Abstract
Azadirachta indica A. Juss. (neem), native to India, is well known worldwide for its insecticidal and ethanopharmacological properties. Although endophytic microbes are known from this plant as only leaves and stems were the subjects of past reports. Now, a variety of procedures and a number of different media were used to isolate the maximum number of endophytic fungi from unripe fruits and roots. A total of 272 isolates of 29 filamentous fungal taxa were isolated at rate of 68.0% from 400 samples of three different individual trees (at locations-Az1, Az2, Az3). Mycological agar (MCA) medium yielded the highest number of isolates (95, with a 14.50% isolation rate) with the greatest species richness. Mycelia Sterilia (1, 2, 3) accounted for 11.06%, Coelomycetes 7.25%, while Hyphomycetes showed the maximum number of representative isolates (81.69%). Mycelia-Sterilia (1, 2, 3), based on their 5.8S ITS 1, ITS2 and partial 18S and 28S rDNA sequences were identified as Fusarium solani (99%), Chaetomium globosum (93%) and Chaetomium globosum (93%) respectively. Humicola, Drechslera, Colletotrichum, and Scytalidium sp. were some of the peculiar fungal endophytes recovered from this plant.
Keywords: Anti-microbial activity, Biodiversity, Fungal endophytes, Isolation media, Azadirachta indica
Introduction
Azadirachta indica A. Juss (Meliaceae) ‘The Indian Lilac’ having its origin from south East Asia now has worldwide presence. It is native to India and significantly contributes to the forest cover of the northern areas. All parts of this plant show an array of negative effects on insects including ovipositor deterrent, anti-feedant, and other inhibitory activities [1, 2]. More than 100 compounds have been isolated from various parts of the neem tree [3–5] and most of the active principles (Limnoids) belong to the group of tetranortriterpinoids especially ‘Azadirachtin’ and its analogs [6]. The people of India have known the useful properties of neem since time immemorial, and only recently have other people in more developed countries realized the value and importance of this tree to human activity [7]. For instance, various researchers have studied the medicinal properties of Azadirachta indica including its anti-pyretic effects [8, 9], anti-malarial effects [10, 11], anti-tumor effects [12], anti-ulcer effects [13], anti-diabetic effects [14], anti-fertility effects [15], CNS effects [16], and cardiovascular effects [17]. Preliminary investigations of leaves and bark of neem [18–20] cited the fact that living tissues of neem can successfully harbor endophytic fungi.
Several reports in the recent years show that the endophytic fungi from this host produce several bioactive compounds [21–24]. An endophytic fungus, Phomopsis sp., isolated from the stems of the neem plant produces some 10-membered lactones, these lactones have very promising activity against plant pathogens Ophiostoma minus and Botrytis cinerea with MIC values 31.25 and 62.50 µg/ml respectively [21]. Again, an endophytic Geotrichum sp., isolated from the leaves of the neem tree, has been reported to produce two new chlorinated epimeric 1,3-oxazinane derivatives, that have significant activity against the nematodes Bursaphelenchus xylophilus and Panagrellus redivevus [22]. ‘Javanicin’ an antibacterial nephthaquinone was isolated and characterized from the endophytic Chloridium sp. obtained from root tissues of the Azadirachta indica A Juss., this highly functionalized nephthaquinone exhibits strong antibacterial activity against Pseudomonas spp., representing pathogens to both humans and plants [23]. Two new solanapyrone analogues were isolated from the fermentation culture of Nigrospora sp. YB-141, an endophytic fungus isolated from Azadirachta indica A. Juss. The structures of the new compounds were elucidated on the basis of spectroscopic analysis. Most of the compounds exhibited no or only weak antifungal activities [24]. Thus with these examples it was established that endophytes from neem plant have potential bioactive compounds that need to be characterized.
Thus in anticipation of finding new bioactive compounds we have taken initiative to isolate endophytes from root and fruits of this vital plant. Endophytic fungi have been reported from leaves, and stems earlier [20], but in this report the isolates have been reported from roots and fruits of neem.
Materials and Methods
Collection Sites and Plant Materials
Varanasi [25.5° N 82.9° E, elevation 279 ft/85 m] is located into the foothills of Himalayan region of northern India and has an annual mean temperature of 31°C (maximum 38°C, minimum 28°C) with about 110 cm precipitation per annum. Woody perennials dominate the tropical deciduous forest cover of the region. Three neem plants (Az1, Az2, Az3) being 10 years old were selected for the study from this region. The first tree designated-Az1 is located on the campus of Banaras Hindu University, Varanasi India. The second tree is Az2 and located in the forest cover of Chandraprabha Sanctuary range of Varanasi India, while tree 3-Az3 is located in the Excavation belt of Sarnath Varanasi India. Roots tissues were recovered by digging the soil adjacent the main trunk down to 1.5 ft and root samples, approximately 0.5–1.5 cm diameter and about 3–5 cm length were collected. The unripe fruits were collected directly from trees. All samples were then brought to the laboratory in an icebox, and used to screen endophytic fungi within 48 h of collection.
Surface Treatment of Plant Samples
To eliminate the epiphytic fungal mycelia, the effectiveness of various surface decontamination methods was tested in preliminary experiments. The small segments of roots (3–5 cm length) and fruits (10 unripe pieces from each location) were subjected to surface treatments. All samples were thoroughly washed into running tap water for about 5–8 min respectively before surface treatments. Two sterilization regimes were adopted, depending upon varying the disinfectant and length of surface treatment. In Regime 1 Sodium Hypochlorite (5.0% w/v), and in Regime 2 H2O2 (35% v/v) were used as surfactants, for 15 min sterilization length. The samples (50% in five equal parts) were treated by soaking into 90% ethanol for 1 min, then placed in sodium hypochlorite (5.0% w/v) for 1, 3, 5, 10, and 15 min respectively, followed by rinsing in 90% ethanol for 10 s. While the remainder of the samples were treated by soaking into concentrated H2O2 (35% v/v), for 1, 3, 5, 10, and 15 min followed by the same ethanol rinsing treatment. The samples were then placed on to PDA, and the plates were placed in an incubator at 25 ± 2°C for at least 25 days. Tissue (%) from which endophytic mycelia emerged was recorded each for two treatment conditions at different lengths of exposure to treatment.
Culture Method and Media
Four culture media used for isolation and identification of endophytic fungi, were prepared in 1 l each of distilled water. Malt yeast extract agar (MYA): 10 g malt extract, 2 g yeast extract, 50 mg streptomycin sulphate, 50 mg chlortetracycline, and 20 g agar. Mycological agar (MCA): 10 g papain-digest of soybean meal, 10 g dextrose, 15 g agar, 0.4 g cyclohexamide, and 50 mg chloramphenicol. Difco Potato dextrose agar (PDA): 200 g potato infusion, dextrose 20 g, and agar 15 g with 100 ppm each of streptomycin sulphate and penicillin. Nutrient agar (NA): 5 g Peptone, 3 g Beef extract, 5 g NaCl and 15 g Agar. This medium was specifically chosen for the isolation of actinomycetes and allied genera.
A disc of about 2–3 mm in diameter was cut from the middle of each root sample, for inoculation. Many discs of different segments of root samples were taken together instead from one segment, so as to obtain the greater diversity of endophytic fungi. This method was also applied to the fruits samples as well. After surface treatment, 200 discs each from root and fruit samples were inoculated on to four selected media. All Petri dishes were sealed with sterile parafilm™ to protect them from contamination during repeated handling, while examining endophytes from desiccation. The plates were incubated at 25 ± 2°C and 98% relative humidity (under 12 h fluorescent light/12 h dark light), enclosed in translucent white covered plastic boxes, in BOD cum humidity incubator (L.K Scientific Inc.) for about 25 days.
The emerging endophytic fungi were sub cultured on PDA, for enumeration and identification. All endophytic fungal isolates were deposited to the Centre of Advanced study in Botany, Banaras Hindu University, India [Accession code: MPCLF/R-1001-1272].
Fungal DNA Isolation and Acquiring ITS-5.8S rDNA Sequence Information
The fungus was grown on potato dextrose broth for 7 days and the mycelium was harvested and the nucleic acid (DNA) was extracted using DNeasy Plant and Fungi Mini Kit (Qiagen) according to the manufacturer’s directions. The ITS regions of the fungus were amplified using PCR and the universal ITS primers ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′). All other procedures were carried out as previously described by Ezra et al. [25]. The DNA was sequenced at the W.M. Keck Facility at Yale University. The sequence data of this fungus are deposited in GenBank and are available on the NCBI web site (http://www.ncbi.nlm.nih.gov). Sequences obtained in this study were compared to the GenBank database using the BLAST software on the NCBI web site: http://www.ncbi.nlm.nih.gov/BLAST/).
Data reduction and Statistics
The isolation frequencies (IR %) were represented by the ratio of the number of segments/tissues from which the particular endophytic mycelia emerged and the total segment/tissues inoculated. One-way analysis of variance (ANOVA) and Tukey’s multiple range test (TMRT) were also performed using the SPSS (ver. 10) to determine which culture media had a significant effect on the isolation of endophytes.
Results
After consecutive treatments in ethanol/hypochlorite for 1, 3 and 5 min, the relative frequency of tissues from which endophytic mycelia emerged was calculated. The emergence of endophytic fungi from tissues consistently increased at 20–30% for 1 min treatment time 37–44% at 3 min, and 40–65% for 5 min from the root and fruits, respectively (Fig. 1). Thereafter as we increased the length of treatment time beyond 5 min and there was a gradual decrease in relative fungal isolation frequency. Interestingly, 35% (v/v) H2O2 (Regime 2) was toxic and seemed too harsh as some fungal endophytes were killed after 5 min and during 8–10 min treatment times, the relative frequency of endophyte isolation dropped significantly up to 50% (Fig. 1). Overall, the efficacy of 5.0% (w/v) NaOCl treatment for destruction of epiphytes was reaffirmed by the results. Adding some alcohol can enhance the wetting, penetrating and killing properties of NaOCl. After optimizing the surface treatment conditions, the method employing NaOCl at 5% of available chlorine for 5 min (Regime 1) was then used throughout all remaining experiments. From 400 segments of roots and fruits of Azadirachta indica, a total of 272 fungal isolates [MCPLF/R-1001-1272] were recovered representing 29 species of filamentous fungi (Table 1). Among 272 isolates maximum 165 were obtained from root samples with isolation frequency of 41.25%, while it was only 26.75% in fruits. The fruit samples exhibit less species richness (20) as compared to roots (26) (Table 4). From root samples the maximum number of endophytes was recovered on the PDA medium (63 isolates, 8 species richness, 28.5% Isolation rate). However, from fruit samples MCA medium (38 isolates, 6 species richness, and 15% Isolation rates) favors the optimal recovery of endophytes. Despite the maximum recovery of endophytes from root tissues on to PDA, the maximum species richness (11) was obtained on the MCA medium and similarly with the fruit tissues the maximum species richness (9) was obtained on the PDA medium despite the maximum endophytic recovery on MCA medium. Species such as Chaetomium, Chloridium, Scytalidium, Nigrospora and Verticillium were exclusively isolated from the root samples, while Humicola, Drechslera and Colletotrichum spp. were obtained exclusively from fruits samples irrespective of medium used in isolation (Table 1). The maximum species richness (25 species, 3.27 ± 3.23 species/media) was obtained from MCA medium. The maximum numbers of isolates also were recovered on MCA medium (95). Lowest species richness was obtained with NA medium (26 species, 0.89 ± 1.47 species/media). The number of species isolated from a particular medium was variable as reflected by the significant standard deviations (Tables 2, 3). Maximum isolation frequency was obtained on MCA medium (23.75%) while the least was obtained on the NA medium (6.50%). Isolation frequencies on PDA (23.25%) and MCA (23.75%) were almost similar in contrast to MYA (14.50%) and NA (6.50%) media (Table 3). Acremonium acutatum, Cladosporium cladosporioides, Curvularia lunata, and Trichoderma sp. were preferentially isolated on PDA. Alternaria alternata, A. longipes, and Aspergillus niger, were isolated more frequently on MCA than others. However, genera like Chloridium virescens and Drechslera sp. were exclusively isolated on MCA medium. Alternaria alternata was isolated more frequently on MCA than PDA and MYA. Alternaria dennisii, Cladosporium cladosporioides, were equally isolated on MYA medium, followed by Scytalidium and mycelia sterilia (Table 5). Hyphomycete members also dominated in number over ascomycetes and zygomycetes (Table 1). Those organisms that were listed as Mycelia Sterilia (1, 2, 3) (Table 1) after ITS-5.8 S rDNA analysis showed that 1 was genetically similar to Fusarium solani (99%) while 2 was close to Chaetomium globosum (93%) and 3 was similar to Chaetomium globosum (93%) respectively, with 99–93% sequence similarities. These sequences are deposited in GenBank as accession EU 80424, EU 780425 and EU 780423, respectively. However, Alternaria along with Aspergillus and Cladosporium sp., showed their presence on the NA medium as well (Tables 1, 5).
Fig. 1.
Influence of surface sterilization on the isolation frequency of the segments from which endophytic mycelia emerged, depending upon two sterilents and varying length of sterilization
Table 1.
Occurrence of endophytic fungi from roots and fruits of Azadirachta indica on to four different isolation media from three host plants
aThe symbol represents, 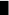 for PDA,
for PDA, 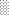 for MCA,
for MCA, 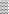 for MYA and
for MYA and 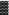 for NA
for NA
bFungi that didn't sporulate on media selected for the study
Table 4.
A comparison of recovery of endophytic fungi from roots and fruits of Azadirachta indica
| Culture mediaa | Isolates recovered | Isolation frequency | Species richness | |||
|---|---|---|---|---|---|---|
| Root | Fruit | Root | Fruit | Root | Fruit | |
 MYA
MYA |
35 | 23 | 17.5 | 11.5 | 4 | 3 |
 MCA
MCA |
57 | 38 | 31.5 | 15.0 | 11 | 6 |
 PDA
PDA |
63 | 30 | 28.5 | 19.0 | 8 | 9 |
 NA
NA |
10 | 16 | 05.0 | 08.0 | 3 | 2 |
| All media | 165 | 107 | 41.25 | 26.75 | 26 | 20 |
aSee materials and method for abbreviation
Table 2.
The effect of culture media on recovery of endophytic fungal isolates and their respective species richness
| Culture mediaa | Segment cultured | Total isolates recovered | Species richness | Species/media (Mean ± SD)b |
|---|---|---|---|---|
 MYA
MYA |
100 | 58 | 18 | 2.00 ± 3.86cd |
 MCA
MCA |
100 | 95 | 25 | 3.27 ± 3.23d |
 PDA
PDA |
100 | 93 | 23 | 3.20 ± 3.86d |
 NA
NA |
100 | 26 | 08 | 0.89 ± 1.47c |
| Total | 400 | 272 | 29 | 9.37 ± 9.05e |
aSee materials and method for abbreviation
bThe values are mean of isolates from three trees at 4 different culture media. Means followed by different letters (c, d and e) indicates significant difference at P = 0.05, according to one-way ANOVA and Tukeys multiple range test (TMRT)
Table 3.
Host comparisons on recovery of endophytic isolates from four different culture mediums and their respective isolation frequency
| Culture mediaa | Total isolates recovered | Isolation frequency (%) | Species/media (Mean ± SD)b | ||
|---|---|---|---|---|---|
| Az1 | Az2 | Az3 | |||
 MYA
MYA |
23 | 17 | 18 | 14.50 | 2.00 ± 3.86cd |
 MCA
MCA |
29 | 40 | 26 | 23.75 | 3.27 ± 3.23d |
 PDA
PDA |
38 | 25 | 30 | 23.25 | 3.20 ± 3.86d |
 NA
NA |
06 | 12 | 08 | 06.50 | 0.89 ± 1.47c |
| Total | 96 | 94 | 82 | 68.00 | 9.37 ± 9.05e |
aSee materials and method for abbreviation
bThe values are mean of isolates from three trees at 4 different culture media. Means followed by different letters indicates significant difference at P = 0.05, according to one-way ANOVA and Tukeys multiple range test
Table 5.
Isolation frequency of endophytic fungi isolated on four different culture media
aNo. of Petri dishes with 5 segments of inoculum's in each
Discussion
The root endophytes are apparently quite common with their geographic and host distribution, although with the paucity of morphological characters and intricacy of inducing sporulation, they were not always easy to identify. In preliminary experimentation, we optimized the tissue treatment protocols among the two treatment regimes (Regimes 1 and 2), because tissue treatment is a crucial step in endophytic microbe isolation. These regimes differ in the nature of surface sterilizers (Sterilants) and the length of treatment. Under Regime 1 the emergence of endophytic fungi consistently increases from 30 to 65% up to a sterilization time of 5 min (Fig. 1). Thereafter as the length of the treatment time increased beyond 5 min there was a gradual decrease in relative fungal isolation frequency which might be attributed to the enhanced toxicity of sterilants and sometimes due to the wetting agents (ethanol in this case), although ethanol has limited penetrating and antibiotic activity [26].
Adding some alcohols seems to enhance the wetting, penetrating and killing properties of NaOCl. Finally, the efficacy of 5.0% (w/v) NaOCl treatment for destruction of epiphytes was reaffirmed by the results. Many other reports also support this conclusion as they find NaOCl at 5% for about 5 min as the most effective treatment protocol [20], as in Picea abies the serial washing of root tissues was done to compare endophytic population, colonizing roots of the host, in relation to site and soil characteristics [27]. At this point, it can be concluded that the endophytic fungi which we have recovered, most have tissue specificity toward the root. This result supports the role of substrate availability which confined the fungi to specific parts of host. From root samples, the PDA medium (63 isolates, 8 species richness, 28.5% isolation rate) shows maximum indices of endophytic recovery in its favor. Similarly, from fruit samples the MCA medium (38 isolates, 6 species richness 15.0% isolation rates) favors the optimal recovery of endophytes. Species such as Chaetomium globosum, Chloridium, Scytalidium, Nigrospora and Verticillium were exclusively isolated from the root, while Humicola, Drechslera and Colletotrichum sp. were obtained exclusively from fruit samples irrespective of media used in isolation (Tables 1, 4).
In case of the MYA medium it was observed that two or three fast growing fungal species over grew the plate in a few days and reduced the opportunity for the recovery of other slow growing fungi. This would be a major factor resulting in comparatively fewer species (18 species, 2.00 ± 3.86 species/media) from this medium. This may be attributed to the strong media preference of a few endophytes than the rest. Thus, media preference is another factor what can directly influence the endophytic recovery which was observed in this experiment. The number of species isolated from a particular medium was variable as reflected by the significant standard deviations (Table 2). Some earlier workers also investigated the effects of isolation media on species richness in twigs and leaves of Chamaecyparis thyoides. It was found that 1% malt extract and 2% yeast extract with 50 ppm each of Streptomycin and chlortetracycline gave the highest species richness for endophytic isolation [28]. One source of variation in the number of species could be the composition of the medium, as well as suitability of their constituents for fungal growth. In spite of this fact the maximum isolation frequency was obtained on the MCA medium (23.75%) while least on to the NA medium (6.50%). Isolation frequencies of PDA (23.25%) and MCA (23.75%) were almost similar in contrast to the MYA (14.50%) and NA (6.50%) media. Acremonium acutatum, Cladosporium cladosporioides, Curvularia lunata, Trichoderma sp. were preferentially isolated on PDA. Alternaria alternata, A. longipes, Aspergillus niger, were isolated more frequently on MCA than others. However, genera like Chloridium sp. and Drechslera sp. were exclusively isolated on MCA medium. Alternaria alternata was isolated more frequently on MCA than PDA and MYA. Alternaria dennisii, Cladosporium cladosporioides, were equally isolated on MYA media, followed by Scytalidium. Rare or incidental species (defined in this paper as those species that only represented by two or three isolates, total 13 species) constitute a high proportion of overall species richness. These species are nearly uniform in their recovery from root and fruits (Table 5). Among fungi recovered in our study Alternaria sp., Acremonium acutatum, Cladosporium, and Aspergillus spp. were found to be dominant. Other dominant genera were Pestalotiopsis, Trichoderma, Curvularia and Penicillium sp. Genera, such as Humicola, Chloridium, Scytalidium, and Collitotrichum spp. were obtained for the first time as endophytes in this plant (Table 1). A considerable number of strains often remain unidentified in most cases and this report is no exception to this fact. We too have recovered Mycelia-Sterilia (MS), which are separated into three categories based on their morphology. In order to identify those (MS) we used molecular tools, based on ITS-5.8 S rDNA sequences followed by BLAST searches. The mycelia-sterilia (1–3) was identified as Fusarium solani (1) and Chaetomium Globosum (2, 3) respectively. Leaf isolated mycelia-sterilia 1, was identified as F. solani (soil fungus), and it shows the vertical traveling nature of this fungus from root to upper tissues of the host.
Several species we have isolated appear to be rarely reported from Azadirachta indica including Humicola, Chloridium, Colletotrichum, and Scytalidium whereas Choridium and Humicola were reported from twigs of Terminalia arjuna as well [29]. Many species such as Alternaria, Colletotrichum, Vertcillium, Aspergillus, and Penicillium are known to be potential pathogenic fungi to several hosts and also reported as endophytes to variety of plants as well. Colletotrichum gloeosporioides is a pathogenic fungus of Cashew tree, but it was also found as an endophyte in many cases [25]. Colletotrichum along with Fusarium were also reported to impair the photosynthetic activity in Maize and Banana [30] and the presence of (MS-1) Fusarium solani in this experiment corroborates this opinion. The exploration of woody perennials for endophytic microorganisms that might produce microbial metabolites for use as therapeutic agents needs much attention. Now with the completion of this work it seems as if we have a more complete picture of the endophytic composition of the neem tree when placed in context with our previous work on neem endophytes [20]. Thus, we are in a position to carefully screen this array of microorganisms for their bioactive metabolites in order to learn if they hold as much promise as the host that supports them.
Acknowledgements
The authors are thankful to CSIR and UGC New Delhi for providing financial assistance. Author (Gary Stroble) expresses his appreciation to the Montana Agricultural Experiment Station and the US national Science Foundation for their support of this work. Author (Lori Baulanger) appreciates the H. Hughes grant to Scott Strobel of Yale University for its support of on this project. Author (Ravindra Kharwar) is thankful to CSIR for financial assistance (File No.EMR II 05-38/1104) and also to DST New Delhi, for award of ‘BOYSCAST fellowship’ (SR/BY/L-02/06) 2006–2007).
References
- 1.Butterworth JH, Morgan ED, Percy GR. The structure of azadirachtin; the functional groups. J Chem Soc Perkin Trans. 1972;1:2445–2450. doi: 10.1039/p19720002445. [DOI] [Google Scholar]
- 2.Koul O, Isman MB, Ketkar CM. Properties and uses of neem, Azadirachta indica. Can J Bot. 1990;68:1–11. doi: 10.1139/b90-001. [DOI] [Google Scholar]
- 3.Champagne DE, Koul O, Isman MB, Scudder GGE, Towers GHN. Biological activity of limonoids from the Rutales. Phytochem. 1992;31:377–394. doi: 10.1016/0031-9422(92)90003-9. [DOI] [Google Scholar]
- 4.Siddiqui S, Faizi S, Siddiqui BS, Mahmood J. Tetracyclic triterpenoides and their derivatives from Azadirachta indica. J Nat Prod. 1988;51:30–43. doi: 10.1021/np50055a003. [DOI] [Google Scholar]
- 5.Ley SV, Denholm AA, Wood A. The chemistry of azadirachtin. Nat Prod Rep. 1993;10:109–157. doi: 10.1039/np9931000109. [DOI] [Google Scholar]
- 6.Kaushik B, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activity and medicinal properties of neem (Azadirachta indica) Curr Sci. 2002;82:1336–1345. [Google Scholar]
- 7.Schmutterer H. Properties and potential of natural pesticides from the neem tree. Azadirachta indica-Rev Entomol. 1990;35:271–297. doi: 10.1146/annurev.en.35.010190.001415. [DOI] [PubMed] [Google Scholar]
- 8.Okpanyi SN, Ezeukwk GC. Anti-inflammatory and anti- pyretic activities of Azadirachta indica. Planta Med. 1981;41:34–49. doi: 10.1055/s-2007-971670. [DOI] [PubMed] [Google Scholar]
- 9.Khattak SG, Gilani SN, Ikram M. Antipyratic studies on some indigenous Pakistani medicinal plants. J Ethnopharmacol. 1985;14:45–51. doi: 10.1016/0378-8741(85)90027-3. [DOI] [PubMed] [Google Scholar]
- 10.Tella A. The effects of Azadirachta indica in acute Plasmodium berghei malaria. Nig Med J. 1977;7:258–263. [PubMed] [Google Scholar]
- 11.Rochankij S, Thebraranonth Y, Yenjal CY. Nimbolide a constituents of Azadirachta indica inhibits Plasmodium falciparum in culture. SEA J Trop Med Pub Health. 1985;16:66–72. [PubMed] [Google Scholar]
- 12.Fujiwara T, Takeka TO, Gihara Y, Shimizu M, Nomura T, Tomuta Y. Studies on the structure of polysaccharides from the bark of Melia azadirach. Chem Pherma Bull. 1982;30:4025–4030. doi: 10.1248/cpb.30.4025. [DOI] [PubMed] [Google Scholar]
- 13.Pillai NR, Santhakumari G. Effects of Nimbidin in acute and chronic gastroduodinal ulcer models in experimental animals. Planta Med. 1984;50:143–146. doi: 10.1055/s-2007-969654. [DOI] [PubMed] [Google Scholar]
- 14.Shukla R, Singh S, Bhandari CR. Preliminary clinical trials on anti diabetic actions of Azadirachta indica. Med Surg. 1973;13:11–12. [Google Scholar]
- 15.Sinha KC, Riar SS, Tiwary RS, Dhawan AK, Bhadhan J, Thomas P, Kain AK, Jain PK. Neem oil as a vaginal contraceptive. Ind J Med Res. 1984;79:131–136. [PubMed] [Google Scholar]
- 16.Singh PP, Junnarkar AY, Reddi GS, Singh KV. Azadirachta indica neuro psychopharmacological and anti-microbial studies. Fitoterapia. 1987;58:233–238. [Google Scholar]
- 17.Thomson EB, Anderson CC. Cardiovascular effects of Azadirachta indica extract. J Pharma Sci. 1978;67:1476–1478. doi: 10.1002/jps.2600671044. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopal R, Suryanarayanan TS. Isolation of endophytic fungi from leaves of neem (Azadirachta indica) Curr Sci. 2000;78:1375–1378. [Google Scholar]
- 19.Mahesh B, Tejesvi MV, Nalini MS, Prakash HS, Kini KR, Subbiah V, Hunthrike SS. Endophytic mycoflora of inner bark of Azadirachta indica A. Juss. Curr Sci. 2005;88:218–219. [Google Scholar]
- 20.Verma VC, Gond SK, Kumar A, Kharwar RN, Strobel G. The endophytic mycoflora of bark, leaf and stem tissues of Azadirachta indica A. Juss (Neem) from Varanasi (India) Microb Ecol. 2007;54:119–125. doi: 10.1007/s00248-006-9179-9. [DOI] [PubMed] [Google Scholar]
- 21.Wu SH, Chen YW, Shao SC, Wang LD, Li ZY, Yang LY, Li SL, Huang R. Ten-membered lactones from Phomopsis sp., an endophytic fungus of Azadirachta indica. J Nat Prod. 2008;71(4):731–734. doi: 10.1021/np070624j. [DOI] [PubMed] [Google Scholar]
- 22.Li GH, Yu ZF, Li X, Wang XB, Zheng LJ, Zhang KQ. Nematicidal metabolites produced by the endophytic fungus Geotrichum sp. AL4. Chem Biodivers. 2007;4:1520–1524. doi: 10.1002/cbdv.200790131. [DOI] [PubMed] [Google Scholar]
- 23.Kharwar RN, Verma VC, Kumar A, Gond SK, Harper JK, Hess WM, Lobkovosky E, Ma C, Ren Y, Strobel GA. Javanicin, an antibacterial nephthaquinone from an endophytic fungus of neem, Chloridium sp. Curr Microbiol. 2009;58(3):233–238. doi: 10.1007/s00284-008-9313-7. [DOI] [PubMed] [Google Scholar]
- 24.Wu SH, Chen You-Wei, Shao Shi-Cheng, Wang Li-Dong, Ying Yu, Zhi-Ying Li, Yang Li-Yuan, Shao-Lan Li, Huang Rong. Two new solanapyrone analogues from the endophytic fungus Nigrospora sp. YB-141 of Azadirachta indica. Chem Biodivers. 2009;6:79–85. doi: 10.1002/cbdv.200700421. [DOI] [PubMed] [Google Scholar]
- 25.Ezra D, Hess WM, Strobel GA. New endophytic isolates of Muscodor albus, a volatile antibiotic-producing fungus. Microbiol. 2004;150:4023–4031. doi: 10.1099/mic.0.27334-0. [DOI] [PubMed] [Google Scholar]
- 26.Bills GF, Polishook JD. Abundance and diversity of microfungi in leaf litter of a lowland rainforest in Costa Rica. Mycologia. 1994;86:187–198. doi: 10.2307/3760635. [DOI] [Google Scholar]
- 27.Bills GF, Polishook JD. Recovery of endophytic fungi from Chamaecyparis thyoides. Sydowia. 1992;44:1–12. [Google Scholar]
- 28.Sturz AV, Christie BR. Potential benefits from cultivar specific red clover-potato sequences. Ann Appl Biol. 1998;133:365–373. doi: 10.1111/j.1744-7348.1998.tb05836.x. [DOI] [Google Scholar]
- 29.Tejesvi MV, Mahesh B, Nalini MS, Prakash HS, Kini KR, Subbiah V, Hunthrike SS. Endophytic fungal assemblages from inner bark and twig of Terminalia arjuna W&A. (Combretaceae) World J Microbiol Biotechnol. 2005;21:1535–1540. doi: 10.1007/s11274-005-7579-5. [DOI] [Google Scholar]
- 30.Costa Pinto LSR, Azevedo JL, Pereira JO, Carneiro Vieira ML, Labata CA. Symptom less infection of banana and maize by endophytic fungi impairs photosynthetic efficiency. New Phytol. 2000;147:609–615. doi: 10.1046/j.1469-8137.2000.00722.x. [DOI] [PubMed] [Google Scholar]





