Source
The low-grade copper ore procured from Malanjkhand, Madhya Pradesh, India was used as leaching material. The ore was crushed, grinded and sieved to obtain a mean particle size of 75. Also representative samples were subjected to acid digestion and analyzed by Atomic Absorption Spectrophotometer (AAS). The mineralogical studies indicated that the granite constitutes of both feldspar (aluminum silicate mineral) and quartz (silica mineral) as major phases. The major copper-bearing minerals present are chalcopyrite and covellite. The former mineral occurs as minute disseminated grains independently or as inclusion within pyrite or as secondary vein in the contact of pyrite and quartz. The covellite appears as veins/vein let’s traversing magnetite. The other associated ore minerals are iron-bearing phases like magnetite (Fe3O4) and pyrite (FeS2). The mineralogical and chemical composition of the sample is summarized in Tables 2 and 3.
Table 2.
Chemical analysis of Malanjkhand low-grade copper ore (values are in percentage)
| Name of the material | Cu | Ni | Co | Zn | Fe | S | TiO2 | AI |
|---|---|---|---|---|---|---|---|---|
| Malanjkhand low-grade copper ore | 0.27 | 0.23 | 0.05 | 0.05 | 3.9 | 2.83 | 0.6 | 91.2 |
Cu copper, Ni nickel, Co cobalt, Zn zinc, Fe iron, S sulphur, TiO2 titanium oxide, AI acid insoluble
Table 3.
Mineral phase analysis from XRD and TEM study
| Sample | XRD | TEM |
|---|---|---|
| Raw ore | Chalcopyrite Covellite Magnetite Pyrite Feldspar Quartz |
Chalcopyrite Covellite Feldspar Quartz CuS |
| Leached residue | Covellite Copper |
KAlsilicate Covellite Copper |
Micro-organism and Growth Media
Individual colonies were obtained by streaking and re-streaking it on nutrient agar plate and incubated at 60°C for 24 h. Purity of the colonies was checked microscopically. The culture was deposited to the Microbial Type Culture Center (MTCC), IMTECH, Chandigarh, India for getting an accession number and further identification. There several modified medium used for leaching of copper from ores by different chemoorganotrophic microorganism [11]. For bioleaching experiments, the bacterial culture was grown on nutrient agar plates at 60°C for 18 h. Bacterial cells were harvested by centrifugation at 4000 rpm (15 min), subsequently washed twice with saline solution (0.9% NaCl) and added in a concentration of 109 cells per ml to mineral growth yeast extract (MGY) liquid medium consisting of g/l: NH4(NO3)-0.1, KH2PO4-0.5, MgSO4·7H2O-0.5, Glucose-20, Yeast Extract-0.02 and pH was adjusted to 6.8 (Table 4).
Table 4.
Composition of mineral growth yeast extract medium
| Component | Amount (g/l) |
|---|---|
| (NH4)NO3 | 0.1 |
| KH2PO4 | 0.5 |
| MgSO4·7H2O | 0.5 |
| Glucose | 20.0 |
| Yeast extract | 0.02 |
| pH | 6.8 |
Growth Substrate
Prior to leaching experiments, the thermopile was initially adapted to low-grade copper ore samples as such at 10% (w/v) pulp-density in the required medium to improve the tolerance of the strain and hence increased in leaching efficiency.
Bioleaching Assay
Leaching experiments were run in 250 ml of Erlenmeyer flasks containing 90 ml of liquid media inoculated with 10 ml bacterial suspension. The culture added to the flasks was in active logarithmic phase of growth under aseptic condition. The flasks were continuously agitated on a rotary shaker at 150 rpm. Bioleaching assay were also performed with different pulp-density of 10–30% (w/v) and at different temperatures ranging from 40 to 65°C. For prolonged leaching the medium was changed under aseptic condition at 7 days intervals. All the experiments were carried out in duplicates to minimize the experimental errors. After 31 days of incubation the experiments were terminated, as there was no significant recovery of copper, nickel, cobalt, zinc and manganese. Control experiments were conducted for all sets and 0.1 N of 10 ml of mercuric chloride was added to control flask to prevent the growth of bacteria. Samples were taken at regular intervals from the flasks for analysis of copper, nickel, cobalt, zinc and manganese in 50 ml volumetric flasks by using ASS.
Parameters Variations for Bioleaching Experiment
Pulp-Density Study
The experiments were carried out at different pulp-densities such as 10, 15, 20 and 30% (w/v) inoculated with the cell culture to a concentration ~109 cells/ml. The pH was maintained at 6.8. Before inoculation, flasks containing the liquid broth along with the ore were sterilized in an autoclave for 20 min at 15 lb pressure to avoid any contamination. The flasks were then incubated at 60°C, at 150 rpm for a period of 30 days. For better leaching efficiency, the medium was changed under aseptic condition at 7 days intervals. Samples were taken at regular intervals for analysis of copper, nickel, cobalt, manganese and zinc by AAS.
Temperature Study
In this study, the leaching experiments were conducted at different range of temperatures such as 40, 50, 60 and 65°C at 10% (w/v) pulp-density. The pH was maintained at 6.8 for a period of 30 days in a rotary shaker (Kunner shaker) running at 150 rpm. The medium was changed under above mention conditions to increase the efficiency of leaching. Samples were taken for analysis of copper nickel, cobalt, manganese and zinc by AAS. Loss by evaporation was readjusted by adding sterile distilled water.
Results and Discussion
Physio-Chemical Characteristics of Atri Water Sample
The water collected from hot sulphur spring was taken for detailed study such as measurement of pH, Eh, ambient temperatures etc. It was found that the pH of the water sample was 6.8, Eh–108 and ambient temperature was 60°C.
Bacterial Identification and Characteristics
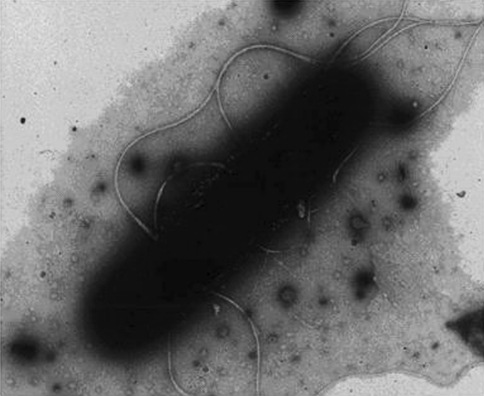
After repeated sub culturing on nutrient agar at 60°C, the isolate is identified as Bacillus stearothermophilus (MTCC No.8052). It is a gram positive, aerobic, rod shaped, endospore forming, motile bacteria having peritrichous flagella, actively prevalent in hot springs as shown in Fig. 1.
Fig. 1.
Photograph of Bacillus Stearothermophilus
Regular examination of bacterial morphology shows that the size of the bacterial cells decreases and endospore formation occurs within 7–8 days of incubation. As a result the dissolution of copper decreases from the mineral sulphide and after a period of one weak bacterial leaching stops. Therefore the culture medium was regularly changed during 30 days of bioleaching at 7-day intervals. The aseptic conditions were maintained as describe earlier. Adaptation of the thermophilic strain to higher temperature i.e. at 60°C, pH-6.8 was obtained through repeated sub culturing on Malanjkhand low-grade copper ore.
Effect of Pulp-Density
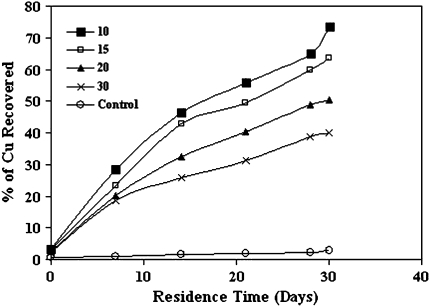
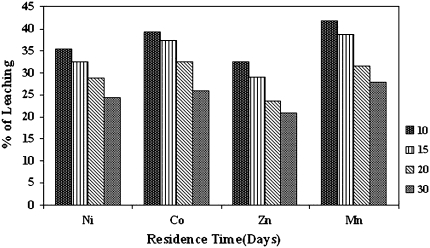
Figure 2, shows the progress of extraction of copper from Malanjkhand low-grade ore decrease with increase in pulp-density. It was found that Bacillus stearothermophilus solubilize copper up to 73.34% at 10% pulp-density, pH 6.8 at 60°C within 30 days of incubation where as the dissolution of copper in other pulp-densities were 63.67, 50.25 and 39.88% at 15, 20 and 30% (w/v) respectively. Comparing these data with control set shows a maximum of 2.78% of Cu was recovered along with the residence time. Figure 3 shows the highest percentage of extraction of other metals (Ni, Co, Mn, and Zn) from Malanjkhand low-grade copper ore with 10% (w/v) pulp-density were 35.4, 39.2, 32.52 and 41.84% respectively after 30 days of incubation.
Fig. 2.
Effect of pulp-density on extraction of copper from Malanjkhand low-grade copper ore. (Conditions pH 6.8, temp 60°C, speed 150 rpm, residence time 30 days)
Fig. 3.
Effect of pulp-density on extraction of Ni, Co, Mn and Zn from Malanjkhand low-grade copper ore. (Conditions pH 6.8, temp 60°C, speed 150 rpm, residence time 30 days)
Effect of Temperature
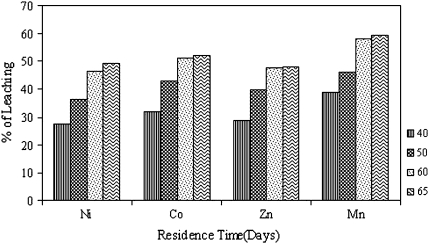
The increase of the temperature leads to an increase of copper extraction rates from low-grade copper ore as shown in Fig. 4. A maximum of 81.25% Cu was recovered at 60°C as compared to other three temperatures i.e. 56.92% at 40°C, 60.37% at 50°C and 78.92% at 60°C respectively. Copper dissolution was higher at 60°C than at 50 or 40°C. There is not much difference in extraction rates of copper between 60 and 65°C. It may be due to adaptation of bacterial strain, towards temperature, medium, pH and to the ore itself. Comparing these data with control set shows a maximum of 6.12% of Cu recovered along with the residence time. Figure 5 illustrate the progress of extraction of other metals from low-grade ore. It was found that a maximum of 46.59% of Ni, 51.2% of Co, 47.68% of Mn and 58.1% of Zn were recovered at 60°C after 30 days. It can be seen that there was a significant difference in the percentage of Cu recovery with increase in the reaction temperature. This can be explained from the fact that, increase in the temperature of the reaction medium leads to an increase in the activation energy of the reacting molecules. Due to an increase in the activation energy the reaction kinetics also increases [12].
Fig. 4.
Effect of temperature on extraction of copper from Malanjkhand low-grade copper ore. (Conditions pH 6.8, pulp-density 10% (w/v), speed 150 rpm, residence time 30 days)
Fig. 5.
Effect of temperature on extraction of Ni, Co, Mn and Zn from Malanjkh and low-grade copper ore. (Conditions pH 6.8, temp 60°C, speed 150 rpm, residence time 30 days)
For more than a decade, thiobacillus sps had been considered for metals recovery and detoxification of industrial wastes especially in bioleaching of sulphides. Several methods were developed for extraction of valuable metals from oxide, sulphides and silicate minerals and ore using heterotrophic microorganisms. It is known that bacilli solubilize ores/concentrates by reduction, which thereby promotes selective leaching [13]. The process of biological removal of elements from ore was result of microbial surface attachment and organic acid production by Bacillus sps however the bacterial strain also synthesizes polysaccharides during bioleaching [14]. Bacilli have the ability to form endospore that is resistance to survive under unfavorable conditions and subsequently suitable to transform their spores to vegetative cells and continue their life cycle. Hence applications of theses native microbial species under controlled conditions can enhance their effectiveness in these areas.
Conclusion
The chemical analysis of Malanjkhand low-grade ore shows 0.27% of copper. Mineralogical studies by X-Ray Diffractometer of Malanjkhand low-grade ore reveals that the copper-bearing minerals present are chalcopyrite and covellite as major phase along with other associated iron-bearing phases like magnetite (Fe3O4) and pyrite (FeS2). It was observed that the potentiality of Bacillus stearothermophilus to leach copper was found to be a maximum of 81.25% at 10% pulp-density, pH-6.8 at 60°C in compare to the control set i.e. in absence of the bacteria which shows a maximum of 6.12% of Cu recovered along with the residence time.
Acknowledgments
The author is thankful to Institute of Minerals and Materials Technology, Council of Scientific and Industrial Research, Bhubaneswar for funding and their cooperation to carry out the work. One of the author wishes to express her gratitude to CSIR for awarding SRF fellowship.
References
- 1.Ziad E, Anis M, Wajih O, Ahmad K. Isolation and characterization of new thermophilic bacteria in Jordan. Internet J Microbiol. 2007;3:2. [Google Scholar]
- 2.Vieille C. Thermozymes. Biotechnol Annu Rev. 1996;2:1–83. doi: 10.1016/S1387-2656(08)70006-1. [DOI] [PubMed] [Google Scholar]
- 3.Hisotsuyanagi K. Stepwise introduction of regulatory genes stimulating production of α -amylase into Bacillus subtilis: construction of α-amylase extra hyper producing strain. Agric Biol Chem. 1979;43:2343–2349. doi: 10.1271/bbb1961.43.2343. [DOI] [Google Scholar]
- 4.Groudev SN, Genchev FN, Guidarjiev SS. Observations on the microflora in an industrial copper dump leaching operation. In: Murr LE, Torma AE, Brierley JA, editors. Metallurgical applications of bacterial leaching and related microbiological phenomenon. New York: Academic Press; 1978. pp. 253–273. [Google Scholar]
- 5.Trudinger PA, Walter MR, Ralph BJ. Biochemistry of ancient and modern environments. Canberra: Australian Academy of Sciences; 1980. [Google Scholar]
- 6.Ehrlich HL. Geomicrobiology. New York: Marcel Dekker; 1981. [Google Scholar]
- 7.Torma AE (1981) Impact of biotechnology on metal extractions. In: Mineral processing and extractive metallurgy review, Gordon and Breach, London
- 8.Krumbein WE. Microbiol Geochemistry. Oxford: Blackwell Scientific Publications; 1983. [Google Scholar]
- 9.Berry DR, Henderson MEK, Taylor IF. The microbiology of rocks and weathered stones. J Soil Sci. 1963;14:102–112. doi: 10.1111/j.1365-2389.1963.tb00935.x. [DOI] [Google Scholar]
- 10.Pradhan N, Nathasharma K, Rao KS, Sukla LB, Mishra BK. Heap bioleaching of chalcopyrite: a review. Miner Eng. 2007;21(5):355–365. [Google Scholar]
- 11.Zastrow P, Straube G. Leaching of copper ore by chemoorganotrophic microorganism. Appl Microbiol Biotechnol. 1991;35:696–698. doi: 10.1007/BF00169640. [DOI] [Google Scholar]
- 12.Laidler KJ. Chemical kinetics. 3. San Francisco: Benjammin-Cummings; 1997. pp. 40–47. [Google Scholar]
- 13.Ehrlich HL. Bacterial leaching of manganese ores. In: Trudinger PA, Walter MR, Ralph BJ, editors. Biogeochemistry of ancient and modern environments. Canberra: Australian Acad. of Sci; 1980. pp. 609–614. [Google Scholar]
- 14.Styriakova I, Styriak I (1999) The release of sulphidic minerals from aluminosilicates by Bacillus strains. In: Proceedings of Biohydrometallurgy and environment towards the mining of the 21st century, Process Metallurgy 9A, Madrid, pp 587–596