Abstract
This study examined the growth of expressive language skills in children who received cochlear implants (CIs) in infancy. Repeated language measures were gathered from 29 children who received CIs between 10 and 40 months of age. Both cross-sectional and growth curve analyses were used to assess the relationship between expressive language outcomes and CI experience. A beneficial effect of earlier implantation on expressive language growth was found. Growth curve analysis showed that growth was more rapid in children implanted as infants than those implanted as toddlers. Age at initial stimulation accounted for 14.6% of the variance of the individual differences in expressive language growth rates.
Keywords: cochlear implants, infants, language growth, yearly hearing detection and intervention, growth curve analysis
Infants born with normal hearing thresholds possess a number of auditory skills crucial to fostering language growth; many of these proficiencies appear to be present as early as birth or beforehand (DeCasper & Fifer, 1980; DeCasper, Lecanuet, Busnel, & Granier-Deferre, 1994; Kuhl & Miller, 1982; Mehler et al., 1988; Moon, Panneton-Cooper, & Fifer, 1993). Neonates with bilateral sensorineural hearing loss (SNHL), however, do not have this benefit of early spoken language exposure. As a result, children with severe to profound SNHL who are born to hearing parents often demonstrate great lags in spoken language development due to limited linguistic input (Brasel & Quigley, 1977; Carney & Moeller, 1998; Lederberg & Spencer, 2001; Mayne, Yoshinaga-Itano, & Sedey, 2000a, 2000b; Pipp-Siegel, Sedey, VanLeeuwen, & Yoshinaga-Itano, 2003; Spencer & Meadow-Orlans, 1996). However, nearly 20 years ago, pediatric cochlear implantation emerged as a surgical option that could provide acoustic information to the auditory system by means of direct electrical stimulation of the auditory nerve. Despite the fact that the typical cochlear implant (CI) device provides frequency and temporal resolution information that is considerably different from that of acoustic hearing, the auditory information provided by the CI appears to improve the rate of spoken language development of children with severe to profound SNHL who are born to hearing parents (Blamey & Sarant, 2000; Geers & Moog, 1994; Geers, Nicholas, & Sedey, 2003; Svirsky, Robbins, Kirk, Pisoni, & Miyamoto, 2000; Tomblin, Spencer, Flock, Tyler, & Gantz, 1999). Indeed, studies have shown that the rate of language development in school-age children with CIs typically approaches that of children with normal hearing. Svirsky and colleagues (Svirsky, 2003; Svirsky et al., 2000) have shown average postimplant-growth rates that match those of children with normal hearing, whereas others have reported rates that ranged from 45% to 67% of normal growth rates (Blamey, 2001; Connor, Hieber, Arts, & Zwolan, 2000). Yet, although these and further data (El-Hakim, Papsin, et al., 2001; Kirk et al., 2002; Ouellet, Le Normand, & Cohen, 2001; Robbins, Osberger, Miyamoto, & Kessler, 1995) suggest that the CI device appears to significantly facilitate language development in children who use it, the device alone does not account for the variability in growth noted across pediatric CI users. In fact, many investigators (Connor et al., 2000; Geers, 2002; Geers, Brenner, & Davidson, 2003; Geers et al., 2002; Hodges, Ash, Balkany, & Schloffman, 1999; Osberger, Zimmerman-Phillips, & Koch, 2002; Tobey, Geers, Brenner, Altuna, & Gabbert, 2003; Tobey et al., 2000) have documented a number of additional factors (e.g., length of CI experience, amount of rehabilitation, device technology, educational setting) that account for the variability in pediatric CI users' performance across assorted speech and language tasks. One factor that continues to require further study is the effect of a child's age at implantation on the individual differences in language development.
In 1993, the National Institutes of Health (NIH) stated that all infants should be screened for hearing impairment following birth (National Center for Hearing Assessment and Management, n.d.). With this implementation of universal newborn hearing screening and the availability of objective measures, such as otoacoustic emissions (OAEs), auditory brainstem response (ABR), and auditory steady-state response (ASSR), professionals are now able to identify infants with profound SNHL within the first few months of their lives. Additionally, the Food and Drug Administration recently lowered the minimum age of cochlear implantation eligibility to 12 months. These two policy decisions have enabled surgeons to consider cochlear implantation at much earlier ages than was the case even 5 years ago. Many of the children now receiving CIs are between 12 and 18 months of age.
One prominent reason for the move toward earlier implantation has been the view that in addition to providing an earlier start in language learning, earlier implantation capitalizes on brain systems that are better suited for language learning during infancy. Two related notions of sensitive periods and neural plasticity appear to provide the basis for this view. A sensitive period in development is defined as “the time during development when a specific manipulation or experience changes the developmental trajectory of a system” (Bruer, 2001, p. 10) and is supported by classic studies of visual deprivation in kittens (Hubel & Wiesel, 1963, 1965; Wiesel & Hubel, 1963, 1965) and more recent studies of second language acquisition (Newport, 1990) and late onset sign language learning (Neville et al., 1998; Newman, Bavelier, Corina, Jezzard, & Neville, 2002).
Neural plasticity also appears to make significant contributions to sensitive periods in the maturation of the auditory system; these contributions were initially noted in barn owls (Miller & Knudsen, 2003) and recent, parallel work has demonstrated the presence of similar contributions in humans as well (Kral, Hartmann, Tillein, Heid, & Klinke, 2000, 2002; Manrique et al., 1999; Ponton, Moore, & Eggermont, 1999; Ponton et al., 1996; Sharma, Dorman, & Spahr, 2002). In particular, the ideal conditions for neural organization of the auditory system appear to occur from birth to toddlerhood. Furthermore, if a child does not have access to auditory stimuli before reaching 84 months of age, central neural organization for audition will likely be affected (Manrique et al., 1999; Ponton et al., 1996; Sharma et al., 2002). This sensitive period is supported by research examining the effects of cochlear implantation on the maturation of the human auditory system. Children who were born with SNHL and implanted before the age of 42 months demonstrated auditory evoked P1 response latencies (by 6 months, postinitial stimulation) that mirrored those of children who were born with normal hearing. However, children who were born with SNHL and implanted after the age 84 months demonstrated auditory evoked P1 response latencies that were significantly longer than those of their normal-hearing peers (Sharma et al., 2002).
This body of research supporting sensitive periods in children with both normal hearing and hearing loss has encouraged researchers and clinicians involved with pediatric cochlear implantation to expect that the same underlying principles are at work in the neurological systems of children with profound SNHL. That is, neural development relies on the receipt of sufficient auditory experience in conjunction with spoken language input to ensure “normal” language and listening development. A delay in input could subsequently reduce learning and alter development. It is further reasoned that if a child undergoes cochlear implantation in early infancy, such early implantation will take advantage of the bidirectional nature between neurological development and experience, thus facilitating overall language development. Earlier research examining the speech and language outcomes of children implanted within the first 60 months of life supports the advantages of early neural plasticity and has contributed to the decline in the age at which children are implanted. For example, Tye-Murray, Spencer, and Woodworth (1995) compared pediatric CI users' scores from a variety of speech production tasks and found that children implanted before the age of 60 months had significantly more accurate speech production than those children implanted after the age of 60 months. These results were later paralleled in work examining speech perception (Fryauf-Bertschy, Tyler, Kelsay, Gantz, & Woodworth, 1997). Finally, the age at which children undergo cochlear implantation has also been noted to affect language outcomes in this population—children implanted before the age of 60 months had significantly higher expressive and receptive vocabulary scores (Connor et al., 2000), higher verbal reasoning scores (Geers, Nicholas, et al., 2003), and better reading skills (Geers, 2003) than children implanted after the age of 60 months.
Similar advantages of early implantation on spoken language acquisition continue to be supported by research evaluating the skills of children implanted even younger than 60 months. In their study, Svirsky and colleagues (Svirsky et al., 2000) developed a predictive growth model to measure the expressive and receptive language growth of pediatric CI recipients before and after cochlear implantation. This model incorporated both observed and predictive scores for each child (the predictive scores estimated the language scores the participants would have obtained if they had never received CIs). Using the model, the authors demonstrated that children implanted (on average) at 54 months of age had mean rates of language growth quite close to those of children with normal hearing. Hammes et al. (2002) obtained data further supporting the idea that early implantation yields spoken language benefits. They conducted a retrospective study of 47 pediatric CI recipients evaluated on several spoken language measures. These children all received their implants before the age of 48 months (range = 9–48 months). After looking at the plotted data, the authors noted that the 10 children implanted before the age of 18 months consistently demonstrated rates of language growth similar to those of their normal-hearing peers. Four children had acquired spoken language skills commensurate with peers who were within 6 months of their chronological ages. Finally, work from El-Hakim and colleagues (El-Hakim, Levasseur, et al., 2001; El-Hakim, Papsin, et al., 2001) also lends support to the notion that implanting a child within the first years of life yields better language outcomes.
Given the research to date, it appears that cochlear implantation facilitates language development, but the question remains whether or not there is a greater learning potential if implantation occurs even earlier in life. Preliminary research within the field suggests that surgically placing a CI within the first 12 months of life may result in a greater language-learning potential; however, the literature examining the development of language skills in infants implanted early in life remains incomplete. Many of the studies to date have examined only a small number of children in this age group (Brinton, 2001; Hammes et al., 2002; Richter, Eissele, Laszig, & Lohle, 2002; Wright, Purcell, & Reed, 2002). Such small sample sizes make it challenging to generalize the findings across the infant CI population. Other studies have used retrospective designs, which have resulted in inconsistent and incomplete language measures. Thus the language measures tended to be neither consistent nor complete across children in these studies (El-Hakim, Levasseur, et al., 2001; El-Hakim, Papsin, et al., 2001; Hammes et al., 2002).
Finally, it is necessary to characterize the growth trajectory or rate. Previous studies typically have not distinguished between the benefits of early implantation that come from simply “starting early,” versus those benefits that come from faster growth rates. Only one study (Svirsky et al., 2000) to date has formally examined both the pediatric CI users' mean differences in language test scores and their language growth over time, but this particular study did not address age at initial stimulation as a factor in these children's growth; thus, cochlear implantation's long-term benefit on language development in infants implanted before the age of 36 months remains unclear.
The present study examined the growth of expressive language skills in 29 children who were implanted within the first few years of life. The study explored the contribution of a child's age at initial stimulation on expressive language growth given the constraints of a clinical population. Explicitly, we were interested in determining how age at initial stimulation is associated with individual differences in expressive language achievement as measured by the Minnesota Child Development Inventory (MCDI; Ireton & Thwing, 1974) and the Preschool Language Scale—3 (PLS–3; Zimmerman, Steiner, & Pond, 1992). It was predicted that age at initial stimulation would significantly affect the rate at which expressive language develops in addition to each child's initial language skill level.
Method
Participants
A total of 29 children (15 males, 14 females) with profound, bilateral SNHL participated in this study. All participants were born to hearing parents and were identified as having SNHL within the first year of life. The infants' and toddlers' ages at the time of surgery for placement of their CI devices ranged from 11 months, 5 days to 40 months, 11 days with an average age at implantation of 21 months, 6 days (SD = 7 months, 2 days). Twelve participants received the Nucleus 24M device and 17 participants received the Nucleus 24R device. All participants in the present study were followed longitudinally as part of a comprehensive, CI center study. These participants were selected from the larger, longitudinal cohort for this study because they had received a CI before the age of 40 months and had a minimum of 12 months of implant experience.
Twenty-six families described their philosophy of communication with their children as total communication; in every instance the sign system used was signed English. The philosophy of total communication can be briefly defined as “the combined use of aural, manual and oral modalities in communicating with and teaching hearing impaired individuals” (Garretson, 1976, p. 89). The 3 remaining families reported that they followed an oral/aural communication philosophy. American English was the primary language spoken in each child's home (i.e., English was spoken more than 50% of the time in the infant's listening environment). Infants and toddlers had no known visual abnormalities. Participants' individual profiles are presented in Table 1.
Table 1.
Individual participant profiles.
| RP # | Sex | Etiology | Age at HA Fit | IS | Communication Mode | CI/HA Combo |
|---|---|---|---|---|---|---|
| RP 1 | M | Unknown | — | 25.5 | TC | N |
| RP 2 | M | Cochlear malformation | 29 | 35.5 | TC | Y |
| RP 3 | F | Genetic: Nonspecific | — | 28.0 | TC | N |
| RP 4 | F | Genetic: Usher's | — | 19.5 | TC | N |
| RP 5 | M | Ototoxicity | 16.5 | 19.5 | TC | N |
| RP 6 | F | Genetic: Connexin-26 | 16 | 19.5 | TC | N |
| RP 7 | F | Unknown | 11 | 19.0 | TC | Y |
| RP 8 | M | Genetic: Nonspecific | — | 31.0 | OA | N |
| RP 9 | F | Unknown | — | 40.0 | TC | N |
| RP 10 | F | Unknown | 22 | 27.0 | TC | N |
| RP 11 | F | Unknown | 17 | 19.0 | TC | N |
| RP 12 | M | Unknown | 4 | 11.0 | OA | N |
| RP 13 | M | Unknown | 18 | 21.0 | TC | N |
| RP 14 | M | Unknown | 5.5 | 20.0 | TC | Y |
| RP 15 | M | Unknown | 17 | 10.5 | TC | N |
| RP 16 | F | Unknown | — | 14.0 | TC | N |
| RP 17 | M | Unknown | 5 | 16.0 | TC | Y |
| RP 18 | F | Unknown | 5 | 28.0 | TC | N |
| RP 19 | M | Unknown | — | 14.0 | TC | N |
| RP 20 | F | Genetic: Nonspecific | 12 | 16.0 | TC | N |
| RP 21 | F | Unknown | 13.5 | 28.0 | TC | N |
| RP 22 | M | CMV | 4 | 15.0 | TC | N |
| RP 23 | F | Unknown | 22.5 | 31.0 | TC | N |
| RP 24 | M | Unknown | — | 39.0 | TC | N |
| RP 25 | F | Meningitis | — | 16.0 | TC | N |
| RP 26 | F | Cochlear malformation | 10 | 19.0 | TC | N |
| RP 27 | M | Genetic: Connexin-26 | — | 14.0 | TC | N |
| RP 28 | F | Genetic: Nonspecific | 4 | 13.0 | OA | N |
| RP 29 | M | Unknown | — | 14.0 | TC | N |
Note. RP # = research participant's identification number; age at HA fit = age initially fit with bilateral hearing aids (in months); IS = age at initial stimulation of CI (in months); TC = total communication; OA = oral/aural communication; CI/HA combo = denotes whether the child uses a hearing aid in combination with a cochlear implant on the opposite ear, Y = yes, N = no; CMV = cytomegalovirus. Dashes indicate age at initial fit is not available.
Language Measures
Children participating in the comprehensive, CI center study are followed systematically with regard to their development of communication skills. Two instruments have been used at this center to measure language growth during the first few years of life: the MDCI and the PLS–3. It is important to note that all children were tested on these measures using each child's preferred modality of communication. For example, if a child's family adhered to a total communication philosophy and simultaneously used spoken and signed English to communicate, then both spoken and signed English was used to test the child. Both spoken and/or signed responses were acceptable. For the present study, it was important to include both spoken and signed responses in the measures of expressive language because the majority of the study's participants subscribed to a total communication philosophy. An alternative study could isolate one modality from the other (e.g., examine the speech growth in children following initial stimulation). In the present study, however, speech and sign are interdependent and to separate speech and sign would yield an inaccurate measure of these children's expressive language growth.
Minnesota Child Development Inventory (MCDI)
The MCDI is a parental response inventory designed as an educational and assessment tool for children with normal hearing. Parental reports of ability, particularly expressive language ability, are generally regarded as valid indices of language status in children with normal hearing (Dale, 1991; Dale, Bates, Reznick, & Morisset, 1989; Tomblin, Shonrock, & Hardy, 1989); additionally, several researchers have adopted this view with children who have hearing loss (Mayne et al., 2000a; Pipp-Siegel et al., 2003; Stallings, Gao, & Svirsky, 2002; Yoshinaga-Itano, Sedey, Coulter, & Mehl, 1998; Yoshinaga-Itano, Snyder, & Day, 2000). Specifically, the inventory poses questions to a parent-reporter regarding various aspects of his or her child's development from the ages of 6 months to 72 months. Participants' ages at testing ranged from 5 months to 69 months, with the exception of 1 child whose age at her final testing point was 89 months. Her data were included in the final analysis because her functional level of communication placed her language abilities within the scope of the test. The following are examples of questions taken from the MCDI:
[Does your child] have a word for `drink'?
[Does your child] ask for `more' or `another one'?
[Does your child] take part in conversations?
Parents record their responses to the questions on designated response forms by answering “yes” or “no” to each question regardless of their children's communication modality. For the six test questions that specifically asked about vocalizing (e.g., [Does your child] make sounds like da, ka, ga, ba?), the parent was instructed to give the participant credit only if he or she actually vocalized the particular sound(s) in question. The test consists of eight subscales, but only the Expressive Language subscale was used in the present study. This subscale is designed to measure expressive language skills ranging from simple gestural behaviors to complex language expression.
Preschool Language Scale—3 (PLS–3)
The PLS–3 is a diagnostic instrument typically used to measure the language development of children with normal hearing aged 0 months to 83 months. Participants' ages at testing ranged from 5 months to 78 months, with the exception of the same, aforementioned child whose age at her final testing point was 89 months.
All observations of expressive communication development were made and recorded by an experimenter during each child's test sessions; expressive communication was noted regardless of the modality in which it occurred. In other words, manual communication was equally weighted with oral communication; children received credit for PLS–3 items regardless of the communication modality used to successfully complete the item. Again, for the seven test questions that specifically asked about vocalizing, the participant was only given credit if he or she actually vocalized the particular sound(s) in question. The test consists of two subscales, but only the Expressive Communication subscale was administered in the present study. This subscale is designed to evaluate young children's expressive language skills in a variety of areas: vocal development, social communication, semantics (content), structure (form), and integrative thinking skills.
Procedure
Repeated measures were gathered on each child. Children were assessed at different ages, and assessment occurred as often as monthly or as seldom as yearly. Data were collected from all participants at least once preceding cochlear implantation and at least once following stimulation. The schedule of data collection postimplantation called for data collection at the time of each child's initial stimulation, at 2-month intervals during the first year after initial stimulation, and at 6-month intervals during the second and third year after initial stimulation. Given the challenges associated with collecting longitudinal data from a clinical population (e.g., missed visits, failure for parents to complete and return MCDI, failure to complete the test session for the PLS–3 due to fatigue on the part of the child), the actual intervals for which data were available deviated from the aforementioned collection schedule. Figure 1 contains a summary of the time points, following implantation, for which MCDI and PLS–3 data were obtained and the children's chronological ages at the time of these observations. It can be seen that many children did not have scores for both tests during the same observation point.
Figure 1.
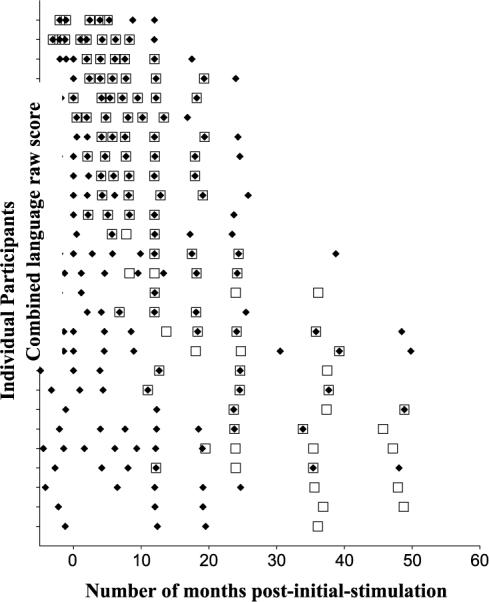
Number and time of expressive language observations gathered from each participant. On the y-axis, each child is represented by a single tick mark; data from the youngest children implanted are located toward the top of the axis while data from the older children implanted are located toward the bottom of the axis. Time is represented on the x-axis and is measured relative to the number of months following initial stimulation of each child's device. The ◆ represents observations during which data from the Minnesota Child Development Inventory were collected from the child; □ represents observations during which data from the Preschool Language Scale—3 were collected from the child.
Each child's development was assessed during his or her individual test session during which the child's parent(s) completed an MCDI. Concurrently, an experimenter administered the PLS–3 for that particular child. The experimenter was experienced in both working with young children who are deaf or hard of hearing and communicating in a manual mode.
Conversion of Scores for Analysis
Both the MCDI and PLS–3 were scored in accordance with their respective Examiner's Manuals. Raw scores were converted into age-equivalent scores based on the norms provided by the tests. The raw scores were used in two ways in this study. First, the raw scores were converted to language quotient scores to provide a descriptive characterization of the functional, expressive language levels attained by the participants. Second, the raw scores were used in the analysis of the language growth rates of the children as a function of age at initial stimulation.
Creation of Norm Referenced Scores
The raw scores from the two measures of expressive language were converted to norm referenced scores using the normative data provided with each instrument. Language achievement was represented in a different format for each test measure. The PLS–3 provides language quotients in the form of standard scores where a score of 100 represents the mean for each child's chronological age and 15 represents the standard deviation. These deviation quotients were used to represent the norm referenced scores from the PLS–3. The MCDI provides age equivalence scores. The interpretation of these age equivalence scores requires that the child's chronological age be considered. Thus, expressive language quotients (ELQs) were yielded by dividing a child's age equivalent score by the child's chronological age at the time the MCDI was completed; the quotient was then multiplied by 100. Similar to the deviation quotients, a score of 100 represents the expected score for children with normal hearing; however, unlike deviation quotients, the standard deviation is not fixed. In both cases, it must be emphasized that these norm-referenced scores can serve only as functional descriptions of the expressive language skills of the children in this study. As was noted above, these tests were adapted to use both spoken and signed English; therefore, the measures were not used strictly according to the tests' standardized procedures. We believe that it can be said that raw scores from the participants in this study reflect the same functional language performance as would be found in the normative population; however, the means by which the participants accomplished this functional level may have been different from the normative population. This approach is consistent with the PLS–3 Examiner's Manual's (Zimmerman et al., 1992, p. 36) philosophy on modifying the test's administration for special populations.
Equating of MCDI and PLS–3 Scores
Our primary research question called for an examination of the growth rates of expressive language after initial stimulation. As shown in Figure 2, for each observation interval at which a child participated in testing, we had an expressive language measure based on the PLS–3 and/or the MCDI. Because each of these tests presumably measures the same thing, rather than analyzing the growth rate of these tests, we chose to use these measures jointly. This approach supplies a more powerful test because it provides more observations for each individual and avoids multiple hypothesis testing. In order to use both tests in the same analysis, the raw scores needed to be combined in those cases where both measures were available at an observation interval. Then the two individual observations needed to be equated to the combined observation. The equating method was based on Crocker and Algina's (1986) work using the following equation:
Figure 2.
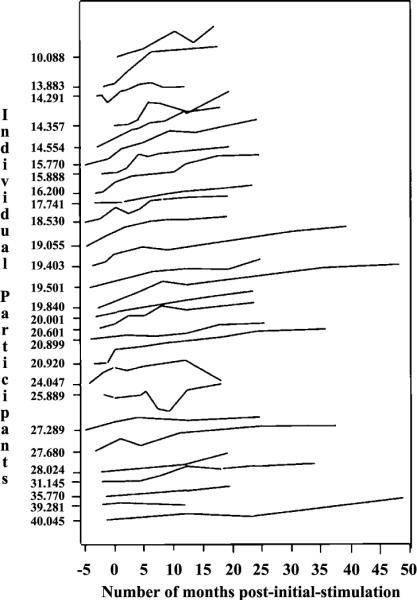
Pattern of expressive language growth curves across individual participants. On the y-axis, each child is represented by a single tick mark; to the left of each tick mark is that particular child's chronological age at initial stimulation (in months). The plots are arranged in order relative to each child's age at initial stimulation of the cochlear implant device; data from the children implanted at the youngest age are located toward the top of the plot while data from the children implanted at older ages are located toward the bottom of the plot. Time is represented on the x-axis and is measured relative to the number of months following initial stimulation of each child's device.
Thus, equating two tests requires computing the means and variances of each of the tests. In this case, these parameter values were computed using the observations where both tests were administered; consequently, this conforms to a design in which parallel measures are both obtained on the same participants. This algorithm resulted in a mean and variance parameter for the sum of the two tests (Y) and for each of the individual tests (X1 = MCDI, X2 = PLS–3). Thus, the observations with either the MCDI or the PLS–3 were equated to the observations comprised of the sum of the two.
Results
The data used for analysis in this study consisted of repeated measures of expressive language derived from the MCDI and the PLS–3. The initial analysis consisted of a descriptive characterization of the language achievement of the participants relative to hearing children. The second analysis tested the primary hypothesis of this study by examining the within-child growth parameters of scores derived from both the MCDI and the PLS–3. These growth parameters characterizing each child's development were then associated across children with the time of initial stimulation of the CI device (Singer & Willett, 2003).
Cross-Sectional Outcomes at Three Intervals of Implant Experience
Table 2 provides the ELQs for children who were tested using the MCDI at three observation points: closest to initial stimulation (peri-implantation), closest to 1 year following initial stimulation, and closest to 2 years following initial stimulation. Table 3 provides the same summary information concerning these observations based on the PLS–3. A comparison of the data in these two tables shows that the MCDI was administered to more of the children during the peri-implantation period than was the PLS–3. This discrepancy resulted because the PLS–3 required direct assessment and was dependent on child compliancy, or scheduling issues, which were sometimes not optimal. Each of the three observation times shown in these tables represents an observation made close to, but not necessarily at, the nominal post-initial-stimulation period. The actual average and range of implant experience at each interval are shown in the respective tables. The amount of data available from the PLS–3 at the peri-implantation time point was too limited to be informative. The ELQ for each measure at each of these peri-intervals reveals a pattern of decline in language status (relative to the participants' hearing peers) as the amount of implant use increases. These cross-sectional data suggest that during the early months of implant experience, although expressive language development lagged behind that of the children with normal hearing who were used to norm these measures, the CI children's levels of language achievement were substantially better than those reported by Svirsky and colleagues (2000) for deaf children who did not receive CIs.
Table 2.
Comparison of expressive language status (based on the Minnesota Child Development Inventory [MCDI]) of children with cochlear implants at three intervals relative to initial stimulation.
| Interval Relative to IS | n | Mean Relative to IS | Range Relative to IS | Mean CA(SD) | Mean ELQ (SD) | Correlation of Age at IS With ELQ |
|---|---|---|---|---|---|---|
| Peri-implant | 27 | −0.83 | 3.23 | 21.07 (7.57) | 84.44 (22.64) | −.27 |
| 12 months | 27 | +11.92 | 4.83 | 33.82 (7.82) | 74.40 (17.73) | −.53* |
| 24 months | 21 | +23.00 | 7.85 | 45.44 (7.26) | 72.81 (24.35) | −.56* |
Note. IS = date of initial stimulation of CI; mean relative to IS = mean number of months before (−) or after (+) initial stimulation of CI; range relative to IS = range of months before/after initial stimulation; mean CA (SD) = mean chronological age, in months (standard deviation) of subsample; correlation of age at IS with ELQ = results of Pearson correlation comparing age at IS with MCDI expressive language quotient score.
p < .05.
Table 3.
Comparison of expressive language status (based on the Preschool Language Scale—3 [PLS-3]) of children with cochlear implants at three intervals relative to initial stimulation.
| Interval Relative to IS | n | Mean Relative to IS | Range Relative to IS | Mean CA(SD) | Mean ELQ (SD) | Correlation of Age at IS With ELQ |
|---|---|---|---|---|---|---|
| Peri-implant | 7 | −0.81 | 2.30 | 14.97 (5.24) | 89.38 (22.64) | −.51 |
| 12 months | 24 | +12.14 | 5.40 | 36.65 (12.56) | 78.21 (17.73) | −.65* |
| 24 months | 21 | +22.31 | 7.09 | 45.63 (9.63) | 74.19(19.71) | −.67* |
Note. IS = date of initial stimulation of CI; mean relative to IS = mean number of months before (−) or after (+) initial stimulation of CI; range relative to IS = range of months before/after initial stimulation; mean CA (SD) = mean chronological age, in months (standard deviation) of subsample; correlation of age at IS with ELQ = results of Pearson correlation comparing age at IS with PLS-3 expressive language quotient score.
p < .05.
The mean ELQs shown in Tables 2 and 3 show a pattern of general decline in language status relative to children with normal hearing, across the three observation intervals. If earlier implantation benefits language status, there should be a negative relationship between age at initial stimulation and language performance. Furthermore, the ELQs of children implanted at younger ages should be higher than the ELQs of children implanted at older ages. The decline should be less pronounced in the children who were implanted at earlier ages. The results of the correlational analysis are included in Tables 2 and 3. During the peri-implantation interval, no significant association was documented between age of initial stimulation at this time point and ELQ based on the MCDI (r = −.27, p >.05). A nonsignificant correlation at the peri-implant interval was also shown for the PLS–3. At 1 year following initial stimulation, the association between ELQ and age at initial stimulation was strong and now significant for both measures (MCDI: r = −.53, p = .004; PLS–3: r = −.65, p = .0005). After an additional 10 months of implant experience, the association continued to be strong and significant for each measure (MCDI: r = −.56, p < .009; PLS-3: r = −.67, p < .0009). This negative correlation suggests that children who were implanted earlier in life fared better (relative to their normal-hearing peers) than did the children who were implanted later in life. This was further demonstrated by comparing the mean MCDI ELQ scores for the 14 children implanted between 12 and 20 months of age with the MCDI ELQ scores for the 13 children implanted between 21 and 48 months of age (see Table 4). Although the ELQs were lowest for those for the children implanted later in life, at both intervals, this difference only reached statistical significance at the 12-month, post-initial-stimulation interval, t(25) = −2.22, p = .03, d = .856). These results suggest that children implanted at older ages fell behind the children implanted at younger ages, who showed only a small decline in ELQ scores following initial stimulation.
Table 4.
Comparison of the mean, MCDI ELQ scores for the relatively younger and older implantees.
| CA at IS Range | n | Mean ELQ (SD) at Peri-Implant | Mean ELQ (SD) at 12 months |
|---|---|---|---|
| 12–20 | 14 | 86.97 (25.13) | 81.20 (17.42) |
| 21–48 | 13 | 82.10 (17.42) | 67.08 (15.51) |
Note. IS = date of initial stimulation of CI; CA at IS range = range of chronological ages at initial stimulation, in months; mean ELQ (SD) at peri-implant = mean ELQ and standard deviation during the peri-imlant interval; mean ELQ (SD) at 12 months = mean ELQ and standard deviation during the 12 months following initial stimulation.
Growth Curve Analysis
As noted in the introduction, if early cochlear implantation is to allow the young child to exploit a sensitive period it should affect the growth slope since growth rate is an indicator of learning efficiency. In order to test for the impact of earlier implantation on expressive language growth rates, the data were subjected to a growth curve analysis using hierarchical linear modeling (HLM; Raudenbush, Bryk, Cheong, & Congdon, 2000). This analysis was performed on raw scores equated to be equivalent to the sum of the MCDI and PLS–3 as described earlier in this article. These equated raw scores are shown in Figure 2 for each child at each postimplantation period for which data were available. This figure shows that the pattern of combined expressive language raw-scores changes during the postimplantation periods for each of the participants. This series of individual plots shows that the children's language scores generally improved with advancing age and implant experience. All the plots in this figure used the same scale for the abscissa and ordinate; therefore, they can be accurately compared. First, it can be seen that the rates of growth varied across children and that the slopes decrease with increments in age of initial stimulation. Second, it can be seen that the rates of growth have a somewhat decelerating, nonlinear quality.
Models of Expressive Language Growth
The data in Figure 2 were analyzed using the HLM: 5 software program (Raudenbush et al., 2000). HLM provides a means of estimating the linear growth rate and intercept of each individual, describing the average group values of these parameters (unconditional model), and provides for tests of the association between predictors and individual differences in these predictors. Because the relationship between language scores and postimplant ages was somewhat nonlinear and HLM was modeling linear growth, a log transform on the equivalent language raw scores was performed. As a result, the data could be analyzed using a linear model with a slope and an intercept within the HLM program.
The results of an unconditional model of expressive language growth (regardless of age at initial stimulation) are provided in Table 5. These results indicate that the average expressive language score at the time of implantation, in log transformed units of the combined language raw scores, was 1.83, which is equivalent to an untransformed value of 3.79. Because the scale of the expressive language scores is in standardized raw score units, this value is not informative; however, we know from Tables 2 and 3 that this score is reflective of expressive language quotients in the mid 80s. The unconditional model also revealed a significant linear growth trend. In this case the log transform resulted in a significant negative slope of −.03 points per month, indicating that expressive language was, on average, improving for these children. It is important to note that there was a significant variance component in this growth parameter; therefore, growth rates varied across children in this sample.
Table 5.
Hierarchical linear modeling results for the unconditional model of expressive language change as a linear function of months post-initial-stimulation of the cochlear implant device.
| Fixed Effects | Coefficient | SE | t |
|---|---|---|---|
| Intercept for log (10-expressive raw score) | 1.826 | .046 | 39.1* |
| Intercept for linear slope, post-IS (months) centered at IS | −0.026 | .004 | −6.43* |
| Random Effects | Variance | df | χ 2 | P |
|---|---|---|---|---|
| Intercept | .0521 | 26 | 239.22 | <.001 |
| Time following implantation | .0004 | 26 | 332.35 | <.001 |
| Level-1 | .01 |
Note. IS = initial stimulation; deviance = −72.72. Approximate df for f = 26. Reliability: intercept = .89, slope = .90.
p < .001.
The data were then submitted to a conditional model in which age at initial stimulation was included in the model along with the parameters of intercept and slope. This model provided a test of whether age at initial stimulation was associated with individual differences within these parameters. Table 6 provides the resulting model from this analysis. Age at initial stimulation was significantly associated with both intercept, t(25) = 6.03, p < .001, and linear growth rate, t(25) = 3.16, p < .005, d = .62 (proportion of variance accounted for). With respect to intercept, children who were implanted at younger ages had higher log transformed combined language raw scores, indicating that the untransformed scores for these children would have been lower than those of the children implanted at older ages. Likewise, the slopes for children who were implanted at younger ages were lower than the slopes for children implanted at older ages; thus, the untransformed slopes would be steeper for the children implanted earlier in life versus later in life. When comparing the residual variance for the time following initial stimulation in the unconditional model in Table 5 (residual variance = .00041) and in the conditional model in Table 6 (residual variance = .00035), age of implantation in the model accounted for 14.6% (.00006/.00041) of the variance of the individual differences in expressive language growth rates. Figure 3 provides a three-dimensional plot based on the parameters derived from this analysis using the untransformed, combined language raw scores. This plot clearly shows that the children implanted at a younger ages initially had lower language scores than those children implanted at older ages. The plot also shows that after nearly 3 years of implant experience, the children implanted earlier in life reached higher levels of expressive language proficiency than the children implanted later in life, despite being at least 3 years younger. The differential growth rates associated with age at initial stimulation are reflected in the tilt of the plane resulting in steeper slopes for the earlier implanted children.
Table 6.
Hierarchical linear modeling for a model of expressive language growth conditioned on age at initial stimulation of the cochlear implant device.
| Fixed Effects | Coefficient | SE | t |
|---|---|---|---|
| Intercept for log (10-expressive raw score) | |||
| Intercept | 1.824 | .032 | 58.11** |
| Age at IS | −0.023 | .0038 | 6.03* |
| Intercept for linear slope, post-IS (months) centered at IS | |||
| Intercept | −0.206 | .004 | −6.78** |
| Age at IS | 0.001 | .0004 | 3.159* |
| Random Effects | Variance | df | χ 2 | p |
|---|---|---|---|---|
| Intercept | .02 | 25 | 111.97 | <.0001 |
| Time following implantation | .0004 | 25 | 270.52 | <.0001 |
| Level-1 | .01 |
Note. IS = initial stimulation; deviance = −71.62. Approximate df for f = 25. Reliability: intercept = .768, age at IS = .88.
p < .005.
p < .001.
Figure 3.
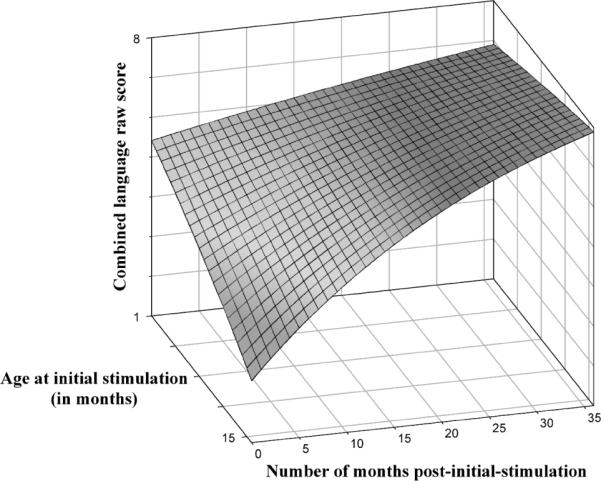
This figure displays a three-dimensional plot of expressive language growth based on the parameters derived from the combined language raw scores. Time is represented on the x-axis and is measured relative to the number of months following initial stimulation of each child's device. The combined, expressive language raw scores are on the y-axis and age at initial stimulation is on the z-axis, where children implanted earlier in life (around 12 months of age) are toward the front and children implanted later in life are toward the back.
Discussion
The present study questioned whether there was evidence of a benefit to expressive language development from efforts at providing CIs during early infancy. Early implantation is challenging because of the complexity of accurate determination of hearing abilities, hearing-aid benefit, as well as the inherent risks of surgery with very young children. Hence, it is important to determine whether there are measurable benefits of early implantation that offset these challenges.
Earlier Implantation Yields Expressive Language Benefits
Early implantation can benefit long-term development in two ways. First, it can shorten the interval of deafness with its associated poorer rate of language development, thus reducing the degree to which a child has fallen behind normal-hearing peers at the time of implantation. This benefit, therefore, would be reflected in the language status at the time of initial stimulation and represents the intercept in postimplantation growth. The data obtained in this study suggested that delays in implantation might have resulted in an ever-increasing gap between the implant candidates and their normal-hearing peers.
Secondly, early implantation can provide benefit for language development by altering the rate of development after initial stimulation. The conditional model of the growth curve analysis provided a direct test of this interaction between age at initial stimulation and language growth rates. This analysis showed that children who were implanted early in childhood had more rapid language growth rates than children implanted later in childhood. In addition, it was found that this relationship had a significant exponential quality such that the relationship between rate of language growth and age at initial stimulation increased as age at initial stimulation decreased. Accordingly, it appears that earlier implantation does provide benefits for expressive language outcomes. We suggest that early implantation provides the child with earlier access to an improved auditory experience. This experience provides the child with access to spoken language at an earlier age, thus facilitating an earlier start in rapid language learning and resulting in a more rapid growth.
The effect of early implantation on improved growth rates is important because alteration of growth rate has a cumulative, multiplicative effect on a child's achievement levels over time. The manner in which the parameters of intercept and slope affect long-term development are very similar to the notions of initial deposit and compound interest rate in a bank account. For example, the earlier start in accelerated spoken language learning due to implantation is equivalent to making an earlier or larger initial deposit on a savings account. That is the deposit sets the effective intercept at a higher level. Steeper growth rates are the same as having a higher interest rate. Over time, variation in the growth rate (interest), even small ones, can have large effects on the overall achievement level (amount saved).
Explaining the Relative Decline in Language Achievement Immediately Following Implantation
The data from this study showed that at the time of initial stimulation, the average language quotient, for the group of children with CIs, was 84 on the MCDI and 89 on the PLS–3—well within the normal range of achievement expected for the group of children with normal hearing of the same age. This index of relative achievement displayed a downward trend over the approximately 24-month period after initial stimulation and was particularly noticeable during the 12 months after initial stimulation. This pattern of decline suggests the expressive language skills of these children are initially similar to children with normal hearing thresholds, but the rates of language growth following CI stimulation do not keep pace with the normal-hearing child within the first years after initial stimulation. The correlation data in Tables 2 and 3, and the comparison of the means shown in Table 4, show that this decline could be attributed to later implantation, which is a conclusion supported by the growth curve analysis.
The conclusions concerning the performance of these children require the caveat that we are comparing the children in this study to normal-hearing children who were administered these language measures in an auditory-speech-only context. In contrast, the children in this study were administered language measures using spoken and signed English. This practice is common in the clinic and in research (Mayne et al., 2000a; Pipp-Siegel et al., 2003; Stallings et al., 2002; Yoshinaga-Itano et al., 1998, 2000); however, it is also the case that this practice does alter the standardized methods of test administration and the relative sensitivity of the measures. We do believe, however, that it is acceptable to conclude that spoken and signed English accomplishment on these tasks (as reflected by a given raw score) can be assumed to be functionally equivalent to the same raw score obtained using oral-speech communication even if the manner of accomplishment was not equivalent in form.
Finally, cochlear implantation in these infants was likely to be followed by a time of transition and acclimation due to the introduction of electrical stimulation to the auditory nerve. It is unclear what the short-term sequelae are of this new auditory stimulus. Secondary effects may include changes in parenting strategies, communication modalities, parental expectations, intervention, or infant responses. Consequently, this transition period may have altered the trajectory of the infants' expressive language development, resulting in the apparent decline in relative performance.
Theoretical Implications
The data from this study indicate that improvements in auditory function in infancy result in more rapid language gains. This would suggest that earlier access to sound alters some fundamental systems involved in language learning in such a way that the long-term trajectory for development is altered. At least three possibilities for such systematic change exist. First, it has long been assumed that language learning depends on neurological systems that are experience-dependent. Thus, these neurological systems require input during a sensitive period early in development in order to retain their functional capacity. The aforementioned study by Sharma and colleagues (2002) corroborated this idea by demonstrating that variation in the age of cochlear implantation results in long-term changes in the morphology of cortical evoked potentials to speech. Therefore, the auditory input provided via earlier implantation might yield improved neurological systems for the support of more efficient language learning.
A second factor could come from the environment. Adults are known to modify their speech to young children by slowing, exaggerating, and simplifying the speech. It is believed by some that these modifications facilitate early language learning (Snow & Ferguson, 1977). It is less clear whether parents of older children who are delayed in language development exhibit these modifications in their speech and instead appear to adopt more directive and corrective language.
Applicability of the Present Results
The present study is the first of its kind to demonstrate the beneficial effect of earlier implantation on expressive language growth in children as young as 12 months of age. In other words, children implanted at 12 months of age had better expressive language skills than children implanted at 15 months of age, children implanted at 15 months of age had better expressive language skills than children implanted at 18 months of age, and so forth.
The present data are certainly compelling as they provide a foundation for understanding the role of a child's age at initial stimulation. This study in particular provides information regarding differential language growth in infants who were implanted as young as 12 months of age. Although the results of this study found that age at initial stimulation accounted for 14.6% of the variance of the individual differences in expressive language growth rates—the earlier implantation occurred, the sooner the children were likely to develop expressive language at a rate commensurate with normal-hearing peers. More research is required, however, before we have an in-depth understanding of all the variables that are likely to be contributing to very young infants' success with their CIs. Future research directions could include examining factors that may be tightly linked to the age at which a CI recipient's device is initially stimulated. To begin with, this study demonstrated an advantage of early initial stimulation in a very general area of inquiry—expressive language. However, future studies could further explore more specific aspects of language, such as phonology, morphology, syntax, and semantic development. Additional studies could examine the affect of early implantation on factors outside of the field of language. For example, are formally educated parents likely to begin the implant process for their children with SNHL much earlier than the parents who have less formal education? Are children with SNHL and no additional disabilities more likely to be implanted earlier than children with SNHL seeking immediate treatment for multiple disabilities? Another line of research could include a comparison of these young implantees' language outcomes and speech perception outcomes. Finally, it is important to note that the current data were based on infants receiving their implant at or after 12 months of age. We do not believe that these results can be extrapolated to infants receiving implants before 12 months of age. Carefully designed research with this population is needed in this regard.
To conclude, the results of this article highlight the importance of the National Early Hearing Detection and Intervention Program. When an infant is identified at birth as having profound SNHL, intervention can begin soon after the child leaves the birthing hospital. Many activities accompany this intervention, such as family education, family grieving, family acceptance, infant hearing-aid fittings, completing a reasonable hearing-aid trial, and measuring hearing-aid benefit. All of these preliminary activities become telescoped into a very short time interval as the age of cochlear implantation is moved downward. Implantation during infancy ultimately “speeds up” the pre-implant, decision-making process. However, it is important to acknowledge that it is unclear at this point whether this early expressive language advantage will be robust later in these children's lives. Thus, when faced with the decision to implant an infant one must consequently continue to weigh the relative, expressive language benefit against the individual needs of each family and the challenges associated with the clinical management of very young children.
Acknowledgments
This research was supported in part by Research Grant 2 P50 DC00242 from the National Institutes on Deafness and Other Communication Disorders; by Grant RR00059 from the General Clinical Research Centers Program, Division of Research Resources, National Institutes of Health; by the Lions Clubs International Foundation; and by the Iowa Lions Foundation. We would like to thank Jillian K. Evans, Courtney M. Smith, Meaghan McCavitt, Cynthia J. Bergan, Victoria C. Klein, and Elon Parker for their help with scoring the MCDI and PLS–3 test items. We would also like to thank all of the infants and their families who have volunteered so much of their time to the University of Iowa Children's Cochlear Implant Program.
Portions of this article were presented at the 23rd Annual Symposium on Research in Child Language Disorders, Madison, WI, July 2002, and at the 7th International Cochlear Implant Conference, Manchester, England, September 2002.
References
- Blamey PJ. Relationships among speech perception, production, language, hearing loss, and age in children with impaired hearing. Journal of Speech, Language, and Hearing Research. 2001;44:264–285. doi: 10.1044/1092-4388(2001/022). [DOI] [PubMed] [Google Scholar]
- Blamey P, Sarant J. Speech perception and language criteria for paediatric cochlear implant candidature. Audiology & Neuro-Otology. 2000;7:114–121. doi: 10.1159/000057659. [DOI] [PubMed] [Google Scholar]
- Brasel KE, Quigley SP. Influence of certain language and communication environments in early childhood on the development of language in deaf individuals. Journal of Speech and Hearing Research. 1977;20:95–107. doi: 10.1044/jshr.2001.95. [DOI] [PubMed] [Google Scholar]
- Brinton J. Measuring language development in deaf children with cochlear implants. International Journal of Language and Communication Disorders. 2001;36(Suppl.):121–125. doi: 10.3109/13682820109177870. [DOI] [PubMed] [Google Scholar]
- Bruer J. A critical and sensitive primer. In: Bailey D, Bruer J, Symons F, Lichtman J, editors. Critical thinking about critical periods. Brookes; Baltimore: 2001. pp. 3–26. [Google Scholar]
- Carney AE, Moeller MP. Treatment efficacy: Hearing loss in children. Journal of Speech, Language, and Hearing Research. 1998;34:565–571. doi: 10.1044/jslhr.4101.s61. [DOI] [PubMed] [Google Scholar]
- Connor CM, Hieber S, Arts HA, Zwolan TA. Speech, vocabulary, and the education of children using cochlear implants: Oral or total communication. Journal of Speech, Language, and Hearing Research. 2000;43:1185–1204. doi: 10.1044/jslhr.4305.1185. [DOI] [PubMed] [Google Scholar]
- Crocker L, Algina J. Introduction to classical and modern test theory. Holt, Rinehart and Winston; Fort Worth, TX: 1986. [Google Scholar]
- Dale PS. The validity of a parent report measure on vocabulary and syntax at 24 months. Journal of Speech and Hearing Research. 1991;34:565–571. doi: 10.1044/jshr.3403.565. [DOI] [PubMed] [Google Scholar]
- Dale PS, Bates E, Reznick JS, Morisset C. The validity of a parent report instrument of child language at twenty months. Journal of Child Language. 1989;16:239–249. doi: 10.1017/s0305000900010394. [DOI] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers' voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- DeCasper AJ, Lecanuet J-P, Busnel M-C, Granier-Deferre C. Fetal reactions to recurrent maternal speech. Infant Behavior and Development. 1994;17:159–164. [Google Scholar]
- El-Hakim H, Levasseur J, Papsin BC, Panesar J, Mount RJ, Stevens D, et al. Assessment of vocabulary development in children after cochlear implantation. Archives of Otolaryngology–Head & Neck Surgery. 2001;127:1053–1059. doi: 10.1001/archotol.127.9.1053. [DOI] [PubMed] [Google Scholar]
- El-Hakim H, Papsin BC, Mount RJ, Levasseur J, Panesar J, Stevens D, et al. Vocabulary acquisition rate after pediatric cochlear implantation and the impact of age at implantation. International Journal of Pediatric Otorhinolaryngology. 2001;59:187–194. doi: 10.1016/s0165-5876(01)00481-5. [DOI] [PubMed] [Google Scholar]
- Fryauf-Bertschy H, Tyler R, Kelsay D, Gantz B, Woodworth G. Cochlear implant use by prelingually deafened children: The influences of age at implant and length of device use. Journal of Speech, Language, and Hearing Research. 1997;40:183–199. doi: 10.1044/jslhr.4001.183. [DOI] [PubMed] [Google Scholar]
- Garretson MD. Total communication. In: Frisna R, editor. A bicentennial monograph on hearing impairment: Trends in the USA. A. G. Bell Association; Washington, DC: 1976. pp. 88–95. [Google Scholar]
- Geers AE. Factors affecting the development of speech, language, and literacy in children with early cochlear implantation. Language, Speech, and Hearing Services in the Schools. 2002;33:172–183. doi: 10.1044/0161-1461(2002/015). [DOI] [PubMed] [Google Scholar]
- Geers AE. Predictors of reading skill development in children with early cochlear implantation. Ear & Hearing. 2003;24:59S–68S. doi: 10.1097/01.AUD.0000051690.43989.5D. [DOI] [PubMed] [Google Scholar]
- Geers AE, Brenner C, Davidson LS. Factors associated with development of speech perception skills in children implanted by age five. Ear & Hearing. 2003;24:24S–35S. doi: 10.1097/01.AUD.0000051687.99218.0F. [DOI] [PubMed] [Google Scholar]
- Geers AE, Brenner C, Nicholas J, Uchanski R, Tye-Murray N, Tobey E. Rehabilitation factors contributing to implant benefit in children. Annals of Otolaryngology, Rhinology, and Laryngology. 2002;111:127–130. doi: 10.1177/00034894021110s525. [DOI] [PubMed] [Google Scholar]
- Geers A, Moog J. Spoken language results: vocabulary, syntax, and communication. Volta Review. 1994;96:131–148. [Google Scholar]
- Geers AE, Nicholas JG, Sedey AL. Language skills of children with early cochlear implantation. Ear & Hearing. 2003;24:46S–58S. doi: 10.1097/01.AUD.0000051689.57380.1B. [DOI] [PubMed] [Google Scholar]
- Hammes DM, Novak MA, Rotz LA, Willis M, Edmondson DM, Thomas JF. Early identification and cochlear implantation: Critical factors for spoken language development. Annals of Otology, Rhinology, and Laryngology–Supplement. 2002;189:74–78. doi: 10.1177/00034894021110s516. [DOI] [PubMed] [Google Scholar]
- Hodges A, Ash MD, Balkany T, Schloffman J. Speech perception results in children with cochlear implants: Contributing factors. Otolaryngology–Head and Neck Surgery. 1999;121:31–34. doi: 10.1016/S0194-5998(99)70119-1. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. Journal of Neurophysiology. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Hubel D, Wiesel T. Binocular interaction in striate cortex of kittens reared with artificial squint. Journal of Neurophysiology. 1965;28:1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Ireton H, Thwing E. Minnesota Child Development Inventory. University of Minnesota; Minneapolis, MN: 1974. [Google Scholar]
- Kirk KI, Miyamoto RT, Lento CL, Ying E, O'Neill T, Fears B. Effects of age at implantation in young children. Annals of Otology, Rhinology, and Laryngology–Supplement. 2002;189:69–73. doi: 10.1177/00034894021110s515. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer-specific manner. Cerebral Cortex. 2000;10:714–726. doi: 10.1093/cercor/10.7.714. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: Central auditory plasticity and sensory deprivation. Cerebral Cortex. 2002;12:797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Miller JD. Discrimination of auditory target dimensions in the presence or absence of variation in a second dimension by infants. Perception & Psychophysics. 1982;31:279–292. doi: 10.3758/bf03202536. [DOI] [PubMed] [Google Scholar]
- Lederberg AR, Spencer PE. Vocabulary development of deaf and hard of hearing children. In: Clark MD, Marschark M, editors. Context, cognition, and deafness. Gallaudet University Press; Washington, DC: 2001. pp. 88–112. [Google Scholar]
- Manrique M, Cervera-Paz FJ, Huarte A, Perez N, Molina M, Garcia-Tapia R. Cerebral auditory plasticity and cochlear implants. International Journal of Pediatric Otorhinolaryngology. 1999;49:S193–S197. doi: 10.1016/s0165-5876(99)00159-7. [DOI] [PubMed] [Google Scholar]
- Mayne AM, Yoshinaga-Itano C, Sedey AL. Expressive vocabulary development of infants and toddlers who are deaf or hard of hearing. Volta Review. 2000a;100:1–28. [Google Scholar]
- Mayne AM, Yoshinaga-Itano C, Sedey AL. Receptive vocabulary development of infants and toddlers who are deaf or hard of hearing. Volta Review. 2000b;100:29–52. [Google Scholar]
- Mehler J, Jusczyk PW, Lambertz G, Halsted N, Bertoncini J, Amiel-Tison C. A precursor of language acquisition in young infants. Cognition. 1988;29:143–178. doi: 10.1016/0010-0277(88)90035-2. [DOI] [PubMed] [Google Scholar]
- Miller GL, Knudsen EI. Adaptive plasticity in the auditory thalamus of juvenile barn owls. Journal of Neuroscience. 2003;23:1059–1065. doi: 10.1523/JNEUROSCI.23-03-01059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, Panneton-Cooper R, Fifer WP. Two-day-olds prefer their native language. Infant Behavior and Development. 1993;16:494–500. [Google Scholar]
- National Center for Hearing Assessment and Management Universal newborn hearing screening. n.d. Retrieved October 10, 2005, from http://www.infanthearing.org/screening/index.html.
- Neville HJ, Bavelier D, Corina D, Rauschecker J, Karni A, Lalwani A, et al. Cerebral organization for language in deaf and hearing subjects: Biological constraints and effects of experience. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:922–929. doi: 10.1073/pnas.95.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AJ, Bavelier D, Corina D, Jezzard P, Neville HJ. A critical period for right hemisphere recruitment in American Sign Language processing. Nature Neuroscience. 2002;5:76–80. doi: 10.1038/nn775. [DOI] [PubMed] [Google Scholar]
- Newport EL. Maturational constraints on language learning. Cognitive Science. 1990;14:11–28. [Google Scholar]
- Osberger MJ, Zimmerman-Phillips S, Koch DB. Cochlear implant candidacy and performance trends in children. Annals of Otolaryngology, Rhinology, and Laryngology. 2002;111:62–65. doi: 10.1177/00034894021110s513. [DOI] [PubMed] [Google Scholar]
- Ouellet C, Le Normand M-T, Cohen H. Language evolution in children with cochlear implants. Brain & Cognition. 2001;46:231–235. doi: 10.1016/s0278-2626(01)80073-7. [DOI] [PubMed] [Google Scholar]
- Pipp-Siegel S, Sedey AL, VanLeeuwen AM, Yoshinaga-Itano C. Mastery motivation and expressive language in young children with hearing loss. Journal of Deaf Studies and Deaf Education. 2003;8:133–145. doi: 10.1093/deafed/eng008. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Don M, Eggermont JJ, Waring MD, Kwong B, Masuda A. Auditory system plasticity in children after long periods of complete deafness. NeuroReport. 1996;8:61–65. doi: 10.1097/00001756-199612200-00013. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Moore W, Eggermont JK. Prolonged deafness limits auditory system developmental plasticity: Evidence from an evoked potentials study in children with cochlear implants. Scandinavian Audiology. 1999;28:13–22. [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Cheong Y, Congdon R. HLM 5: Hierarchical Linear and Nonlinear Modeling [Computer software] Scientific Software International; Chicago, IL: 2000. [Google Scholar]
- Richter B, Eissele S, Laszig R, Lohle E. Receptive and expressive language skills of 106 children with a minimum of 2 years' experience in hearing with a cochlear implant. International Journal of Pediatric Otorhinolaryngology. 2002;64:111–125. doi: 10.1016/s0165-5876(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Robbins AM, Osberger MJ, Miyamoto RT, Kessler KS. Language development in young children with cochlear implants. Advances in Oto-Rhino-Laryngology. 1995;50:160–166. doi: 10.1159/000424453. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear & Hearing. 2002;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; London: 2003. [Google Scholar]
- Snow C, Ferguson CA. Talking to children: Language input and acquisition. Cambridge University Press; Cambridge, MA: 1977. [Google Scholar]
- Spencer PE, Meadow-Orlans KP. Play, language, and maternal responsiveness: A longitudinal study of deaf and hearing infants. Child Development. 1996;67:3176–3191. [PubMed] [Google Scholar]
- Stallings L, Gao S, Svirsky M. Assessing the language abilities of pediatric cochlear implant users across a broad range of ages and performance abilities. Volta Review. 2002;102:215–235. [Google Scholar]
- Svirsky MA. Outcomes of pediatric cochlear implantation as a function of age at implant. Paper presented at the annual convention of the American Speech-Language-Hearing Association; Chicago. 2003, November 13–15. [Google Scholar]
- Svirsky MA, Robbins AM, Kirk KI, Pisoni DB, Miyamoto RT. Language development in profoundly deaf children with cochlear implants. Psychological Science. 2000;11:153–158. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey EA, Geers AE, Brenner C, Altuna D, Gabbert G. Factors associated with development of speech production skills in children implanted by age five. Ear & Hearing. 2003;24:36S–45S. doi: 10.1097/01.AUD.0000051688.48224.A6. [DOI] [PubMed] [Google Scholar]
- Tobey EA, Geers AE, Douek BM, Perrin J, Skellet R, Brenner C, et al. Factors associated with speech intelligibility in children with cochlear implants. Annals of Otolaryngology, Rhinology, and Laryngology–Supplement. 2000;185:28–30. doi: 10.1177/0003489400109s1212. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Shonrock C, Hardy J. The concurrent validity of the Minnesota Child Development Inventory as a measure of young children's language development. Journal of Speech and Hearing Disorders. 1989;54:101–105. doi: 10.1044/jshd.5401.101. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Spencer L, Flock S, Tyler R, Gantz B. A comparison of language achievement in children with cochlear implants and children using hearing aids. Journal of Speech, Language, and Hearing Research. 1999;42:497–511. doi: 10.1044/jslhr.4202.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye-Murray N, Spencer L, Woodworth GG. Acquisition of speech by children who have prolonged cochlear implant experience. Journal of Speech and Hearing Research. 1995;38:327–337. doi: 10.1044/jshr.3802.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. Journal of Neurophysiology. 1963;26:1002–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wiesel T, Hubel D. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. Journal of Neurophysiology. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wright M, Purcell A, Reed VA. Cochlear implants and infants: Expectations and outcomes. Annals of Otology, Rhinology, and Laryngology–Supplement. 2002;189:131–137. doi: 10.1177/00034894021110s526. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998;102:1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Snyder LS, Day D. The relationship of language and symbolic play in children with hearing loss. Volta Review. 2000;100:135–164. [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale—3. The Psychological Corporation; San Antonio, TX: 1992. [Google Scholar]


