Abstract
Purpose
This study characterized the development of speech sound production in prelingually deaf children with a minimum of 8 years of cochlear implant (CI) experience.
Method
Twenty-seven pediatric CI recipients' spontaneous speech samples from annual evaluation sessions were phonemically transcribed. Accuracy for these speech samples was evaluated in piecewise regression models.
Results
As a group, pediatric CI recipients showed steady improvement in speech sound production following implantation, but the improvement rate declined after 6 years of device experience. Piecewise regression models indicated that the slope estimating the participants' improvement rate was statistically greater than 0 during the first 6 years postimplantation, but not after 6 years. The group of pediatric CI recipients' accuracy of speech sound production after 4 years of device experience reasonably predicts their speech sound production after 5–10 years of device experience.
Conclusions
The development of speech sound production in prelingually deaf children stabilizes after 6 years of device experience, and typically approaches a plateau by 8 years of device use. Early growth in speech before 4 years of device experience did not predict later rates of growth or levels of achievement. However, good predictions could be made after 4 years of device use.
Keywords: cochlear implants, speech development, speech production, speech sound, plateau
Prelingually deaf (born deaf or become deaf before age 3) children who are users of hearing aids or tactile aids exhibit delayed or disordered development in speech production (e.g., Bamford & Saunders, 1991; Geers, Moog, & Schick, 1984; Levitt, McGarr, & Geffner, 1987). However, a substantial body of research has demonstrated that children who are prelingually deaf are able to develop speech production skills with the use of a cochlear implant (CI; Blamey, Barry, & Jacq, 2001; Geers, 1997; Geers & Tobey, 1995; Miyamoto, Kirk, Robbins, Todd, & Riley, 1996; Osberger, Maso, & Sam, 1993; Peng, Spencer, & Tomblin, 2004; Serry & Blamey, 1999; Serry, Blamey, & Grogan, 1997; Spencer, Tye-Murray, & Tomblin, 1998; Svirsky & Chin, 2000; Tobey & Geers, 1995; Tobey, Geers, Brenner, Altuna, & Gabbert, 2003; Tye-Murray & Kirk, 1993; Tye-Murray, Spencer, & Woodworth, 1995). Pediatric CI recipients show higher accuracy in producing speech contrasts than their age-matched peers with comparable sensorineural hearing impairments (at the severe-profound range) who did not receive an implant. These individuals also demonstrate significant improvement in speech sound production, in terms of accuracy and overall speech intelligibility (i.e., how well the speaker can be understood) following cochlear implantation.
Studies of speech development in the pediatric CI population have demonstrated noteworthy benefits with the use of a CI in terms of speech sound production and speech intelligibility during the initial several years of device use (e.g., Blamey, Barry, & Jacq, 2001; Blamey, Barry, Bow, et al., 2001; Chin, Tsai, & Gao, 2003; Miyamoto et al., 1996). However, the ultimate level of achievement possible is unclear, and researchers do not know whether there is a point in time at which further improvement becomes impossible. Blamey and colleagues, for example, assessed the phonetic inventory development in a group of nine pediatric CI recipients and reported systematic improvement in phonetic inventory development over the first 4 years of device use (Blamey, Barry, & Jacq, 2001). On the other hand, phonetic inventory development appeared to decrease between the 5th and 6th years postimplantation. The findings from Blamey, Barry, and Jacq (2001) suggested that there may be constraints on the development of speech sound production in pediatric CI recipients with extended device experience. That is, following 6 years of device experience, their CI participants' mean chronological age was 9.75 years. This age is older than the age at which most children with normal hearing (NH) master speech sound production (i.e., phonetic inventory of their native language). Thus, the loss of plasticity in the nervous system as children grow in age may result in the reduced improvement rate in these individuals' phonetic inventory development. Blamey and colleagues attributed this slowing rate in speech development to the effect of a sensitive period associated with pediatric CI recipients' chronological age and age at implantation (Blamey, Barry, & Jacq, 2001).
Interestingly, in another study on the same group of 9 pediatric CI recipients by Blamey and colleagues (Blamey, Barry, Bow, et al., 2001), the findings regarding the existence of a plateau in speech sound development appeared to be contradictory to the conclusion presented in Blamey, Barry, and Jacq (2001). That is, Blamey, Barry, and Jacq (2001) reported a plateau at the 6th year post-implantation, whereas Blamey, Barry, Bow, et al. (2001) reported no plateau. The discrepancy in findings is likely attributable to different analysis approaches. The plateau shown in Figure 6 of Blamey, Barry, and Jacq (2001) was based on a 50% correct criterion adopted to define whether the child had “acquired” a particular phone production. On the other hand, Figure 3 of Blamey, Barry, Bow, et al. (2001) reveals no evident plateau for the group of pediatric CI recipients during the first 6 years postimplantation. Percentages of correct scores, rather than a 50% correct criterion, were used in this set of analyses. In addition to the different conclusions of these two studies, some aspects of these studies were also different. For example, Blamey, Barry, and Jacq (2001) investigated the phonetic inventory (i.e., phone/phoneme production) of pediatric CI recipients, whereas Blamey, Barry, Bow, et al. (2001) investigated phoneme and word production, as well as speech intelligibility, in these individuals. To achieve the study purpose, narrow transcription was adopted by Blamey, Barry, and Jacq (2001), whereas broad transcription was adopted in Blamey, Barry, Bow, et al. (2001).
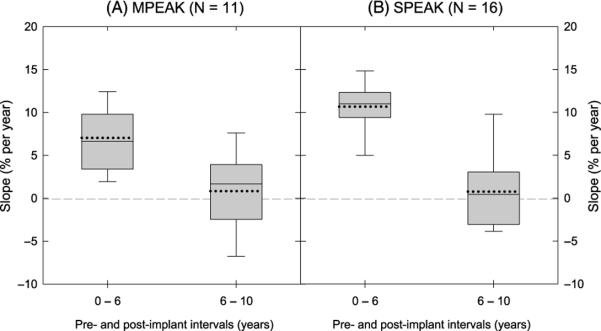
Figure 3.
Distributions of the slopes estimating the pediatric CI recipients' improvement rates of speech sound production from 0–6 to 6–10 years postimplantation. Panels A and B display the slopes estimating the improvement rate for the participants in the MPEAK and SPEAK subgroups, respectively. The x-axis displays the preimplant and postimplant annual sessions. The y-axis displays the slopes; that is, the improvement rate of accuracy in speech sound production (percentage correct). See caption to Figure 1 for details of symbols in the plot. MPEAK = multipeak; SPEAK = spectral peak.
There is a need to assess the developmental trajectories of speech sound production in order to reconcile whether there is an eventual plateau in speech sound development after receiving a CI. In a longitudinal study, Miyamoto et al. (1996) examined speech intelligibility of prelingually deaf children with a CI, indicating improvement in speech intelligibility over time following implantation. These authors reported that pediatric CI recipients' improvement in speech intelligibility does not reach a plateau with up to 5 years of device experience. Similarly, Chin and colleagues suggested that with 5 to 6 years of device experience, there is no asymptotic pattern with regard to speech intelligibility (Chin et al., 2003). In another study, Blamey, Barry, Bow, et al. (2001) suggested no plateau in speech sound production during the initial 6 years postimplantation. These authors suggest a need to further investigate the speech sound development beyond 6 years of CI experience, in order to determine whether the children will continue to improve until they reach near-normal performance levels.
It is unclear whether the growth can continue with extended device experience (i.e., if a plateau will emerge at some point in time, for example, after 6 years post-implantation or by 8 years of age). On the other hand, children with NH demonstrate mastery of producing speech sound contrasts in their native language between 4 and 8 years of age (Menyuk, 1972; Shriberg, Gruber, & Kwiatkowski, 1994). After 8 years of age, persisting speech errors are assumed by Shriberg and colleagues to be unambiguously abnormal as continued developmental growth is unexpected. It would be useful to determine if pediatric CI recipients' speech sound development typically approaches a similar point at which subsequent growth is not expected. That is, knowing the timing and pattern of growth in speech sound production will assist in the planning of speech intervention for pediatric CI recipients who are prelingually deaf. Additionally, we may further relate this plateau (if any) to certain participant-related variables (e.g., age at implantation, communication mode) or device-related variables (e.g., speech-coding strategy). Lastly, within the clinical setting, it is important to know the degree to which long-term outcomes of speech sound production may be predictable based on pediatric CI recipients' mastery levels of speech sound production at earlier stages post-implantation. In other words, there may be a time point when individual differences in speech sound development stabilize, and, hence, reliable predications about the long-term status of pediatric CI recipients' speech sound development may be made.
Our characterization of the long-term growth of speech sound development in pediatric CI recipients has theoretical implications. If a plateau is shown, it is important to consider what might constrain development. Traditional accounts of critical periods in speech development often use some form of maturational processes as first proposed by Lenneberg (1967). Such mechanisms are driven by chronological-age-based processes. There-fore, one should expect to see plateaus in CI recipients associated with chronological age regardless of their age of implantation. In contrast, there are accounts of critical or sensitive periods that are based on the dynamics of learning (Munakata & McClelland, 2003). In these accounts, constraints on learning at any point in time are the product of prior learning history rather than experience independent biological processes. Thus, if a plateau is found, it should be related to the length of experience rather than the age of the child.
The purpose of this study was to characterize the development of speech sound production in prelingually deaf children with extended CI experience (i.e., 8–10 years). Specifically, the issue regarding whether there is a plateau in the development of speech sound production following 6 years of device experience was addressed. Moreover, this study examined the extent to which we could predict pediatric CI recipients' eventual accuracy in speech sound production by their early performance postimplant performance.
Method
Participants
Twenty-seven (14 males and 13 females) children and young adults who are CI recipients in the longitudinal cohort of the Iowa Children's Cochlear Implant Project served as participants in this study. These individuals were all prelingually deaf and native speakers of American English. They all underwent surgery and follow-up assessments of their auditory, speech, and language development at the University of Iowa Hospital and Clinics. To be included in this study, the participant must have had a minimum of 8 years of device experience and had speech samples available from two or more annual evaluation sessions from between both preimplant 6 years postimplantation and 6–10 years postimplantation. These criteria were adopted to permit evaluations of the improvement rates of speech sound production across the annual evaluation sessions. All participants and their parents signed the consent and/or assent documents approved by the University of Iowa Institutional Review Board.
The participants' age at implantation ranged from 2.58 to 7.44 years (M = 4.57 years). All participants received the Nucleus 22 device (Cochlear Americas). Among these participants, 16 were mapped with the spectral-peak (SPEAK) speech-coding strategy, and 11 were mapped with the multipeak (MPEAK) strategy during the most recent annual evaluation session when they had speech samples available. There was no statistically significant difference in the average age at implantation between the two subgroups of the users mapped with the MPEAK and SPEAK strategies (M = 4.94 years, SD = 1.32 years for the MPEAK users; M = 4.32 years, SD = 1.12 years for the SPEAK users), t(25) = 1.322, p = .198.
Among the 27 participants, 2 (CI-25 and CI-26) received their education in a mainstream public school setting where only spoken English was used as the instructional language (oral communication [OC]). All the other participants (n = 25) received their education in a mainstream public school setting where both spoken English and signing exact English were used (total communication [TC]). Classification of communication methods (OC or TC) was based on parental reports, which was confirmed by the educational setting of the participant during the most recent annual evaluation session when the participant's speech sample was available. Table 1 provides a summary of the 27 participants' biographical and audiological information.
Table 1.
The 27 CI participants' biographical and audiological information.
| Preimplant pure-tone thresholds of the better ear (dB HL) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Gender | Etiology of deafness | Implanted ear | Age at implantation (years) | Coding strategy | 500Hz | 1000Hz | 2000Hz | Comm. mode | Samples available |
| CI-1 | Male | Unknown | Left | 4.75 | MPEAK | NR | NR | 110 | TC | 8 |
| CI-2 | Female | Genetic | Right | 4.53 | MPEAK | 110 | 120 | 120 | TC | 5 |
| CI-3 | Male | Meningitis | Left | 3.52 | SPEAK | NR | NR | NR | TC | 8 |
| CI-4 | Female | Unknown | Left | 3.89 | MPEAK | 105 | NR | NR | TC | 6 |
| CI-5 | Female | Unknown | Right | 6.72 | MPEAK | 75 | 110 | NR | TC | 8 |
| CI-6 | Female | Unknown | Right | 5.03 | MPEAK | 110 | NR | NR | TC | 8 |
| CI-7 | Male | Meningitis | Right | 3.82 | SPEAK | 100 | 120 | NR | TC | 9 |
| CI-8 | Female | Unknown | Right | 4.24 | SPEAK | 95 | 105 | NR | TC | 5 |
| CI-9 | Female | Unknown | Right | 5.43 | MPEAK | 100 | 110 | 115 | TC | 6 |
| CI-10 | Male | Genetic | Left | 2.88 | MPEAK | 85 | 95 | NR | TC | 8 |
| CI-11 | Female | Unknown | Left | 3.53 | SPEAK | 115 | NR | NR | TC | 7 |
| CI-12 | Male | Meningitis | Left | 5.58 | SPEAK | 105 | 105 | 105 | TC | 8 |
| CI-13 | Female | Meningitis | Left | 5.16 | SPEAK | 115 | NR | NR | TC | 8 |
| CI-14 | Male | Unknown | Left | 5.55 | MPEAK | 95 | NR | NR | TC | 9 |
| CI-15 | Female | Unknown | Right | 4.84 | SPEAK | 100 | 110 | 110 | TC | 10 |
| CI-16 | Male | Meningitis | Right | 2.58 | SPEAK | 90 | 105 | 100 | TC | 8 |
| CI-17 | Male | Waardenburg's | Left | 4.52 | SPEAK | 95 | 100 | NR | TC | 10 |
| CI-18 | Female | Unknown | Left | 4.38 | MPEAK | 110 | 120 | NR | TC | 8 |
| CI-19 | Male | Genetic | Right | 2.74 | SPEAK | 110 | 115 | NR | TC | 9 |
| CI-20 | Female | CMV | Left | 3.74 | MPEAK | NR | NR | NR | TC | 11 |
| CI-21 | Female | Unknown | Left | 7.44 | MPEAK | 90 | 110 | NR | TC | 8 |
| CI-22 | Male | Unknown | Right | 3.92 | SPEAK | 90 | NR | NR | TC | 6 |
| CI-23 | Male | Genetic | Right | 3.57 | SPEAK | 105 | 115 | 110 | TC | 8 |
| CI-24 | Male | Genetic | Right | 3.39 | SPEAK | 105 | NR | NR | TC | 10 |
| CI-25 | Male | Genetic | Left | 6.35 | SPEAK | 85 | 100 | 95 | OC | 8 |
| CI-26 | Male | Unknown | Right | 5.55 | SPEAK | 90 | 110 | NR | OC | 10 |
| CI-27 | Female | Unknown | Left | 5.75 | SPEAK | 105 | 110 | NR | TC | 7 |
Note. CI = cochlear implant; NR = no response at the output limits (110dB HL at 500Hz, 115dB HL at 1000Hz, and 115dB HL at 2000Hz) of the pure-tone audiometer; Comm mode = communication mode; MPEAK = multipeak; SPEAK = spectral peak; TC = total communication; OC = oral communication; CMV = cytomegalovirus.
Speech Samples
The CI participants contributed a total of 223 samples across all pre- and postimplant annual evaluation sessions. Each participant contributed 5–11 speech samples (M = 7.96) across all sessions (see Table 1, last column for details). The spontaneous speech production samples of each CI participant were elicited using a story–retell task. Detailed descriptions of this task can be found in Tye-Murray et al. (1995) and Tye-Murray (1998). In this task, the examiner described a set of four pictures in a series following a script, using simultaneous communication with spoken English and signing exact English. The participant was instructed to retell the story using oral speech (and sign, if preferred). This procedure was repeated until the participant completed retelling stories with all six sets of pictures, or until he or she was unable to complete the task because of young age or unwillingness to continue. If the participant did not know how to retell a story, the examiner would raise questions such as “What did (the boy) do?” or say “Tell me something about (the boy)” to the participant. Each session was video- and audiotaped onto a videotape, using the Panasonic AG 1330B videotaping system coupled to a Tamron 69YE Zoom lens camera through a Telex WT-700 microphone. This microphone was attached to the participant's clothing about 4.5 in. below his or her mouth. The task was always performed in a quiet testing room (ambient noise level = 40 dB SPL; long-term averaged level, A-weighting).
Transcription and Reliability
The speech samples were phonemically glossed for the intended words based on the participant's signed and spoken utterances, in addition to the contexts of the stories by a certified speech-language pathologist (the primary examiner). Of each sample, only the initial 100 spoken words were phonemically transcribed in order to approximately equalize sample size across participants. On average, there were 261.47 phonemes (ranging from 220.40 to 297.00 phonemes among all participants) averaged across all annual sessions when there were speech samples available. A comparison of the phonemic transcription and the gloss yielded a percentage correct score1 (i.e., accurate number of phonemes produced in proportion to the number of glossed phonemes) that served as the index of mastery of speech sound production (Tye-Murray et al., 1995). Of note, phoneme accuracy, rather than target word accuracy, was analyzed. This is because our primary interest was to assess the performance in speech sound production.
To demonstrate insignificant transcriber bias, inter-judge reliability measures were performed with 26 speech samples that were randomly selected among all CI participants and across all annual evaluation sessions. These selected speech samples constituted 11.66% of total available samples. The measures were completed by two research assistants (designated as Transcribers A and B; both had taken a course in English phonetics prior to transcription). Both transcribers were native speakers of American English and were familiar with the speech of children with hearing impairments. Point-by-point phoneme accuracy was assessed for each speech sample following training, using a small set of speech samples of pediatric CI recipients who were not participants in this study. The average interjudge agreements between the primary examiner (the speech-language pathologist; see previous paragraph) and the Transcribers A and B were 78.58% (SD = 7.04%) and 79.05% (SD = 8.87%), respectively, across the selected samples.2
The observed interjudge reliability, although acceptable, was not great. It has been documented that typical interjudge reliability for phonemic transcription can range from mid-60s to mid-high-90s (Shriberg & Lof, 1991). Investigating the speech sound development of 9 pediatric CI recipients who shared similar participant characteristics with those in the present study, Blamey, Barry, Bow, et al. (2001) reported 72% interrater agreement. Blamey, Barry, Bow, et al. (2001) indicated that this low agreement is likely related to the fact that as the accuracy of phoneme production decreases, the transcription agreement also decreases (Shriberg & Lof, 1991).
Results
The objectives of this study were to characterize the long-term speech sound development of pediatric CI recipients and to assess whether there was a plateau that was characteristic for the group following 6 years of device experience. Figure 1A illustrates the distributions of the 27 pediatric CI recipients' accuracy in speech sound production from the preimplant evaluation session (0 year) to as many as 10 years postimplantation. The total number of speech samples from each annual evaluation session is displayed on the top of each bar, as not all CI participants attended every session and had available speech sound samples across all annual evaluation sessions. As Figure 1A illustrates, the mean accuracy of the group of pediatric CI recipients' speech sound production was 15.51% (SD = 16.12%) prior to implantation, increasing to 62.90% (SD = 20.51%) with 4 years of device experience, 76.28% (SD = 16.24) with 6 years of device experience, and 81.40% (SD = 22.68%) with 10 years of device experience. Although many pediatric CI recipients received high levels of accuracy (e.g., 80% and above) with several years of device experience (i.e., beyond 6 years), these individuals' accuracy of speech sound production, on average, reached the maximal performance level at about 85%. Note that the average improvement rate was 11.85%, 6.69%, and 1.28% per year for the intervals from 0–4, 4–6, and 6–10 years, respectively. The same group of pediatric CI recipients' accuracy in speech sound production was also assessed as a function of these individuals' chronological ages. As Figure 1B displays, the mean accuracy of the group of pediatric CI recipients' speech sound production exhibited steady improvement until these individuals reached 10–12 years of age.
Figure 1.
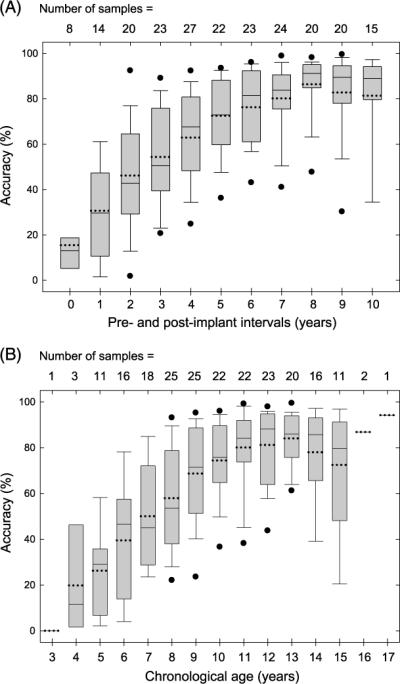
(A): Distributions of the group of pediatric cochlear implant (CI) recipients' speech sound production from 0 year (preimplant) to 10 years (postimplantation). (B): Distributions of the group of pediatric CI recipients' speech sound production as a function of their chronological age. The x-axis displays the preimplant and post-implant annual sessions. The y-axis displays the accuracy in speech sound production (percentage correct). The mean and median are displayed by the dotted and solid lines across each box, respectively. The upper and lower bounds of each box represent the first and third quartiles, the end of the whiskers are located ± 1.25 SD away from the mean, and the filled circles represent the 5th and 95th percentile bounds (if they fall outside of the end of the whisker). The total number of speech samples available from each session is shown on the top of each bar.
The accuracy of speech sound production in each of the annual evaluation sessions was evaluated in relation to the variable and age at implantation by computing the Pearson correlation coefficients (r values) between the accuracy in every annual evaluation session and this variable (age at implantation). The coefficients were found to be statistically significant, with only 2 years of device experience (r = .480, p = .032). The coefficients in all other annual sessions ranged from 6−.434 (with 8 years of device experience, p = .056) to .449 (with 1 year of device experience, p = .107); none were found to be statistically significant (all p values > .05).
Independent sample t tests were performed to evaluate if the difference in average accuracy in each annual evaluation session was significantly different between the subgroups of pediatric CI recipients who were mapped with the MPEAK versus SPEAK speech-coding strategies. The mean accuracy of the SPEAK subgroup was generally higher than that of the MPEAK subgroup, but only reached a statistically significant level (α = .05) with 4 years of device experience, t(25) = 2.334, p = .028, and with 6 years of device experience, t(21) = 2.360, p = .028. With respect to communication modes (TC vs. OC), the two OC users appeared to be among the participants who demonstrated higher accuracy than the group mean accuracy in all annual evaluation sessions when they had speech samples available. They both had mean accuracy higher than 90% across the 6th to 10th year postimplant sessions. Because of the limited number (n = 2) of the OC users, no statistical analyses were performed to evaluate if the accuracy was different in each annual evaluation session between the TC and OC users.
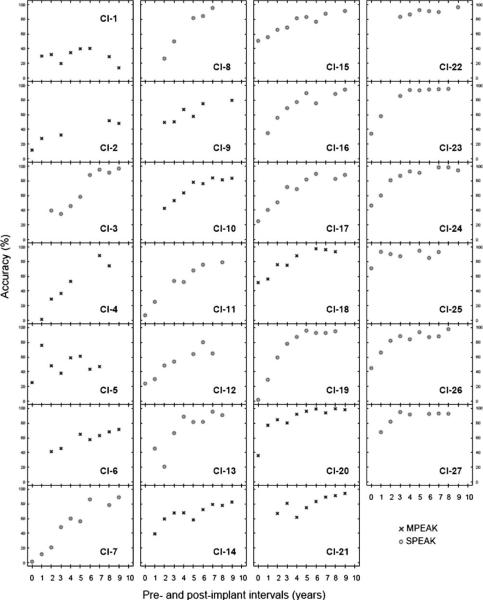
The improvement rates reported earlier were based on group performance. At the individual level, each pediatric CI recipient showed somewhat different developmental patterns in his or her accuracy of speech sound production. To illustrate individual improvement patterns, Figure 2 was plotted to depict the profile of each participant's accuracy in speech sound production as the device experience accrues. Each panel of Figure 2 displays 1 participant's accuracy in speech sound production, as a function of the amount of device experience. Different symbols were used to display which subgroup (SPEAK vs. MPEAK) the participant belonged to (see Figure 2 legends), as the advancement of CI speech-coding strategies has been reported to contribute to interparticipant variability in CI listeners' speech perception or speech production performance (e.g., Peng et al., 2004; Psarros et al., 2002; Skinner et al., 1994; Skinner, Fourakis, Holden, Holden, & Demorest, 1996). With few exceptions (e.g., CI-1), the group of pediatric CI recipients' accuracy in speech sound production exhibited an overall improving trend as their device experience accrued.
Figure 2.
Individual profiles of the participants' development of speech sound production across the preimplant session (0 year) and postimplant annual sessions. The x-axis displays the preimplant and postimplant annual sessions. The y-axis displays the accuracy in speech sound production (percentage correct).
As was noted previously, not all CI participants had speech samples available across the preimplant and annual evaluation sessions (0–10 years). The fact that not all of the CI participants had speech samples available in every evaluation session might have resulted in the decline of the improvement rate as the device experience accrued over the 10-year span. In other words, it was possible that the CI participants with longer device experience were, in fact, systematically poorer users. Hence, growth in the later years was only representing poorer users. This concern was taken into account in the following set of analyses, focusing on the improvement rates of those who had speech samples available during both postimplant periods (i.e., 0–6 and 6–10 years).
To evaluate whether there was a plateau in pediatric CI recipients' speech sound development, a piecewise regression model was fit to assess each participant's accuracy of speech sound production from preimplantation to the 6th year postimplantation and from the 6th to 10th years postimplantation. The two intervals were determined primarily based on the findings in Blamey, Barry, Bow, et al. (2001), where no plateau was identified in the speech sound development of their pediatric CI recipients with the initial 6 years of device experience. In these piecewise regression models, the intercept estimated the participant's accuracy of speech sound production at the time of implantation. If the intercept was less than zero, a piecewise regression model through the origin was fit to the data. Additionally, two estimated slopes were also derived for each participant. One slope estimated the improvement rate (percentage per year) from the preimplant session to the 6th year annual session, and the other estimated the improvement rate between the 6th and 10th years postimplantation.
During the preimplant session to the 6th year post-implantation, the group accuracy showed an average improvement rate at 7.03% (SD = 3.69%) and 10.67% (SD = 3.22%) per year increase of device experience for the users mapped with the MPEAK and SPEAK strategies, respectively (overall = 9.18%, SD = 3.81%). However, between the 6th and 10th years postimplantation, the average improvement rate decreased to 0.84% (SD = 4.56%) and 0.77% (SD = 4.57%) per year increase of device experience for the users mapped with the MPEAK and SPEAK strategies, respectively. One-sample t tests were performed to evaluate if the means of the slope that estimated the improvement rate from 0–6 to 6–10 years postimplantation were significantly different from zero, respectively, for the users of the MPEAK and SPEAK strategies. The results indicated that although the estimated slope was significantly different from zero for both the users mapped with the MPEAK and SPEAK strategies from 0 to 6 years postimplantation—t(10) = 6.327, p < .001 for the MPEAK users; t(15) = 13.246, p < .001 for the SPEAK users—the slope was not statistically different from zero from 6 to 10 years post-implantation, t(11) = 0.609, p = .556 for the MPEAK users; t(15) = 0.671, p = .512 for the SPEAK users. Moreover, the magnitude of the slope from 6 to 10 years postimplantation was statistically more reduced relative to that from 0 to 6 years for both the users mapped with the MPEAK strategy, t(10) = 3.755, p < .001, and with the SPEAK strategy, t(15) = 7.530, p < .001.
Figure 3 displays the distributions of the slopes estimating the pediatric CI recipients' improvement rates of speech sound production from 0–6 to 6–10 years postimplantation for the pediatric CI recipients who were mapped with the MPEAK and SPEAK speech-coding strategies (Panels A and B, respectively). Note that individual differences were observed, despite that the group data indicated that the pediatric CI recipients exhibited different trends of improvement rates from 0–6 to 6–10 years postimplantation. Independent sample t tests indicated that the difference between the estimated slopes of the two subgroups (MPEAK vs. SPEAK) was statistically significant during the initial 6 years of device experience, t(25) = 2.715, p = .012, but was not statistically significant between the 6th and 10th years postimplantation, t(25) = 0.039, p = .969.
As shown in Figure 2, it appeared that several CI participants' sound production accuracy reached high performance levels at around 6 years postimplantation, leaving no room for them to show any further improvement subsequently. We further assessed those who obviously reached rather high levels of performance—that is, 90%–100%, at 6 or 7 years postimplantation; a total of 8 participants (CI-18, CI-19, CI-20, CI-22, CI-23, CI-25, CI-26, and CI-27) fulfilled this operational criterion. We repeated the set of analyses to assess whether the estimated slopes were significantly different from zero, from 0–6 to 6–10 years postimplantation, separately for the two subgroups (i.e., Subgroup A, the 19 individuals who did not reach the high levels of performance, defined as below the accuracy at 90%; Subgroup B, the 8 individuals who exhibited rather high accuracy following 6 years of device experience).
One-sample t tests revealed that the estimated slope was significantly different from zero from 0 to 6 years postimplantation for both Subgroups A and B: Subgroup A, t(18) = 9.170, p < .001; Subgroup B, t(7) = 6.062, p = .001. Furthermore, the estimated slope during the period from 6 to 10 years postimplantation was not significantly different from zero for Subgroup A, t(18) = 1.676, p = .111. As for Subgroup B, although the estimated slope during this period was found to be significantly different from zero, the slope was not positive in value, t(7) = −3.763, p = .007. Taken together, although the plateau observed in the 8 participants who already exhibited high performance levels in speech sound production might be associated with the ceiling effect, the estimated slope for the remaining participants indicated that these pediatric CI recipients' development of speech sound production reached the plateau during the period from 6 to 10 years postimplantation.
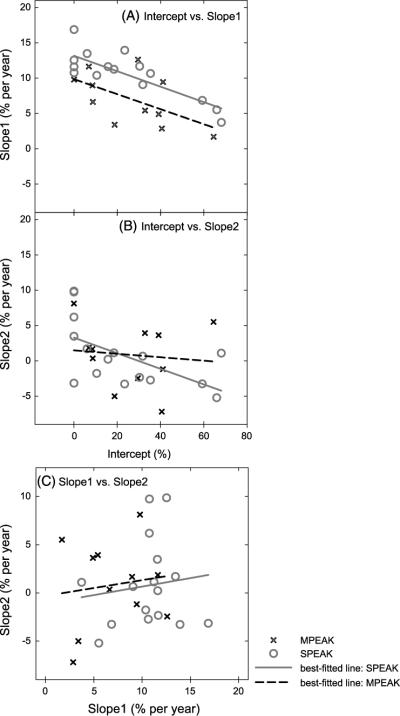
The relations among pairs of the intercept and the two slopes that estimated the improvement rate from 0–6 years (Slope 1) to 6–10 years (Slope 2) were assessed, controlling for age at implantation.3 The partial correlation coefficient was −.436 between the intercept and Slope 1 (p = .026), −.365 between the intercept and Slope 2 (p = .066), and .045 between Slope 1 and Slope 2 (p = .828). The partial correlation coefficients were not evaluated separately for the users of the MPEAK and SPEAK speech-coding strategies, given that similar trends were observed for the coefficients for the pairs (of the intercept, Slope 1, and Slope 2) that were evaluated. That is, the coefficient was positive for Slope 1 and Slope 2, and was negative for the intercept and Slope 1 and for the intercept and Slope 2. Figure 4 depicts the distributions that illustrate the relation between pairs of the intercept, Slope 1, and Slope 2 that was derived from the piecewise regression models. Data points shown with different symbols and the best fitted regression lines are displayed for the pediatric CI recipients that were mapped with different speech-coding strategies.
Figure 4.
Scatterplots that depict the relation between pairs of the intercept and the two slopes. Slope 1 estimated the improvement rate from 0 to 6 years postimplantation; Slope 2 estimated the improvement rate from 6 to 10 years. Panels A–C display Slope 1 versus intercept, Slope 2 versus intercept, and Slope 1 versus Slope 2, respectively. The crosses and circles refer to the data points of the participants in the MPEAK and SPEAK subgroups, respectively. The dashed and solid lines display the best fitted linear regression lines for the MPEAK and SPEAK subgroups, respectively.
These paired comparisons indicated that the group of pediatric CI recipients' performance of speech sound production at the time of implantation (as suggested by the intercept) was inversely associated with the improvement rate of accuracy in speech sound production from 0 to 6 years postimplantation. In other words, the lower the accuracy at the time of implantation, the higher the improvement rate would be during the first 6 years of device experience. On the other hand, the improvement rate during the first 6 years of device experience was not significantly correlated with the rate from 6 to 10 years postimplantation. Similarly, no statistically significant correlation was observed between the early speech sound production performance during the first 6 years of device use and the long-term outcomes in speech sound production in each annual session following the 6th year postimplantation (r values ranged from −.016 to .271; all p values > .05). In short, neither the improvement rate beyond 6 years postimplantation nor the long-term outcome in speech sound production was found to be associated with the intercept that estimated the initial performance level at the time of implantation.
The previous set of analyses addressed the relations among the intercept and two slopes that estimated the group of participants' performance of speech sound production at the time of implantation and the growth in such performance. As was mentioned previously, there was a significant slowing rate of development, or a plateau between the 6th and 10th years following implantation compared with that during the first 6 years of device experience in many pediatric CI recipients' development of speech sound production. In the following set of analyses, the individual patterns in speech sound development were evaluated to determine the extent to which pediatric CI recipients' performance of speech sound production at early stages postimplantation can predict the later performance.
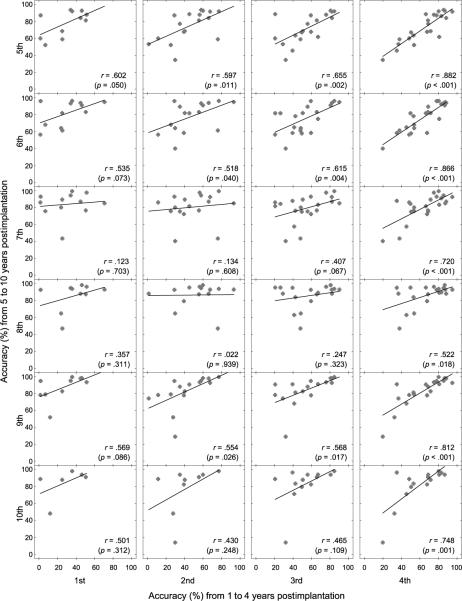
To address this question, the relations between the accuracy of speech sound production were assessed with each of 5–10 years of device experience and the accuracy with each of 1–4 years of device experience. Figure 5 illustrates the distributions of the accuracy of speech sound production in the group of pediatric CI recipients with each of 5–10 years of device experience as a function of the accuracy with each of 1–4 years of device experience. Pearson correlation coefficients for the individuals with both the MPEAK and SPEAK speech-coding strategies were both positive. No specific patterns were observed between the accuracy of speech sound production with each of 5–10 years of device experience and the accuracy with each of 1–4 years of device experience for the two subgroups. Thus, the data of coefficients were collapsed for the users mapped with the MPEAK and SPEAK strategies.
Figure 5.
Distributions of the group of CI participants' accuracy in speech sound production with each of 5–10 years of device experience, as a function of the accuracy with each of 1–4 years of device experience. Pearson correlation coefficients (r values) and p values are indicated in the panels. The line on each panel displays display the best fitted linear regression line.
As Figure 5 displays, there was a systematic increase in the magnitude of coefficients between the accuracy from 1 to 4 years of device experience and the accuracy from 5 to 10 years of device experience. That is, the correlations were all low and nonsignificant with 1 year of device experience. The magnitude of the coefficients increased considerably, with the device experience increasing from the 1st to 2nd year and from the 2nd to 3rd year postimplantation, but these correlation coefficients remained modest. By the 4th year postimplantation, however, the correlation coefficients became much higher compared with those in the previous annual evaluation sessions (i.e., 1st to 2nd year postimplantation). All of these correlation coefficients, ranging from .522 (4th year vs. 8th year) to .882 (4th year vs. 5th year), were found to be statistically significant (4th year vs. 8th year, p = .018; p values ≤ .001 for other pairs).
Linear regression models were fit to the data points with each of 5–10 years of device experience as a function of the accuracy levels with 4 years of device experience. Table 2 provides a summary of these linear regression models and their statistics. All linear regression models reached a statistically significant level (α = .05). In other words, the accuracy in speech sound production of the group of pediatric CI recipients with 5–10 years of device experience can be reasonably predicted by the accuracy with 4 years of device experience. Data from the linear regression models indicated that the accuracy of speech sound production from 5 to 10 years of device experience was associated with the accuracy multiples 0.36–0.82 (depending on the exact annual evaluation session) with 4 years of device experience (p values = .018 or less).
Table 2.
A summary of linear regression models fitted to the data and their statistics.
| Comparison | Linear regression equation | R2 | df | F | p value |
|---|---|---|---|---|---|
| A4 vs. A5 | A5 = 23.88 + 0.75 × A4 | .778 | 1,21 | 70.07 | <.001 |
| A4 vs. A6 | A6 = 29.87 + 0.73 × A4 | .750 | 1,22 | 63.08 | <.001 |
| A4 vs. A7 | A7 = 44.44 + 0.56 × A4 | .496 | 1,23 | 23.62 | <.001 |
| A4 vs. A8 | A8 = 62.05 + 0.36 × A4 | .272 | 1,19 | 6.73 | .018 |
| A4 vs. A9 | A9 = 41.58 + 0.65 × A4 | .660 | 1,19 | 34.94 | <.001 |
| A4 vs. A10 | A10 = 32.41 + 0.82 × A4 | .560 | 1,14 | 16.51 | <.001 |
Note. A4 to A10 refer to the accuracy of speech sound production in the group of pediatric cochlear implant (CI) recipients, each with 4–10 years of device experience.
Discussion
This study characterized the development of speech sound production in prelingually deaf children with long-term device experience (i.e., beyond 6 years of CI experience). We were particularly interested in examining the pattern of speech growth in pediatric CI recipients and determining whether (and if so, when) developmental trajectories stabilize and ultimately plateau. As suggested by the present findings, the magnitude of the group of pediatric CI recipients' improvement in speech sound production was quite large from the preimplant session to the 4th year postimplantation (on average 11.85% per year increase of device experience). However, this improvement rate appeared to decline between the 4th and 6th years following implantation (on average 6.69% per year increase of device experience), and decreased even more following the 6th year (on average 1.28% per year increase of device experience). The higher improvement rate during the initial several years (through the 4th to 6th years) postimplantation suggests that there is a period when the auditory experience provided by a CI has noticeable effects on its users' development of speech sound production.
These findings were consistent with the results reported by Blamey, Barry, Bow, et al. (2001): There is no plateau in speech sound development during the initial 6 years postimplantation. As was mentioned in the introduction, Blamey, Barry, and Jacq (2001) reported a plateau in speech sound development at the 6th year postimplantation, whereas Blamey, Barry, Bow, et al. (2001) reported no plateau. In this study, we reported the percentage of correct scores for the phoneme production of pediatric CI recipients. Consistent with the findings by Blamey, Barry, Bow, et al. (2001), who adopted the same scoring approach, we did not observe a plateau during the first 6 years following implantation.
In contrast to the magnitude of the present pediatric CI recipients' rate of improvement in speech sound production during the first 6 years postimplantation, the rate appeared to decline substantially from about 6 to 10 years postimplantation (on average 1.28% per year increase of implant experience). During this period (6–10 years), many of the pediatric CI recipients began to approach mid-to-high levels of performance (i.e., mean accuracy higher than 75%). By the 6th year postimplantation, the median of these individuals' accuracy in speech sound production was 81.48%. The accuracy remained at around the same level (83.85%) with 7 years of device experience, and improved to 91.21%, 89.48%, and 88.97% with 8–10 years of device experience. The group of CI recipients' accuracy of speech sound production exhibited substantial amounts of improvement, when compared with that during the initial several years postimplantation.
The performance levels of the pediatric CI recipients' speech sound production during the first 6 years were nowhere near perfect (Blamey, Barry, & Jacq, 2001), leaving these individuals room to improve in their performance. In other words, the “ceiling” issue was not a concern when assessing the existence of a plateau in that study. On the other hand, in the present study, the observed “plateau” during 6–10 years postimplantation might be related to the fact that some of the CI participants' sound production accuracy reached high performance levels, and there was no room for them to show any further improvement. To examine this possibility, we further assessed those who obviously reached rather high levels of performance (90%–100%, an operational definition) at 6 or 7 years postimplantation (n = 8). The analyses were performed separately for the two subgroups (i.e., these 8 participants who exhibited rather high accuracy following 6 years of device experience vs. the remaining participants) to evaluate the whether the estimated slope was significantly different from zero: from 0–6 to 6–10 years postimplantation. Consistent with the original conclusion, these results indicated no further improvement following 6 years of implant experience for both subgroups, regardless of the possible ceiling performance observed in some pediatric CI recipients with rather high performance levels in accurate speech sound production.
The present findings can assist in extending researchers' understanding of the effects of the long-term device experience on pediatric CI recipients' development of speech sound production. Tye-Murray et al. (1995) assessed the accuracy of speech sound production in prelingually deaf children who used a CI. They reported that with 3 years of device experience, pediatric CI recipients received 53% accuracy in speech sound production. The present findings suggest that with long-term device experience, pediatric CI recipients are likely able to achieve satisfactory levels of accuracy in speech sound production. On the other hand, these individuals' performance levels of speech sound production are still lower when compared with the performance levels of their NH peers. That is, NH children's accuracy in speech sound production achieves slightly below 100% by 6–8 years of age (Menyuk, 1972; Shriberg et al., 1994).
The present data suggest that speech sound development may continue beyond age 8, which is often thought to be the terminus of normal speech development in NH children. Interestingly, our results indicate that the age when the plateau occurred is associated with a hearing age of around 8 years in pediatric CI recipients (Figure 1B). These findings would support an account of sensitive periods that emphasizes experience dependence rather than maturational mechanisms. However, it should be acknowledged that these individuals' age when the plateau occurred coincides with the onset of adolescence, which is considered as the ending time point for the speech development period (Lenneberg, 1967). Thus, at this time it is difficult to use these data to resolve what mechanisms might account for the plateau. In fact, it is possible that the plateau represents simply a ceiling or saturation point in learning. In that regard, the peak level of performance for each child (i.e., the level of asymptote) represents the limits of the speech information provided by the auditory system. If so, we might expect to find differences in the plateau level for different devices.
In this study, we examined whether there was a plateau in the development of speech sound production from 6 to 10 years postimplantation by fitting a piecewise regression model to each CI participant's chronological data. The results were evaluated separately for the subgroups of individuals who were mapped with the MPEAK and SPEAK speech-coding strategies, as advancements in technology might contribute to interparticipant variability in CI users' postimplant speech development (Peng et al., 2004). The results indicated that mean improvement rates (reflected by the estimated slope in each model) from the preimplant session to the 6th year postimplantation for both the MPEAK and SPEAK subgroups were positive and significantly different from zero. However, the improvement rates of both subgroups of participants declined and were not significantly different from zero from 6 to 10 years postimplantation. The average improvement rate of the users mapped with the SPEAK speech-coding strategy was significantly higher when compared with that of those mapped with the MPEAK speech-coding strategy. The significant difference in the improvement rates between the users with different speech-coding strategies, however, was limited to the initial 6 years of device experience. With 6–10 years of device experience, there was no statistically significant difference in the improvement rates between the pediatric CI recipients that were mapped with the MPEAK and SPEAK strategies. These findings suggest that the plateau level is not, at least solely, driven by the auditory information provided.
Taken together, the present findings suggest the steady improvement in pediatric CI recipients' speech sound development during the initial 6 years postimplantation. However, these individuals' speech development reaches a plateau following the 6th year postimplantation. Interestingly, the pediatric CI users mapped with a more advanced speech-coding strategy (i.e., SPEAK) show advantages, only in the improvement rates, over those with a less advanced strategy (i.e., MPEAK), during the first 6 years postimplantation. However, these individuals' speech sound development reaches the plateau after 6 years postimplantation, regardless of the speech-coding strategy (SPEAK vs. MPEAK) that the individual is mapped with.
The present findings indicated that the improvement rate between the 6th and 10th year of CI experience was not significantly correlated with the rate prior to the 6th year or the initial performance level at the time of implantation. A subsequent critical question raised in this investigation was the extent to which pediatric CI recipients' mastery of speech sound production with extended device experience can be predicted by early accuracy levels in speech sound production. More specifically, the question of interest was as follows: When in development do researchers find sufficient stability in the relative standing among pediatric CI recipients that they can make decisions with regard to whether long-term outcomes will be favorable or at risk?
To answer this question, the accuracy of speech sound production with 5–10 years of device experience was evaluated in relation to the accuracy with each of the initial 4 years postimplantation. The results indicated that there is a systematic increase in the magnitude of correlation coefficients between the accuracy with 1–4 years of device experience and that with 5–10 years of device experience. Moreover, the correlations were the strongest between the accuracy with 4 years of device experience (among all the annual sessions from the 1st to 4th year) and that with each of 5–10 years of device experience. Each of the correlation coefficients (r values) between the accuracy with 4 years of device experience and the accuracy with each of 5–10 years of device experience was statistically significant (see Figure 5).
Further linear regression analyses indicated that pediatric CI recipients' accuracy in speech sound production from each of the 5th to 10th year postimplantation can be reasonably predicated by these individuals' performance with 4 years of device experience (see Table 2). These findings have significant clinical applications in managing pediatric CI recipients' development of speech sound production. Specifically, accuracy of speech sound production after 4 years of device experience can be informative with respect to the prognosis for long-term outcomes. Early speech production skills can be used as an index for clinicians, educators, and other professionals in determining if a pediatric CI user is on track to achieve good long-standing outcomes in speech sound production. A caveat to this is that it takes at least 4 years of CI experience before one can project the long-term efficiency of pediatric CI recipients' speech sound production.
The present findings have other important clinical implications: It is imperative to maximize pediatric CI recipients' development of speech sound production during the initial 6 years postimplantation. Moreover, pediatric CI recipients' performance levels of speech sound production with extended device experience (i.e., 5–10 years) can be reasonably predicted using the accuracy in speech sound production of these individuals after 4 years of device experience. The present findings also revealed that individuals who were mapped with the SPEAK strategy (as opposed to MPEAK) exhibited higher improvement rates during the initial 6 years of device experience. That is, maximizing the development of speech sound production at early stages postimplantation may be achieved by providing pediatric CI recipients with more advanced speech-coding strategies or other technological advancements.
This study has some inevitable limitations. It was not possible to further define the roles of certain participant variables, such as education settings (or communication modality) of our participants. Moreover, one might hypothesize that individuals who depend more heavily on sign may have a more shallow developmental trajectory of speech sound production and/or lower peak accuracy than children who depend more heavily on auditory–oral communication. Unfortunately, this hypothesis could not be tested because of our limited information regarding the participants' amount of reliance on sign. It was only clear to us that the CI participants who were in the TC program received their education at school with signing exact English and oral English (simultaneous), and all used oral speech at home with their family (because none of these individuals had deaf parents or siblings). Additional studies will be needed to test this hypothesis.
Another limitation of this study was that all participants in this study were mapped with either MPEAK or SPEAK speech-coding strategies. Given that these two strategies were relatively old, when compared with more current speech-coding strategies, such as advanced combination encoder or continuous interleaved sampler (Loizou, 1998; Psarros et al., 2002), it is possible that pediatric CI recipients who are users of a more advanced speech-coding strategy may show different profiles in their development of speech sound production (e.g., maximal performance levels or timing of the plateau, if it exists). With the trends of CI technological advancements and early implantation in infants as young as 12 months of age (American Speech-Language-Hearing Association, 2003), the extent to which the present findings regarding development of speech sound production can be applied to the current generation of pediatric CI recipients remains to be demonstrated in future studies. Finally, another future study could examine the role of vocabulary acquisition and how that might be related to the developmental trajectories of speech sound production in the pediatric CI population.
Conclusions
The present findings suggest that pediatric CI recipients exhibit steady improvement in the development of speech sound production during the first 6 years postimplantation, but as a group, these individuals' performance appears to stabilize (or reaches a plateau) afterward. Moreover, pediatric CI recipients' accuracy of speech sound production with 4 years of device experience can reasonably predict the long-term outcomes of speech sound production (i.e., with 5–10 years of device experience). These findings provide indices for clinical applications when long-term advancements in pediatric CI recipients' development of speech sound production are considered.
Acknowledgments
Funding of this research was provided by the National Institute on Deafness and Other Communication Disorders (Grant 2 P50 DC00242) and the General Clinical Research Centers Program of the National Institutes of Health (Grant RR00059). We appreciate the participation of all pediatric cochlear implant recipients and their families. We thank Sarah Knoll and Kara Zielinski for assisting in the transcription of speech samples (for reliability measurements). We acknowledge all constructive suggestions provided by Craig Champlin and Peggy Nelson.
Footnotes
All results reported in this study were not different when statistical analyses were performed using the scores on the rationalized arcsine unit scale, as described in Studebaker (1985).
The transcriptions obtained from Transcribers A and B were used exclusively for the purpose of reporting interjudge agreements. All of the results reported in this study are based on the judgments by the primary examiner.
The variable, age at implantation, was controlled. This is because those with a younger age at implantation were also younger in age and, hence, were likely to have a lower phoneme accuracy score to begin with.
References
- American Speech-Language-Hearing Association Technical report: cochlear implants. ASHA Supplement. 2003:24. [Google Scholar]
- Bamford J, Saunders E. Hearing impairment, auditory perception and language disability. 2nd ed. Singular Publishing Group; San Diego, CA: 1991. [Google Scholar]
- Blamey PJ, Barry JG, Jacq P. Phonetic inventory development in young cochlear implant users 6 years postoperation. Journal of Speech, Language, and Hearing Research. 2001;44:73–79. doi: 10.1044/1092-4388(2001/007). [DOI] [PubMed] [Google Scholar]
- Blamey PJ, Barry J, Bow C, Sarant J, Paatsch L, Wales R. The development of speech production following cochlear implantation. Clinical Linguistics and Phonetics. 2001;15:363–382. [Google Scholar]
- Chin SB, Tsai PL, Gao S. Connected speech intelligibility of children with cochlear implants and children with normal hearing. American Journal of Speech-Language Pathology. 2003;12:440–451. doi: 10.1044/1058-0360(2003/090). [DOI] [PubMed] [Google Scholar]
- Geers AE. Comparing implants with hearing aids in profoundly deaf children. Otolaryngology and Head and Neck Surgery. 1997;117:150–154. doi: 10.1016/s0194-5998(97)70167-0. [DOI] [PubMed] [Google Scholar]
- Geers AE, Moog J, Schick B. Acquisition of spoken and signed English by profoundly deaf children. Journal of Speech and Hearing Disorders. 1984;49:378–388. doi: 10.1044/jshd.4904.378. [DOI] [PubMed] [Google Scholar]
- Geers AE, Tobey EA. Longitudinal comparison of the benefits of cochlear implants and tactile aids in a controlled educational setting. The Annals of Otology, Rhinology & Laryngology. 1995;166:328S–329S. [PubMed] [Google Scholar]
- Lenneberg E. Biological foundations of language. Wiley; New York: 1967. [Google Scholar]
- Levitt H, McGarr NS, Geffner D. Development of language and communication skills in hearing-impaired children. ASHA Monographs. 1987;26:1–158. [PubMed] [Google Scholar]
- Loizou PC. Introduction to cochlear implants. IEEE Engineering in Medicine and Biology Magazine: The Quarterly Magazine of the Engineering in Medicine & Biology Society. 1998;18:32–42. doi: 10.1109/51.740962. [DOI] [PubMed] [Google Scholar]
- Menyuk P. The development of speech. Bobbs-Merrill; New York: 1972. [Google Scholar]
- Miyamoto RT, Kirk KI, Robbins AM, Todd S, Riley A. Speech perception and speech production skills of children with multichannel cochlear implants. Acta Oto-Laryngologica. 1996;116:240–243. doi: 10.3109/00016489609137832. [DOI] [PubMed] [Google Scholar]
- Munakata Y, McClelland JL. Connectionist models of development. Developmental Science. 2003;6:413–429. [Google Scholar]
- Osberger MJ, Maso M, Sam LK. Speech intelligibility of children with cochlear implants, tactile aids, or hearing aids. Journal of Speech and Hearing Research. 1993;36:186–203. doi: 10.1044/jshr.3601.186. [DOI] [PubMed] [Google Scholar]
- Peng S, Spencer JL, Tomblin JB. Speech intelligibility of pediatric cochlear implant recipients with seven years of device experience. Journal of Speech, Language, and Hearing Research. 2004;47:1227–1236. doi: 10.1044/1092-4388(2004/092). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psarros CE, Plant KL, Lee K, Decker JA, Whitford LA, Cowan RSC. Conversion from the SPEAK to the ACE strategy in children using the nucleus 24 cochlear implant system: Speech perception and speech production outcomes. Ear and Hearing. 2002;23:18S–27S. doi: 10.1097/00003446-200202001-00003. [DOI] [PubMed] [Google Scholar]
- Serry TA, Blamey PJ. A 4-year investigation into phonetic inventory development in young cochlear implant users. Journal of Speech, Language, and Hearing Research. 1999;42:141–154. doi: 10.1044/jslhr.4201.141. [DOI] [PubMed] [Google Scholar]
- Serry TA, Blamey PJ, Grogan M. Phoneme acquisition in the first 4 years of implant use. The American Journal of Otology. 1997;18:S122–S124. [PubMed] [Google Scholar]
- Shriberg LD, Gruber FA, Kwiatkowski J. Developmental phonological disorders: III. Long-term speech-sound normalization. Journal of Speech and Hearing Research. 1994;37:1151–1177. doi: 10.1044/jshr.3705.1151. [DOI] [PubMed] [Google Scholar]
- Shriberg LD, Lof G. Reliability studies in broad and narrow phonetic transcription. Clinical Linguistics and Phonetics. 1991;5:225–279. [Google Scholar]
- Skinner MW, Clark GM, Whitford LA, Seligman PM, Staller SJ, Shipp DB, et al. Evaluation of a new spectral peak coding strategy for the Nucleus 22 Channel Cochlear Implant System. The American Journal of Otology. 1994;15:15–27. [PubMed] [Google Scholar]
- Skinner MW, Fourakis MS, Holden TA, Holden LK, Demorest ME. Identification of speech by cochlear implant recipients with the Multipeak (MPEAK) and Spectral Peak (SPEAK) speech coding strategies: I. Vowels. Ear and Hearing. 1996;17:182–197. doi: 10.1097/00003446-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Spencer LJ, Tye-Murray N, Tomblin JB. The production of English inflectional morphology, speech production and listening performance in children with cochlear implants. Ear and Hearing. 1998;19:310–318. doi: 10.1097/00003446-199808000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studebaker GA. A “rationalized” arcsine transform. Journal of Speech and Hearing Research. 1985;28:455–462. doi: 10.1044/jshr.2803.455. [DOI] [PubMed] [Google Scholar]
- Svirsky MA, Chin SB. Speech production. In: Waltzman SB, Cohen NL, editors. Cochlear implants. Thieme Medical Publishers; New York: 2000. pp. 293–309. [Google Scholar]
- Tobey EA, Geers AE. Speech production benefits of cochlear implants. Advances in Oto-Rhino-Laryngology. 1995;50:146–153. doi: 10.1159/000424451. [DOI] [PubMed] [Google Scholar]
- Tobey EA, Geers AE, Brenner CB, Altuna D, Gabbert G. Factors associated with development of speech production skills in children implanted by age five. Ear and Hearing. 2003;24:36S–45S. doi: 10.1097/01.AUD.0000051688.48224.A6. [DOI] [PubMed] [Google Scholar]
- Tye-Murray N. Speech, language, and literacy development. In: Tye-Murray N, Clark W, editors. Foundations of aural rehabilitation: Children, adults, and their family members. Singular Publishing Group; San Diego, CA: 1998. pp. 415–446. [Google Scholar]
- Tye-Murray N, Kirk KI. Vowel and diphthong production by young users of cochlear implants and the relationship between the phonetic level evaluation and spontaneous speech. Journal of Speech and Hearing Research. 1993;36:488–502. doi: 10.1044/jshr.3603.488. [DOI] [PubMed] [Google Scholar]
- Tye-Murray N, Spencer L, Woodworth GG. Acquisition of speech by children who have prolonged cochlear implant experience. Journal of Speech and Hearing Research. 1995;38:327–337. doi: 10.1044/jshr.3802.327. [DOI] [PMC free article] [PubMed] [Google Scholar]