Abstract
The location of neurons generating the rhythm of breathing in mammals is unknown. By microsection of the neonatal rat brainstem in vitro, a limited region of the ventral medulla (the pre-Bötzinger Complex) that contains neurons essential for rhythmogenesis was identified. Rhythm generation was eliminated by removal of only this region. Medullary slices containing the pre-Bötzinger Complex generated respiratory-related oscillations similar to those generated by the whole brainstem in vitro, and neurons with voltage-dependent pacemaker-like properties were identified in this region. Thus, the respiratory rhythm in the mammalian neonatal nervous system may result from a population of conditional bursting pacemaker neurons in the pre-Bötzinger Complex.
The Rhythm of Breathing Animates mammalian life, and the source of this rhythm, the noeud vital (1), is unknown. The basic oscillator lies within the brainstem, but technical limitations of experiments in the mammalian nervous system in vivo have hindered localization of the neurons generating the rhythm (2). An in vitro preparation of neonatal mammalian brainstem and spinal cord that spontaneously generates respiratory rhythm (3, 4) allows application of a broader range of techniques and has provided insights into neural mechanisms controlling breathing (5). Analysis of synaptic mechanisms in this preparation has led to the hypothesis that conditional pacemaker neurons in the medulla are the kernel for rhythm generation (6). Further tests of this hypothesis and network-based models (2, 7) of rhythmogenesis require identification of the sites and specific cells producing the rhythm. In our experiments we have systematically microsectioned the in vitro neonatal rat brainstem and precisely localized regions with neurons critical for rhythmogenesis (8).
Serial transverse microsections (50 to 75 μm thick) were made with a Vibratome, either caudally along the brainstem, starting from the pontomedullary junction, or rostrally from the spinomedullary junction, and perturbations of rhythmogenesis were analyzed (9). Rostral to caudal sectioning (n = 20 experiments) did not perturb the frequency of inspiratory phase motor discharge (of phrenic and other respiratory motoneurons) until the level of caudal retrofacial nucleus; further sectioning induced instabilities in the rhythm and then eliminated rhythmic motor output (10) (Fig. 1). Perturbations of rhythmogenesis occurred only with the removal of sections at this level of the medulla. More caudal medullary regions were not essential for rhythm generation, because sectioning rostrally from the spinomedullary junction (n = 11) did not disrupt respiratory motor output on cranial nerves (11) until a level rostral to the obex, within 200 μm of the level causing cessation of respiratory output in the rostral to caudal sectioning experiments. Transections in horizontal planes (n = 11), which removed regions dorsal to nucleus ambiguus (Fig. 1) but left intact motor circuits of the ventral medulla (2), did not alter the rhythmic discharge of medullary motoneurons (12).
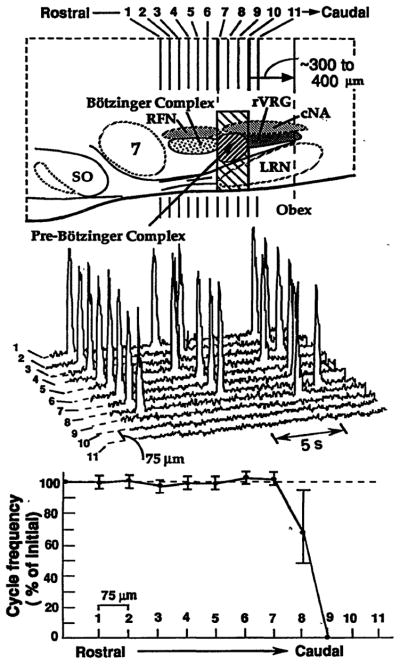
Fig. 1.
Perturbations of respiratory motor pattern with serial microsections of neonatal rat medulla in vitro. (Top) Sagittal view of medulla showing pre-Bötzinger Complex and neighboring regions. Hatched rectangular area indicates critical region for rhythmogenesis. Pre-Bötzinger Complex (shaded area) within the critical region extends from caudal end of retrofacial nucleus ~200 μm toward obex. SO, superior olive; 7, facial nucleus; LRN, lateral reticular nucleus; RFN, retrofacial nucleus; rVRG, rostral ventral respiratory group; cNA, caudal (semicompact) division of nucleus ambiguus. (Middle) Traces show integrated phrenic motoneuron population discharge on C4 spinal ventral roots after 75-μm sectioning in the rostral to caudal direction. The steady-state discharge shown is after sections made from the level of caudal facial nucleus through the caudal end of pre-Bötzinger Complex (1 through 11). Sections at the level of pre-Bötzinger Complex (sections 8 to 10) eliminated rhythmic motor output of all spinal and cranial (IX, X, XII) (not shown) respiratory motoneuron populations. (Bottom) A single 75-μm section through the rostral boundary of the pre-Bötzinger Complex caused a reduction in cycle frequency and instabilities of the rhythm (that is, an increase in cycle-to-cycle variation of period). The mean cycle period in the experiment shown was 7.5 ± 0.4 s (n = 15 cycles) after sectioning just rostral to pre-Bötzinger Complex and 11.1 ± 2.9 s after a single transection within this region.
Neurons essential for respiratory rhythmogenesis thus appear localized in the ventral medulla just caudal to the level of retrofacial nucleus. Rhythmically active neurons are localized within this critical area (2, 4, 13) just caudal to the Bötzinger Complex of respiratory neurons (Fig. 1) (14). We refer to this region with rhythmically active cells as the pre-Bötzinger Complex (8, 15) and propose that it contains neurons that generate respiratory rhythm. This region has not been previously identified as a site for rhythm generation. These results exclude more rostral areas as primary sites for rhythmogenesis (16).
To establish whether neurons in the pre-Bötzinger Complex could generate respiratory rhythm, we prepared transverse medullary slices (350 to 600 μm thick) that included this region (Fig. 2), local circuits for motor output generation, and hypoglossal respiratory motoneurons and nerves (17). The thinner slices (350 μm thick) just enclosed the boundaries of the pre-Bötzinger Complex. These slices generated rhythmic motor output on hypoglossal nerves (Fig. 2) (18), confirming that neurons at the level of the pre-Bötzinger Complex could generate rhythm. The motoneuron population discharge, as well as synaptic drive potentials and currents of medullary neurons, were similar in frequency, duration, and temporal pattern (Fig. 2, A and B) to that generated in the en bloc brainstem (4, 18); thus, the oscillatory neuronal activity of the slice represented respiratory activity. To verify that pre-Bötzinger Complex neurons in these slices could affect the rhythm, we produced local perturbations of neuronal excitability. Transient neuronal depolarization by microinjection (20 to 40 nl) of physiological solutions with high K+ concentrations (25 mM) reversibly increased the motor burst frequency three- to fourfold (Fig. 2C). Reduction of local excitatory synaptic transmission by microinjection of 6-cyano-7-nitroquinoxaline-2,3-di-one (CNQX) (250 fmol), a non–N-methyl-D-aspartate (NMDA) receptor antagonist, transiently reduced the frequency and reversibly eliminated the oscillatory output (Fig. 2C). This result is consistent with findings that excitatory neurotransmission mediated by endogenously released excitatory amino acids is necessary for rhythmogenesis (19).
Fig. 2.
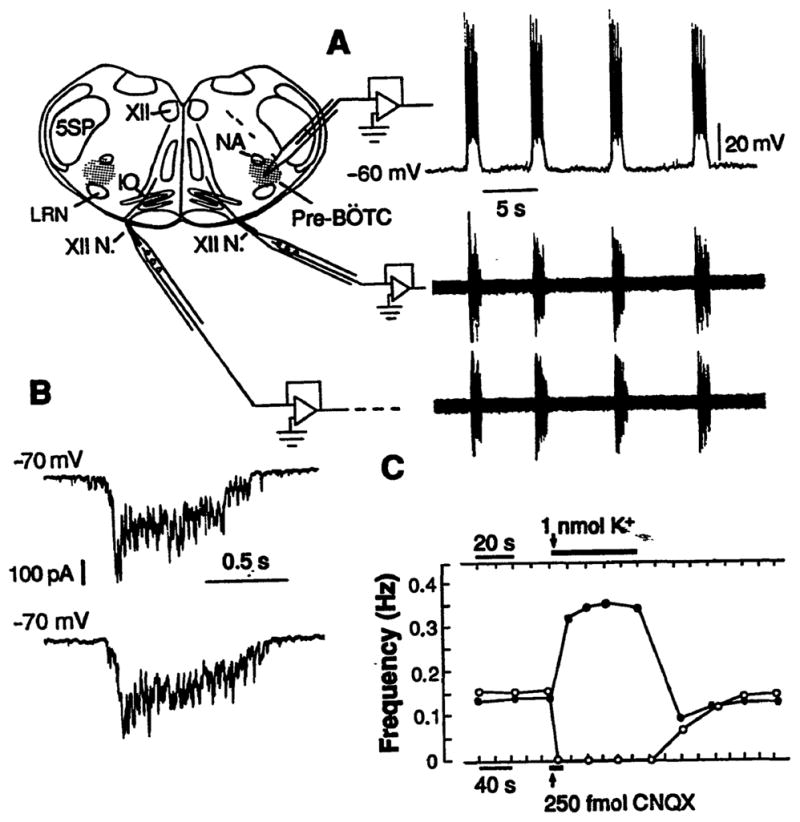
(A) Rhythmic activity of a medullary slice (500 μm thick) containing pre-Bötzinger Complex (pre-BÖTC, shaded regions). NA, nucleus ambiguus; 5SP, spinal trigeminal nucleus; XII, hypoglossal nucleus; XII N., hypoglossal nerve; and IO, inferior olive. Traces at right show respiratory motor discharge recorded bilaterally from hypoglossal nerve roots (lower) (18) and whole-cell recording from a rhythmically active neuron in pre-Bötzinger Complex (upper). (B) (Upper) Synaptic drive currents of pre-Bötzinger Complex neuron under voltage-clamp (−70 mV) consisted of rapidly peaking, slowly decrementing envelope. (Lower) Identical pattern of synaptic currents of respiratory neuron in en bloc medulla. (C) Unilateral microinjection of solution with elevated K+ concentration (upper arrow and bar) (40 nl, 25 mM; 1 nmol) in the pre-Bötzinger Complex in slice reversibly elevated motor burst frequency bilaterally (solid dots). Microinjection of 250 fmol CNQX (10 nl, 25 μM) (lower arrow and bar) in pre-Bötzinger Complex reduced the burst frequency (open points) and transiently blocked rhythmogenesis, which gradually returned with local washout of antagonist. Data are average values computed from continuous recordings of motor output from slice.
Oscillatory properties of neurons in the pre-Bötzinger Complex in the slices were investigated with whole-cell patch-clamp recording techniques (20). Several populations of neurons exhibited periodic membrane potential depolarization synchronous with the motor output (Figs. 2 and 3) (20). To test for voltage-dependent pacemaker properties (19), we depolarized neurons under current-clamp recording conditions. In ~25% (4 of 15) of the rhythmically active cells, small membrane currents (30 to 100 pA) sufficient to depolarize the baseline potential to −55 to −45 mV resulted in oscillatory bursting at a higher frequency than that of the motor output (Fig. 3B). This type of voltage-dependent bursting property is characteristic of conditional pacemaker neurons. These bursting pacemaker-like cells were a distinct class, because other rhythmically active neurons in the region, as well as in adjacent regions, responded to membrane depolarization by generating a continuous stream of action potentials but not bursting oscillations. The rhythmic depolarization of these neurons without intrinsic bursting properties was due to periodic synaptic inputs with 300- to 600-pA peak synaptic currents (Fig. 2B) and reversal potentials near 0 mV.
Fig. 3.
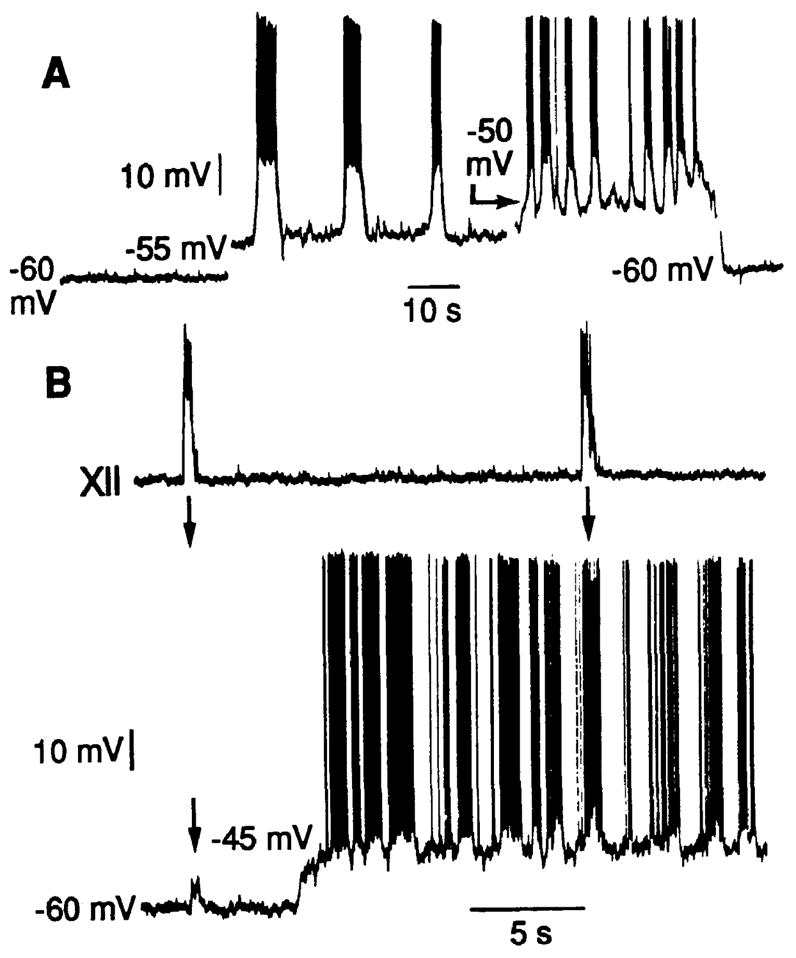
Voltage-dependent oscillatory properties of neurons in the pre-Bötzinger Complex. (A) One population of neurons generated large-amplitude oscillatory membrane potentials in response to membrane depolarization. Recording was obtained in a slice 300 μm thick without rhythmic motor output. (B) In slices generating hypoglossal (XII) motor output (top trace), neurons exhibiting intrinsic bursting properties (bottom trace) received synaptic inputs that synchronize neuron depolarization and bursting (19); synchronizing inputs are evident as small-amplitude (~5 mV) depolarizing potentials (arrow at left in bottom trace), which cause the neuron to generate a burst of action potentials coincident with the rest of the network at more depolarized baseline potentials (arrow at right). Voltage-dependent bursting properties become evident with depolarization to −45 mV, which results in membrane potential oscillations and bursting at a higher frequency than that of slice motor output.
Neurons with voltage-dependent oscillatory properties were also identified in the pre-Bötzinger Complex in thinner slices (250 to 350 μm thick) that did not generate rhythmic motor activity (21). These cells (n = 8) generated large-amplitude (10- to 15-mV) membrane potential oscillations and bursts of action potentials with depolarization into the −55 to −45 mV range. The frequency of these oscillations increased as the holding potential was elevated in this range (Fig. 3A). The periods of the oscillations covered the range of respiratory cycle periods (~3 to 15 s) observed in rhythmically active slices and en bloc brainstem-spinal cord preparations.
To determine if there are unique features of the cellular organization of the pre-Bötzinger Complex, we examined the anatomy of the homologous region in adult rats (Fig. 4), where we could label neurons on the basis of their connections within the respiratory network (22). We established several distinct cytoarchitectonic features. In the pre-Bötzinger Complex there was a marked absence of brainstem output neurons (that is, bulbospinal neurons) compared to adjacent regions. The highest number of bulbospinal neurons was in the ventrolateral reticular formation near the obex, caudal to the pre-Bötzinger Complex (Fig. 4A). The pre-Bötzinger Complex contained the highest percentage of propriobulbar interneurons (23) (Fig. 4B). This distribution of neuron types suggests that the pre-Bötzinger Complex has a distinct neuronal organization. Direct intracellular recordings from respiratory neurons in the pre-Bötzinger Complex in the adult cat (24) also indicate a dense concentration of interneurons. The pre-Bötzinger Complex interneurons have direct connections to the more caudal areas containing bulbospinal neurons (22). These interneurons may represent the substrate for transmission of the locally generated rhythm to the premotoneurons that transmit the oscillatory drive to spinal respiratory motoneurons.
Fig. 4.
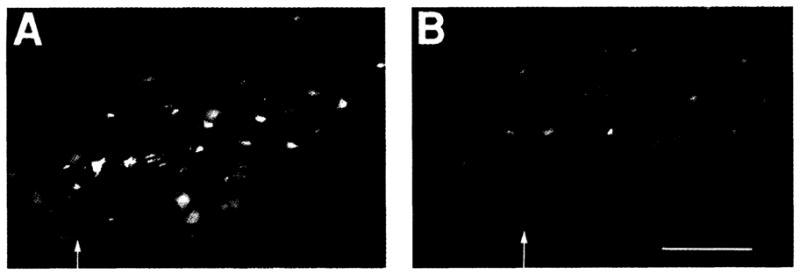
Fluorescence photomicrographs of horizontally sliced sections in the plane of the caudal (A) and contiguous rostral (B) portions of the lateral tegmental field containing the ventral respiratory group (14) and pre-Bötzinger Complex. Note the nonuniform distribution of three classes of retrogradely labeled neurons: (i) bulbospinal premotoneurons labeled with Fluoro-Gold (Fluorochrome, Inc., Englewood, Colorado) (yellow); (ii) propriobulbar interneurons labeled with rhodamine-impregnated latex beads (red); and (iii) vagal motoneurons labeled with fast blue. Bulbospinal neurons and vagal motoneurons are concentrated at caudal levels (A), whereas in the pre-Bötzinger Complex (B) there is a high density of propriobulbar neurons and few bulbospinal neurons and vagal motoneurons. The caudal boundary of pre-Bötzinger Complex is indicated by the arrow at left in (B); rostral boundary is at the right margin. The level of obex is indicated by the arrow in (A); the bottom is toward the midline. Length bar, 200 μm.
We have identified a very limited region of the ventrolateral medulla containing neurons capable of generating respiratory rhythm in the neonatal nervous system. This region may contain the minimal neuronal substrate for rhythmogenesis. The presence of neurons with voltage-dependent oscillatory properties in this region is consistent with the hypothesis that conditional bursting pacemaker neurons are the kernel for rhythm generation in the neonate (6). However, before it can be concluded that the conditionally bursting neurons found in the pre-Bötzinger Complex are in fact pacemaker neurons generating the rhythm, the identified cells must be shown to produce the oscillatory drive to the network. Furthermore, because a transformation of rhythm generation mechanisms could occur during postnatal development, a hybrid of network and pacemaker properties may be essential for rhythmogenesis in the adult mammal (25). The slice preparations developed in our studies contain an isolated, functionally active circuit in vitro and will facilitate further analysis of mechanisms underlying the generation and transmission of respiratory oscillations in neonatal mammals.
Acknowledgments
Supported by NIH grants HL4095, NS24742, Research Career Development Award HL02204 (J.C.S.), DFG Ba 1095/1-1, and an Alexander von Humboldt Foundation Research Fellowship (J.C.S.).
Contributor Information
Jeffrey C. Smith, Systems Neurobiology Laboratory, Department of Kinesiology, University of California, Los Angeles, CA 90024–1527
Howard H. Ellenberger, Systems Neurobiology Laboratory, Department of Kinesiology, University of California, Los Angeles, CA 90024–1527
Klaus Ballanyi, Physiologisches Institut, Universität Göttingen, Humboldtallee 23, D-3400 Göttingen, Federal Republic of Germany.
Diethelm W. Richter, Physiologisches Institut, Universität Göttingen, Humboldtallee 23, D-3400 Göttingen, Federal Republic of Germany
Jack L. Feldman, Systems Neurobiology Laboratory, Department of Kinesiology, University of California, Los Angeles, CA 90024–1527
REFERENCES AND NOTES
- 1.Fluorens P. C R Acad Sci (Paris) 1851;33:437. [Google Scholar]
- 2.Cohen M. Physiol Rev. 1979;59:1105. doi: 10.1152/physrev.1979.59.4.1105. [DOI] [PubMed] [Google Scholar]; Feldman JL. Handbook of Physiology. In: Bloom FE, editor. The Nervous System IV. American Physiological Society; Washington, D.C: 1986. pp. 463–524. [Google Scholar]; Ezure K. Prog Neurobiol (NY) 1990;35:429. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- 3.Suzue T. J Physiol (London) 1984;354:173. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]; Smith JC, Feldman JL. J Neurosci Methods. 1987;21:321. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- 4.Smith JC, Greer JJ, Liu G, Feldman JL. J Neurophysiol. 1990;64:1149. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- 5.Harada Y, Kuno M, Wang YZ. J Physiol (London) 1986;368:679. doi: 10.1113/jphysiol.1985.sp015883. [DOI] [PMC free article] [PubMed] [Google Scholar]; McCrimmon DR, Smith JC, Feldman JL. J Neurosci. 1989;9:1910. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hilaire G, Monteau R, Errchidi S. Brain Res. 1989;485:325. doi: 10.1016/0006-8993(89)90577-5. [DOI] [PubMed] [Google Scholar]; Liu G, Feldman JL, Smith JC. J Neurophysiol. 1990;64:423. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- 6.Smith JC, Feldman JL. Fed Proc. 1986;45:518. [Google Scholar]; Smith JC, Feldman JL. In: Respiratory Muscles and Their Neuromotor Control. Sieck GC, Gandevia S, Cameron W, editors. Liss; New York: 1987. pp. 27–36. [Google Scholar]; Feldman JL, Smith JC. Ann NY Acad Sci. 1989;563:114. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- 7.Richter DW, Ballantyne D, Remmers JE. News Physiol Sci. 1986;1:109. [Google Scholar]
- 8.Smith JC, Greer JJ, Feldman JL. Soc Neurosci Abstr. 1989;15:505. [Google Scholar]; ibid. 1990;16:1130. [Google Scholar]
- 9.Experiments were performed with in vitro neonatal rat brainstem–spinal cord or isolated medulla preparations as described (4, 8). Recordings of spinal or cranial respiratory motoneuron discharge were made simultaneously with microsectioning for analysis of perturbations of the respiratory cycle period (8).
- 10.Bulbospinal premotoneurons transmitting inspiratory drive to phrenic motoneurons are concentrated caudal to the pre-Bötzinger Complex (Fig. 4) (2); thus, perturbations of motor output with rostral to caudal sectioning were not due to removal of bulbospinal neurons.
- 11.Isolated medulla preparations that generated respiratory motor output on glossopharyngeal nerves were used. Sectioning rostral to the obex removed glossopharyngeal (pre)motoneurons [ Bieger D, Hopkins DA. J Comp Neurol. 1987;262:546. doi: 10.1002/cne.902620408. resulting in reduction of the motor discharge amplitude and elimination of motor output, with only minor perturbations of the rhythm, at a level 100 to 200 μm caudal to pre-Bötzinger Complex.
- 12.Horizontal sectioning was done by a single manual cut in isolated medulla preparations. The mean cycle period of glossopharyngeal and vagus nerve inspiratory discharge was 7.8 ± 0.5 s and 8.9 ± 0.6 s (±SD, n = 11 experiments) before and after sectioning, respectively.
- 13.Otake K, Sasaki H, Ezure K, Manabe M. J Comp Neurol. 1990;302:485. doi: 10.1002/cne.903020306. [DOI] [PubMed] [Google Scholar]
- 14.Three contiguous subdivisions of ventral brainstem respiratory neurons are distinguished (2) (Fig. 1): (i) Bötzinger Complex, (ii) rostral ventral respiratory group (rVRG), and (iii) caudal VRG.
- 15.Feldman JL, Connelly CA, Ellenberger HH, Smith JC. Eur J Neurosci Suppl. 1990;3:171. [Google Scholar]
- 16.Onimaru H, Arata A, Homma I. Neurosci Lett. 1987;78:151. doi: 10.1016/0304-3940(87)90624-0. [DOI] [PubMed] [Google Scholar]; Brain Res. 1988;445:314. doi: 10.1016/0006-8993(88)91194-8. [DOI] [PubMed] [Google Scholar]
- 17.The medulla was sectioned from the rostral brainstem to within 150 μm of the rostral boundary of pre-Bötzinger Complex (Fig. 1). Slices 350 to 600 μm thick were cut.
- 18.Slices >500 μm thick generated rhythmic motor output with a cycle frequency that was 40 to 70% of that in the en bloc brainstem–spinal cord before the slice was cut. This frequency could be increased by changing the K+ concentration in the slice bathing solution from 3 to 9 mM; the mean cycle period in en bloc preparations and in slices with 9 mM K+ was 7.6 ± 0.9 s and 6.5 ± 1.3 s (n = 5), respectively. Thinner slices did not generate motor output at 3 mM K+ but generated rhythmic output at K+ concentrations of 9 to 11 mM.
- 19.We have proposed that the rhythm generating cells are a population of synchronized, conditional bursting pacemaker neurons (6) requiring depolarizing synaptic inputs for the activation of voltage-dependent currents responsible for regenerative oscillations. A critical depolarizing conditional input is mediated by excitatory amino acids acting at non-NMDA receptors [ Greer JJ, Smith JC, Feldman JL. J Physiol (London) 1991;437:727. doi: 10.1113/jphysiol.1991.sp018622.
- 20.Blanton MG, Lo Turco JJ, Kriegstein A. J Neurosci Methods. 1989;30:203. doi: 10.1016/0165-0270(89)90131-3. Patch electrodes 4 to 7 megohm contained 120 mM D-gluconate potassium salt, 1 mM CaCl2, 1 mM NaCl, 10 mM Hepes, 11 mM bis-o-aminophenoxyethane-N,N,N′,N′-tetraacetate BAPTA tetrapotassium salt, 1 mM MgCl2, and 0.5 mM NaATP pH 7.4. Approximately 25% of the neurons 15 of 55 recorded in pre-Bötzinger Complex were rhythmically active. [DOI] [PubMed] [Google Scholar]
- 21.Approximately 23% (8 of 35) of the neurons tested had voltage-dependent oscillatory properties.
- 22.Retrograde labeling experiments were done in adult Wistar rats (n = 8) as described [ Ellenberger HH, Feldman JL. J Comp Neurol. 1990;294:202. doi: 10.1002/cne.902940205. In each animal we labeled three classes of neurons: i bulbospinal premotoneurons that project to the phrenic motor nucleus; ii propriobulbar interneurons with direct connections to more caudal medullary regions containing the highest density of bulbospinal neurons; and iii vagal motoneurons.
- 23.Near the level of obex where the bulbospinal neuron density was highest, ~40% of labeled neurons were propriobulbar; in the pre-Bötzinger Complex, ~70% of labeled neurons were propriobulbar.
- 24.Schwarzacher S, Smith JC, Richter DW. Pfluegers Arch. 1991;(suppl 1):418, R17. [Google Scholar]
- 25.Feldman JL, et al. Am J Physiol. 1990;259:R879. doi: 10.1152/ajpregu.1990.259.5.R879. [DOI] [PubMed] [Google Scholar]