Abstract
Objective
To determine if nicotine's effects are influenced by caffeine in nonsmoking, moderate-caffeine consuming individuals (N=20).
Methods
The first 3 sessions included one of 3 randomly ordered, double-blind caffeine doses (0, 75, or 150 mg, oral [po]) and 2 single-blind nicotine gum doses (2 and 4 mg) in ascending order. The fourth session (single blind) repeated the 0 mg caffeine condition.
Results
Nicotine increased heart rate and subjective ratings indicative of aversive effects, and decreased reaction times. These effects were independent of caffeine dose and reliable across sessions.
Conclusions
In nonsmokers, nicotine effects are not influenced by moderate caffeine doses.
Keywords: nicotine, caffeine, interaction, subjective effect, cognition
Nicotine and caffeine are mild psychomotor stimulants and the most widely used drugs in the United States.1,2 Interestingly, 80-97% of nicotine users also use caffeine:3 smokers drink more coffee than nonsmokers and self-administer more nicotine when using caffeine.4,5 This frequent co-administration may reflect the influence of pharmacokinetics6,7 and/or environment.8 Also, the effects of the nicotine-caffeine combination may differ from those of either drug alone.1,9
Individually, nicotine and caffeine increase heart rate,10 produce stimulation and arousal,11,12 and improve attention.13,14 Some of these effects may be enhanced when the drugs are combined. For example, oral caffeine, but not placebo, increased the subjective and reinforcing effects of intravenous (IV) nicotine (eg, higher ratings of “stimulated” and monetary crossover point, or amount of money willing to be paid to receive drug; [N = 9]9; see also [N = 12]1). Also, 2 mg nicotine gum increased accuracy on a digit-recall task when administered with active, but not placebo, caffeine [N = 6].15
Importantly, results from these studies may be influenced by the fact that all participants were regular nicotine users. When regular nicotine users are included in studies examining nicotine effects, results can be influenced by tolerance and dependence. For instance, recent nicotine exposure may result in a marked reduction of drug effect (ie, tolerance),16 whereas dependence can produce withdrawal signs and symptoms after a period of drug abstinence.17 Drug-induced suppression of these signs and symptoms18 can be mistaken for a direct effect of the drug. One way to understand the direct effects of drugs is to include individuals who are not tolerant to or dependent on them.19,20 Accordingly, there are several reports of the combined effects of nicotine and caffeine in nonnicotine users (N = 10).21,22 However, these small-sample studies may have been underpowered. Thus, their failure to report an enhanced effect of the nicotine/caffeine combination, relative to either drug alone, may reflect a Type II error. Although including individuals with no history of caffeine use would also be valuable, this goal is virtually impossible in a society where caffeine use is nearly ubiquitous.23 However, the influence of caffeine tolerance and/or withdrawal might be reduced by recruiting participants with a history of using only low-to-moderate doses (ie, 50-200 mg/day).24,25
Indeed, results from some studies of nicotine/caffeine combinations may be influenced by the range of caffeine doses tested. The typical American consumes an average of 200-250 mg caffeine/day,23 though doses 2 to 4 times greater have been used.6 For example, participants in one study were given 200 mg/70 kg oral caffeine 3 times daily for 12 days, and they also received an additional 200 or 400 mg/70 kg intravenous caffeine on testing days.9 Another study included the administration of 1 or 2 mg/kg oral caffeine every 2 hours for 10 consecutive hours on each study day (ie, 5 administrations/day).6 These doses of caffeine are higher than those typically consumed and therefore may not represent normal caffeine use outside of the laboratory.
The purpose of this study was to determine if concurrent administration of moderate doses of caffeine influenced the physiological, subjective, and cognitive effects of nicotine in 20 nonsmoking, caffeine-consuming individuals. A sample size of 20 participants was considered appropriate because it exceeded that of other studies detecting significant nicotine/caffeine interaction effects.eg, 9,15 A secondary objective was to determine whether the effects of nicotine could be measured reliably; therefore, the placebo caffeine condition was repeated on the fourth and final study day.
METHODS
Participants
All volunteers gave written consent to participate in this study, which was approved by the university's IRB board and conducted in accordance with the Helsinki declaration. Advertisements and word-of-mouth were used to recruit 12 men (11 white; 1 African American) and 8 women (6 white; 2 African American) to complete this study. Volunteers were excluded from participation if they reported recent nicotine/tobacco use (within the past year), daily caffeine intake of > 200 mg, past or current cardiovascular or psychiatric conditions, or current pregnancy or breast-feeding. They were also excluded if breath samples contained > 7 parts per million (ppm) carbon monoxide (CO; an indicator of recent smoke inhalation) at screening (mean = 2.0, SD = 0.9) or prior to the onset of any session. Volunteers were included if they were healthy and between 18 and 50 years of age (mean = 27.1 years, SD = 9.3). Based on previously defined criteria,26 participants’ daily caffeine intake ranged from 0 to 200 mg (mean = 88.2 mg, SD = 74.5).
Of the 20 participants, 4 reported using nicotine/tobacco at some time greater than 2 years prior to participation. Research shows that past smoking status may influence response to nicotine.27 There were no significant differences in any demographic characteristics for these 4 individuals, as compared to the 16 never-smokers. All participants provided informed consent and were paid $360 upon completion of the study.
Experimental Medications
Nicotine (2 and 4 mg) was administered in the form of mint-flavored, polacrilex gum (Nicorette®, GlaxoSmithKline Consumer Healthcare, LP). The selected dose range for nicotine gum was based on previous studies demonstrating clear dose-dependent effects on a variety of measures.28 Dosing was accomplished using a 15-minute gum-chewing procedure.29
Caffeine anhydrous (USP; Spectrum Quality Products, Inc.; 75 and 150 mg) and a lactose placebo were administered orally in identical size 0, opaque gelatin capsules. These doses were chosen because they are within the range of those most commonly administered in studies examining the effects of caffeine.1,10
Prior to enrollment, participants were informed that they would receive one capsule during each session and that the capsule might or might not contain the caffeine equivalent of 1 to 2 cups of coffee or 2 to 4 colas. Participants were also informed that they would be asked to chew 2 pieces of nicotine gum during each session.
Study Design
This laboratory-based study used a 3-condition, within-subject design to examine the separate and combined effects of nicotine and caffeine; a fourth condition was included to assess the reliability of nicotine's effects (placebo caffeine was administered). The first three 4.5-hour conditions included one of 3 randomly ordered, double-blind caffeine doses (0, 75, or 150 mg, po), and both of 2 single-blind nicotine gum doses (2 and 4 mg) presented in ascending dose order. The fourth 4.5-hour condition was identical, except that it always involved single-blind administration of 0 mg caffeine. Conditions were separated by 48 hours, and participants were familiarized with experimental procedures and tasks prior to their first session.
Experimental Sessions
Before each session, participants were instructed to abstain from all caffeine-containing products for 12 hours and from all foods for 1 hour. The session began with the onset of continuous heart rate and blood pressure recording. After 30 minutes, participants swallowed a caffeine capsule (0, 75, or 150 mg) followed immediately by the baseline assessment period in which all subjective and cognitive measures were administered (completed in approximately 20 minutes). The next assessment period began 1 hour after capsule ingestion. At 1.75-2.00 hours after capsule ingestion, participants chewed the 2 mg nicotine gum dose (according to a standardized procedure where they chewed once every 3 seconds for 15 minutes in response to auditory cues)29 followed immediately by another 20-minute assessment period. At 2.75-3.00 hours after capsule ingestion, participants chewed the 4 mg nicotine gum dose followed immediately by the fourth and final assessment period. This schedule of drug administration and assessment periods was constructed to assess no drug conditions (Assessment 1), the effects of each caffeine dose alone (Assessment 2), and the combination of caffeine with 2 mg nicotine (Assessment 3; caffeine effects in capsule form peak 85-110 minutes post administration)30 and with 4 mg nicotine (Assessment 4; caffeine effects in capsule form last for approximately 4 hours).30 Thus, drug administration was timed so that nicotine and caffeine effects would coincide. Importantly, maximizing the likelihood of observing a nicotine/caffeine interaction was balanced with protecting participants from nicotine intoxication (nicotine's cardiovascular effects can remain for up to 90 minutes in nonsmokers).31 Separating the 2 mg and 4 mg nicotine gum doses by 1 hour allowed the effects of the 2 mg dose to dissipate somewhat, prior to the onset of the effects of the 4 mg dose. Admittedly, protecting participants in this manner reduced the chances that the effects of 4 mg nicotine coincided with the peak effects of caffeine.
Outcome Measures
Within each session, the effects of caffeine and nicotine were assessed using physiological, subjective, and cognitive measures.
Physiological measures
Heart rate and blood pressure were monitored continuously (Noninvasive Patient Monitor model 507E, Criticare Systems, Waukesha, WI). Blood pressure was recorded automatically every 5 minutes, and heart rate every 20 seconds.
Subject-rated measures
Participants responded to several computerized (CReSS, Plowshare Technologies, Baltimore, MD) subjective questionnaires: the direct effects scale (DES), the positive and negative affect schedule (PANAS),32 and the profile of mood states (POMS).33 The DES was developed using reports describing the effects of nicotine, caffeine, and other drugs.30,34 The scale consisted of 17 items: “Do you feel any drug effects?”, “Do you like the drug effects?”, “Do you dislike the drug effects”, “Do you feel any good drug effects?”, “Do you feel any bad drug effects?”, “Nausea,” “Dizziness,” “Nervousness,” “Perspiration,” “Headache,” “Excessive salivation,” “Heart pounding,” “Confused,” “Weakness,” “Well-being,” “Confident,” and “Do you want more of the drug(s) that you received today?” These items were presented as visual analog scales (VAS) consisting of a word or phrase above a horizontal line anchored on the left with “not at all” and on the right with “extremely.” Participants used the mouse to make a mark along the horizontal line, and item scores were calculated as a percentage representing the mark's distance from the left anchor. The PANAS consists of 20 items: “Enthusiastic,” “Inspired,” “Attentive,” “Active,” “Alert,” “Excited,” “Determined,” “Strong,” “Proud,” “Interested,” “Upset,” “Hostile,” “Ashamed,” “Distressed,” “Guilty,” “Irritable,” “Afraid,” “Scared,” “Nervous,” and “Jittery.” Participants rated each item on a 5-point scale ranging from 0 (Not at all) to 4 (Extremely). Items are collapsed into 2 factors previously defined by factor analysis: positive affect (PA) and negative affect (NA). The POMS consists of 65 items related to mood that are rated on a 5-point scale ranging from 0 (Not at all) to 4 (Extremely). Items are reported as 6 previously defined factors: anger/hostility, confusion/bewilderment, depression/dejection, fatigue/inertia, tension/anxiety, and vigor/activity.
Cognitive measures
Participants completed the attention network task (ANT)35 and the N-back task.36 Data collection for all cognitive measures was controlled by commercially available software (E-prime),37 and participants were instructed to respond to each item as quickly and accurately as possible. The ANT was designed to measure 3 aspects of attention: alerting, orienting, and executive function.35 Past research has shown that these aspects of attention may be altered by exposure to nicotine.38,39 Participants indicated whether a central arrow on the screen, presented above or below a fixation cross, was pointing to the left or to the right. The arrow could be flanked on one or both sides by (a) additional arrows pointing in the same (congruent) direction, (b) additional arrows pointing in the opposite (incongruent) direction, or (c) dashes (neutral). Further, cueing conditions indicated when and/or where the next arrow presentation was to take place: (a) no cue (fixation cross only), (b) center cue (asterisk at fixation cross location) indicating the arrow presentation will occur soon, (c) double cue (asterisks above and below fixation cross) indicating the arrow presentation will occur soon, or (d) spatial cues (asterisk either above or below fixation cross) indicating the arrow presentation will occur soon and where it will occur. There were 96 trials in total, with 32 trials for each flanking condition and 24 trials of each cue type. The timing (onset and duration) of the arrow and cue conditions were as described elsewhere.35
The N-back task was designed to measure 3 aspects of working memory: the central executive, the phonological loop, and the visuospatial sketchpad. This study used a version that examines the central executive and phonological loop, which may be sensitive to nicotine's effects.40 Specifically, participants saw individual letters appear at a 2-second rate and were asked to indicate whether the presented letter was the same as the nth back letter, with n being 3 for this study. For example, if participants saw “a, d, m, a”, they would respond “yes” (by pressing a specified key), indicating they had already seen the letter “a” 3 trials back. Participants experienced a total of 48 test trials during this task.
Data Analysis
Prior to analysis, physiological data were averaged to produce one value for each of four 10-minute periods: the 10 minutes prior to and the last 10 minutes of each of 2 gum-dose administration periods. The ANT35 was scored using subtractions of the mean response times (RT) for the different conditions to calculate estimates of alerting (no cue RT minus double cue RT), orienting (center cue RT minus spatial cue RT), and executive function (incongruent stimuli RT minus congruent stimuli RT). Higher scores for alerting and orienting reflect better attentional function, whereas larger values for executive function indicate inferior performance. N-back performance was scored as response accuracy (accurate responses minus false positives), response failure (misses; number of times participant failed to respond in time allotted), and response times to accurate responses. Data collected from all measures during the first 3 randomized caffeine sessions were analyzed using a 2-factor (caffeine dose; time), repeated-measures analysis of variance (ANOVA). Huynh-Feldt corrections were used to adjust for potential violations of the sphericity assumption, and differences between means were examined using Tukey's honestly significant difference test (HSD; P<.05). To assess the reliability of nicotine's effects across study days, data collected on each of the 2 placebo caffeine days for each gum dose were correlated using the Pearson correlation coefficient. For physiological measures, difference scores (last 10 minutes of nicotine administration minus 10 minutes before nicotine administration) were computed for each gum dose and correlated; for subjective and cognitive measures, scores collected immediately after 2 and 4 mg gum administration were correlated. Across all analyses, computer malfunction and/or human error resulted in missing data for one participant on physiological and cognitive measures.
RESULTS
Results for all statistical analyses are presented in Table 1 and discussed below. As the table shows, nicotine influenced many physiological and subjective measures, whereas caffeine alone and in combination with nicotine produced minimal effects.
Table 1.
Statistical Analysis Results for All Measures
| Nicotine |
Caffeine |
Nicotine*Caffeine |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| F | P | Partialη2 | F | P | Partialη2 | F | P | Partialη2 | |
| Physiological measuresa | |||||||||
| Heart rate | 35.8 | <.001 | .665 | 1.3 | n.s. | .066 | 0.2 | n.s. | .012 |
| Systolic blood pressure | 3.9 | <.05 | .176 | 1.1 | n.s. | .057 | 1.7 | n.s. | .087 |
| Diastolic blood pressure | 3.9 | <.05 | .176 | 0.8 | n.s. | .044 | 1.0 | n.s. | .054 |
| Subject-rated measuresb | |||||||||
| Direct effects scale (DES) | |||||||||
| Do you feel any drug effects? | 65.3 | <.001 | .775 | 1.7 | n.s. | .080 | 1.2 | n.s. | .059 |
| Do you like the drug effects? | 2.0 | n.s. | .094 | 3.0 | n.s. | .137 | 0.1 | n.s. | .006 |
| Do you dislike the drug effects? | 64.5 | <.001 | .773 | 0.9 | n.s. | .043 | 1.6 | n.s. | .079 |
| Do you feel any good drug effects? | 3.1 | <.05 | .141 | 2.7 | n.s. | .125 | 0.2 | n.s. | .009 |
| Do you feel any bad drug effects? | 61.6 | <.001 | .764 | 1.4 | n.s. | .069 | 1.1 | n.s. | .054 |
| Nausea | 14.7 | <.001 | .436 | 1.2 | n.s. | .061 | 0.7 | n.s. | .034 |
| Dizziness | 19.8 | <.001 | .510 | 1.2 | n.s. | .060 | 0.9 | n.s. | .044 |
| Nervousness | 14.0 | <.001 | .424 | 2.3 | n.s. | .107 | 1.8 | n.s. | .085 |
| Perspiration | 5.5 | <.05 | .224 | 2.5 | n.s. | .115 | 0.8 | n.s. | .038 |
| Headache | 4.4 | <.05 | .187 | 1.4 | n.s. | .066 | 2.2 | n.s. | .101 |
| Excessive salivation | 7.2 | <.05 | .276 | 0.5 | n.s. | .024 | 0.3 | n.s. | .015 |
| Heart pounding | 4.4 | <.05 | .188 | 0.2 | n.s. | .011 | 0.2 | n.s. | .008 |
| Confused | 7.2 | <.05 | .275 | 1.8 | n.s. | .088 | 0.9 | n.s. | .046 |
| Weakness | 6.7 | <.01 | .262 | 1.4 | n.s. | .068 | 0.9 | n.s. | .044 |
| Well-being | 0.2 | n.s. | .013 | 0.6 | n.s. | .029 | 0.5 | n.s. | .026 |
| Confident | 0.8 | n.s. | .038 | 1.5 | n.s. | .072 | 0.8 | n.s. | .041 |
| Do you want more of the drug(s)? | 0.6 | n.s. | .030 | 1.0 | n.s. | .050 | 0.6 | n.s. | .030 |
| Positive and negative affect scale | |||||||||
| (PANAS) | |||||||||
| Negative affect | 8.1 | <.01 | .300 | 2.4 | n.s. | .110 | 0.3 | n.s. | .016 |
| Positive affect | 1.3 | n.s. | .066 | 1.9 | n.s. | .091 | 1.8 | n.s. | .087 |
| Profile of mood states (POMS) | |||||||||
| Anger | 4.9 | <.05 | .206 | 0.1 | n.s. | .006 | 0.3 | n.s. | .014 |
| Confusion | 3.5 | n.s. | .157 | 1.6 | n.s. | .079 | 0.4 | n.s. | .019 |
| Depression | 3.6 | n.s. | .161 | 3.0 | n.s. | .137 | 0.7 | n.s. | .034 |
| Fatigue | 1.2 | n.s. | .059 | 0.8 | n.s. | .038 | 1.5 | n.s. | .075 |
| Tension | 12.7 | <.001 | .401 | 2.3 | n.s. | .109 | 0.4 | n.s. | .022 |
| Vigor | 1.1 | n.s. | .052 | 2.1 | n.s. | .099 | 1.4 | n.s. | .067 |
| Cognitive effectsc | |||||||||
| Attention network task (ANT) | |||||||||
| Alerting | 0.3 | n.s. | .015 | 1.6 | n.s. | .080 | 0.4 | n.s. | .020 |
| Orienting | 1.0 | n.s. | .051 | 3.6 | <.05 | .165 | 1.1 | n.s. | .058 |
| Executive function | 0.1 | n.s. | .007 | 0.2 | n.s. | .011 | 0.6 | n.s. | .032 |
| N-Back | |||||||||
| Accuracy | 0.6 | n.s. | .032 | 4.7 | <.05 | .208 | 3.2 | <.01 | .150 |
| Misses | 2.6 | n.s. | .127 | 1.0 | n.s. | .052 | 1.1 | n.s. | .058 |
| Reaction time | 4.4 | <.05 | .195 | 1.6 | n.s. | .084 | 0.1 | n.s. | .008 |
Note.
N=19; Nicotine df = (4,72), Caffeine df = (2,36), Nicotine*Caffeine df = (8,144).
N=20; Nicotine df = (3,57), Caffeine df = (2,38), Nicotine*Caffeine df = (6,114).
N=19; Nicotine df = (3,54), Caffeine df = (2,36), Nicotine*Caffeine df = (6,108).
Physiological Measures
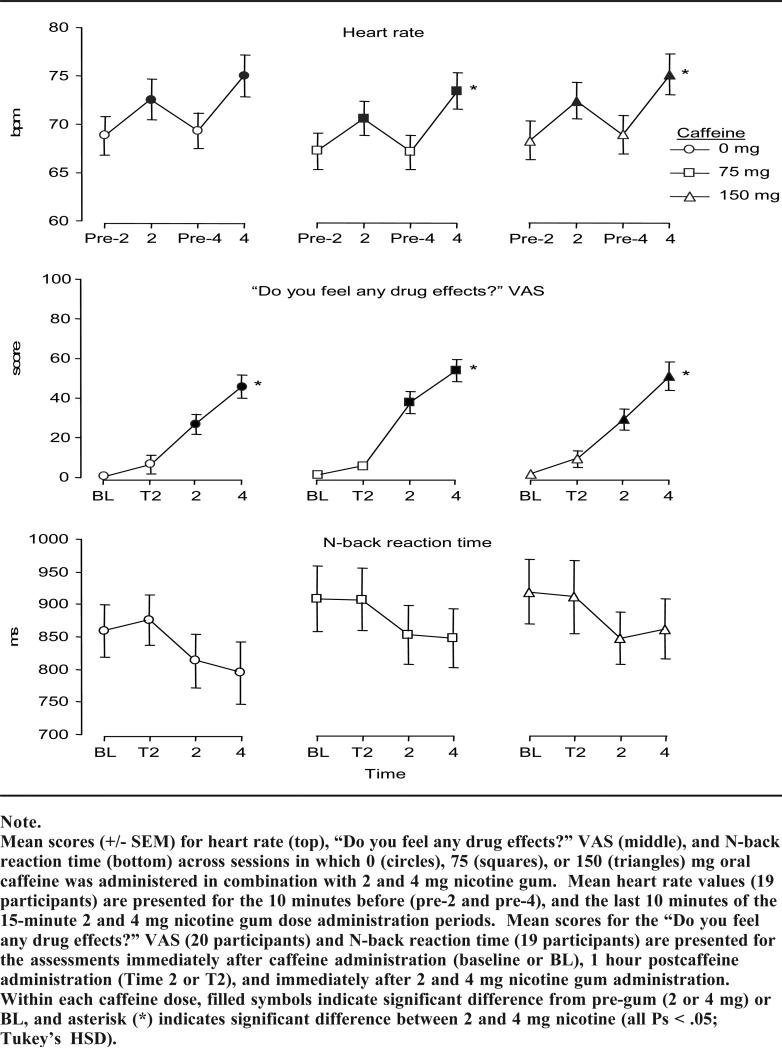
Heart rate and blood pressure were increased significantly by nicotine (ie, Fs > 3.9, Ps < .05), though not by caffeine when presented alone, or in combination with nicotine (Fs < 1.7, Ps > .05). For instance, Figure 1 (top) illustrates the effects of each nicotine dose on heart rate (the physiological measure with the largest F value for the main effect of nicotine) across the 3 caffeine doses. As seen in the figure, the last 10 minutes of the 2 and 4 mg nicotine gum administration periods were associated with increased heart rate, relative to the 10 minutes before administration, and these increases were of similar magnitude across all doses of caffeine. Collapsed across caffeine dose, mean heart rate increased significantly from 68.1 bpm (SD = 8.3) pre-2 mg nicotine to 71.9 bpm (SD = 8.3) during the 2 mg nicotine gum chew period and again to 74.5 bpm (SD = 8.8) during the 4 mg nicotine gum chew period (2 and 4 mg significantly greater than baseline value, and from each other; Tukey's HSD, P < .05). Likewise, mean diastolic blood pressure increased from 70.2 mmHg (SD = 9.8) pre-2 mg nicotine to 71.9 mmHg (SD = 7.9) during 2 mg nicotine, and again to 73.5 mmHg (SD = 8.4) during 4 mg nicotine. A similar pattern of results was observed for systolic blood pressure.
Figure 1.
Physiological, Subjective, and Cognitive Effects of Caffeine Combined with Nicotine
Subjective Measures
Overall, nicotine alone produced significant increases on a variety of DES measures indicative of aversive effects (ie, Fs > 3.1, Ps < .05), including “drug effect,” “bad effects,” “dislike,” “dizziness,” “nausea,” and “nervousness.” No statistically significant main effects of caffeine or nicotine/caffeine interactions were observed on any DES item (Fs < 3.0, Ps > .05). Collapsed across caffeine dose, scores for most DES items on which a significant main effect of nicotine was observed were generally similar at baseline (BL; immediately after capsule administration) and time 2 (T2; one hour after capsule administration), whereas significant increases were observed immediately following the 2 and 4 mg nicotine gum chewing procedure. For instance, Figure 1 (middle) illustrates the effects of each nicotine dose on the DES item “drug effect” (the subjective effect measure with the largest F value) across the 3 doses of caffeine. As seen in the figure, nicotine gum increased ratings relative to pre-gum values, and the higher nicotine dose produced greater effects. Collapsed across caffeine dose, mean scores for drug effect were 1.1 (SD = 4.7) at baseline and 7.1 (SD = 17.6) at time 2, but increased to 31.3 (SD = 23.5) after 2 mg nicotine (significantly greater than baseline value, P < .05, Tukey's HSD), and 50.3 (SD = 27.8) after 4 mg nicotine gum administration (significantly greater than after 2 mg nicotine, P<.05, Tukey's HSD). A similar pattern of results was observed for other DES items on which a significant main effect of nicotine was observed.
Nicotine alone influenced PANAS and POMS results, whereas caffeine alone, or in combination with nicotine, did not (Fs < 3.0, Ps >.05). Collapsed across caffeine dose, negative affect scale scores were low at baseline (mean = 1.5, SD = 2.2) and time 2 (mean = 1.6, SD = 2.8), and then increased after 2 mg (mean = 2.9, SD = 3.7) and 4 mg nicotine (mean = 4.9, SD = 5.9; significantly greater than baseline value, P<.05, Tukey's HSD). Similar results were seen with the POMS anger and tension subscales.
Cognitive Measures
As Table 1 reveals, neither nicotine nor the combination of nicotine and caffeine altered any component of the ANT reliably (ie, alerting, orienting, or executive function; Fs < 1.1, Ps > .05). However, caffeine altered the orienting component of the ANT (ie, main effect of caffeine; F = 3.6, P<.05). Collapsed across all time points, orienting performance increased with increasing caffeine dose: 40.3 (SD = 26.6) for 0 mg caffeine, 41.1 (SD = 30.9) for 75 mg caffeine, and 50.6 (SD = 27.8) for 150 mg caffeine. Table 1 also shows some effects of nicotine and caffeine on N-back performance. For instance, there was a significant nicotine-by-caffeine interaction for accuracy. Scores at 4 mg nicotine were higher in the 150 mg caffeine condition (4 mg mean = 0.76, SD = 0.21), relative to when this dose was administered after 0 (4 mg mean = 0.69, SD = 0.20) and 75 mg caffeine (4 mg mean = 0.64, SD = 0.23; n.s., Tukey's HSD). Also, a significant effect of nicotine was observed for N-back reaction time: across all caffeine doses, reaction times were decreased after nicotine gum, relative to pre-gum values (Figure 1, bottom).
Assessment of Reliability: Correlation Coefficients Across Study Days
Measures on which significant effects of 2 and 4 mg nicotine gum were observed during the randomized portion of the study showed large to moderate correlations across the 2 study days when placebo caffeine was administered (ie, first as part of the 3 randomized caffeine conditions and again during a fourth condition). For example, for heart rate, the cross-day correlation coefficient for 2 mg gum was 0.79 and for 4 mg gum was 0.77 (Ps < .01). Likewise, moderate correlations were observed for self-report measures for which a significant main effect of nicotine is reported in Table 1 (r's > .45, Ps < .05). In addition, N-back reaction time was correlated moderately across study days for 2 mg (r = .49, P < .05) and 4 mg (r = .38) gum doses.
DISCUSSION
Epidemiological and clinical research suggests a potential interaction between nicotine and caffeine.3,4,9,10 In clinical research, the tolerance and dependence associated with frequent nicotine and/or caffeine use may influence study results.19,20 In this study, we eliminated the potential influence of tobacco use and reduced the influence of caffeine use in order to examine the separate and combined effects of these drugs on measures of physiological, subjective, and cognitive responding. In addition, we included doses of caffeine that reflect more accurately those administered during a single episode of caffeine self-administration in the real world. Overall, nicotine produced effects that were independent of caffeine dose and were reliable across session days.
Consistent with results from past research with nonsmokers,31,41 nicotine affected cardiovascular response significantly and produced a variety of aversive subjective effects in nonsmokers.41 In contrast, results provided minimal evidence for nicotine's influence on cognition. Only N-back reaction time was influenced by nicotine, an effect that is consistent with previous findings of nicotine-induced enhancement.38,43 However, other work has shown that nicotine has no effect on cognition in this population28,42 or even produces impairment,41,44 suggesting further exploration of the specific cognitive processes responsive to nicotine.42
The moderate caffeine doses used in this study did not influence either physiological or subjective response. Although these results seem to contradict some previous work,6,9 the higher doses used in those studies (up to 4 times greater than the 150 mg dose used in this study) may account for the discrepancy (but see also1). As for caffeine's influence on cognition, the drug altered the orienting component of attention (improved orienting effect for the ANT) and interacted with nicotine to influence working memory accuracy (nicotine-related performance was best at the highest caffeine dose). These findings contrast with the lack of subjective and physiological results reported above and may indicate that some cognitive indices are more sensitive to the effects of caffeine than are behavioral or physiological measures. Interestingly though, of the available studies examining the cognitive effects of caffeine in light (< 150 mg/day) or nonusers, few significant results have been reported.25,45,46 Thus, caffeine's cognitive enhancing effects may be most evident only for some aspects of processing or when the drug is administered to regular users following a period of abstinence.46,47
This study provided little evidence that the effects of nicotine are influenced by concurrent caffeine (with the exception of working memory accuracy as noted above) and is consistent with other studies examining the combination of these drugs in nonsmoking, moderate-caffeine-consuming individuals.21,22 This observation may suggest that the co-administration of tobacco and caffeine-containing beverages reflects a behavioral rather than pharmacological mechanism. Sensory characteristics associated with common nicotine and caffeine vehicles (ie, cigarettes, coffee) can influence self-administration. For example, regular tobacco users drinking coffee smoked more cigarettes than those not drinking coffee (ie, no drink), but this effect was independent of number of cups/session (1, 2, or 3).48 Perhaps more important, smokers who were drinking coffee smoked more cigarettes than those drinking water, but the number of cigarettes and the number of puffs was independent of whether the coffee was caffeinated or decaffeinated.48 Indeed, participants drinking a 100% caffeine-free coffee substitute or water smoked significantly more cigarettes than did nondrinking controls. Thus, the stimuli associated with the liquid vehicle, rather than caffeine per se, may have influenced tobacco consumption in this experiment.48 Moreover, the failure of these studies, as well as others,49 to report any dose-related effects of caffeine on smoking suggests that the interaction between these 2 drugs may not be the result of a pharmacological mechanism.
Study limitations include the lack of a nicotine placebo, the timing of nicotine and caffeine administration, unverified compliance with caffeine use restrictions, and sample size. Although a placebo nicotine gum condition would have been ideal, commercially manufactured placebo nicotine gum was not available for use in this study. The dose relationship observed on most nicotine effects suggests that these effects were mediated pharmacologically (though the magnitude of this dose-relationship may have been influenced by the cumulative effects at 4 mg nicotine gum administered one hour after 2 mg nicotine gum). Other nicotine delivery methods are available, but are either too slow (ie, transdermal) for the study design or too fast (ie, intravenous) for non-nicotine-using participants. This study's dosing schedule may not have maximized the possibility of observing an interaction: nicotine gum was administered 1.75-2.00 (2 mg) and 2.75-3.00 (4 mg) hours postcaffeine, and, although caffeine's effects can last up to 4 hours, the effects of caffeine in capsule form have been shown to peak at 1 hour.30,50 Overnight caffeine abstinence was required but not verified; noncompliance (ie, recent caffeine consumption) may have reduced the likelihood of observing the direct effects of caffeine or a nicotine/caffeine interaction. Finally, the sample size used here exceeds that of previous studies of the combined effects of nicotine and caffeine in regular users (eg, 9-19 participants) and nonusers of nicotine (eg, 10 participants), but may nonetheless have limited study sensitivity. As shown in Table 1, small effect sizes were observed for the interaction terms (η2 values ranged from 0.01 to 0.15), whereas those for the nicotine main effect terms yielded η2 values as high as 0.78. Thus, future studies examining the combined influence of nicotine and caffeine in nonusers might benefit from a power analysis using these sample sizes, and researchers may want to consider including more rather than fewer participants.
In sum, this study's results do not support the idea that nicotine and caffeine interact to produce effects that are different from those of either drug alone. Based on these results, as well as others examining the role of sensory stimuli in nicotine consumption,48 nonpharmacological factors may mediate the co-administration of these 2 drugs.
Acknowledgments
This work was supported by PHS grants R01 DA011082, R01 CA103827, F31 DA018447, and F31 DA017437. Portions of this work were presented at the Ninth Annual Meeting of the Society for Research on Nicotine and Tobacco, February 19-22, 2003.
REFERENCES
- 1.Rose JE, Behm FM. Psychophysiological interactions between caffeine and nicotine. Pharmacol Biochem Behav. 1991;38:333–337. doi: 10.1016/0091-3057(91)90287-c. [DOI] [PubMed] [Google Scholar]
- 2.Tanda G, Goldberg SR. Alteration of the behavioral effects of nicotine by chronic caffeine exposure. Pharmacol Biochem Behav. 2000;66:47–64. doi: 10.1016/s0091-3057(00)00234-3. [DOI] [PubMed] [Google Scholar]
- 3.Swanson JA, Lee JW, Hopp JW. Caffeine and nicotine: a review of their joint use and possible interaction effects in tobacco withdrawal. Addict Behav. 1994;19:229–256. doi: 10.1016/0306-4603(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 4.Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interactions. Psychol Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- 5.Emurian HH, Nellis MJ, Brady JV, et al. Event time-series relationship between cigarette smoking and coffee drinking. Addict Behav. 1982;7:441–444. doi: 10.1016/0306-4603(82)90016-8. [DOI] [PubMed] [Google Scholar]
- 6.Brown CR, Benowitz NL. Caffeine and cigarette smoking: behavioral, cardiovascular, and metabolic interactions. Pharmacol Biochem Behav. 1989;34:565–570. doi: 10.1016/0091-3057(89)90559-5. [DOI] [PubMed] [Google Scholar]
- 7.Brown CR, Jacob P, Wilson M, et al. Changes in rate and pattern of caffeine metabolism after cigarette abstinence. Clin Pharmacol Therap. 1988;43:488–491. doi: 10.1038/clpt.1988.63. [DOI] [PubMed] [Google Scholar]
- 8.Conway TL, Vickers RR, Jr, Ward HW, et al. Occupational stress and variation in cigarette, coffee, and alcohol consumption. J Health Soc Behav. 1981;22:155–165. [PubMed] [Google Scholar]
- 9.Jones HE, Griffiths RR. Oral caffeine maintenance potentiates the reinforcing and stimulant subjective effects of intravenous nicotine in cigarette smokers. Psychopharmacology. 2003;165:280–290. doi: 10.1007/s00213-002-1262-4. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert DG, Dibb WD, Plath LC, et al. Effects of nicotine and caffeine, separately and in combination, on EEG topography, mood, heart rate, cortisol, and vigilance. Psychophysiology. 2000;37:583–595. [PubMed] [Google Scholar]
- 11.Garrett BE, Griffiths RR. Intravenous nicotine and caffeine: subjective and physiological effects in cocaine abusers. J Pharmacol Exp Therap. 2001;296:486–494. [PubMed] [Google Scholar]
- 12.Perkins KA, Grobe JE, Weiss D, et al. Nicotine preference in smokers as a function of smoking abstinence. Pharmacol Biochem Behav. 1996;55:257–263. doi: 10.1016/s0091-3057(96)00079-2. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman HR, Wurtman RJ, Emde GG, et al. The effects of low doses of caffeine on human performance and mood. Psychopharmacology. 1987;92:308–312. doi: 10.1007/BF00210835. [DOI] [PubMed] [Google Scholar]
- 14.Warburton DM, Mancuso G. Evaluation of the information processing and mood effects of a transdermal nicotine patch. Psychopharmacology. 1998;135:305–310. doi: 10.1007/s002130050514. [DOI] [PubMed] [Google Scholar]
- 15.Cohen C, Pickworth WB, Bunker EB, et al. Caffeine antagonizes EEG effects of tobacco withdrawal. Pharmacol Biochem Behav. 1994;47:919–936. doi: 10.1016/0091-3057(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 16.Perkins KA, Stitzer ML. In: Behavioral pharmacology of nicotine. In Handbook of substance abuse: neurobehavioral pharmacology. Tarter RE, Ammerman RT, Ott PJ, editors. Plenum Press; New York: 1998. pp. 299–317. [Google Scholar]
- 17.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 18.Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: a review of effects on human performance. Exp Clin Psychopharmacol. 1994;2:345–395. [Google Scholar]
- 19.Jarvik ME. Beneficial effects of nicotine. Br J Addict. 1991;86:571–575. doi: 10.1111/j.1360-0443.1991.tb01810.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40:1243–1255. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- 21.Kerr JS, Sherwood N, Hindmarch I. Separate and combined effects of the social drugs on psychomotor performance. Psychopharmacology. 1991;104:113–119. doi: 10.1007/BF02244564. [DOI] [PubMed] [Google Scholar]
- 22.Smits P, Temme L, Thien T. The cardiovascular interaction between caffeine and nicotine in humans. Clin Pharmacol Therap. 1993;54:194–204. doi: 10.1038/clpt.1993.131. [DOI] [PubMed] [Google Scholar]
- 23.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 24.Daniels JW, Mole PA, Shaffrath JD, et al. Effects of caffeine on blood pressure, heart rate, and forearm blood flow during dynamic leg exercise. J Appl Physiol. 1998;85:154–159. doi: 10.1152/jappl.1998.85.1.154. [DOI] [PubMed] [Google Scholar]
- 25.Herz RS. Caffeine effects on mood and memory. Behav Res Ther. 1999;37:869–879. doi: 10.1016/s0005-7967(98)00190-9. [DOI] [PubMed] [Google Scholar]
- 26.Tiffin P, Ashton H, Marsh R, et al. Pharmacokinetic and pharmacodynamic responses to caffeine in poor and normal sleepers. Psychopharmacology. 1995;121:494–502. doi: 10.1007/BF02246500. [DOI] [PubMed] [Google Scholar]
- 27.Hughes JR, Strickler G, King D, et al. Smoking history, instructions, and the effects of nicotine: 2 pilot studies. Pharmacol Biochem Behav. 1989;34:149–155. doi: 10.1016/0091-3057(89)90366-3. [DOI] [PubMed] [Google Scholar]
- 28.Heishman SJ, Snyder FR, Henningfield JE. Performance, subjective, and physiological effects of nicotine in non-smokers. Drug and Alcohol Depend. 1993;34:11–18. doi: 10.1016/0376-8716(93)90041-n. [DOI] [PubMed] [Google Scholar]
- 29.Houtsmuller EJ, Fant RV, Eissenberg TE, et al. Flavor improvement does not increase abuse liability of nicotine chewing gum. Pharmacol Biochem Behav. 2002;72:559–568. doi: 10.1016/s0091-3057(02)00723-2. [DOI] [PubMed] [Google Scholar]
- 30.Liguori A, Hughes JR, Grass JA. Absorption and subjective effects of caffeine from coffee, cola, and capsules. Pharmacol Biochem Behav. 1997;58:721–726. doi: 10.1016/s0091-3057(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 31.Nyberg G, Panfilov V, Sivertsson R, et al. Cardiovascular effects of nicotine chewing gum in healthy non-smokers. Eur J Clin Pharmacol. 1982;23:303–307. doi: 10.1007/BF00613610. [DOI] [PubMed] [Google Scholar]
- 32.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 33.McNair DM, Lorr M, Droppelman L. Profile of Mood States (Manual) Education and Industrial Testing Services; San Diego, CA: 1971. [Google Scholar]
- 34.Dale LC, Hurt RD, Offord KP, et al. High-dose nicotine patch therapy: percentage of replacement and smoking cessation. JAMA. 1995;274:1353–1358. [PubMed] [Google Scholar]
- 35.Fan J, McCandliss BD, Sommer T, et al. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 36.Jonides J, Schumacher EH, Smith EE, et al. Verbal working memory load affects regional brain activation as measured by PET. J Cogn Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- 37.Schneider W, Eschman A, Zuccolotto A. E-Prime User's Guide. Psychology Software Tools Inc; Pittsburgh: 2002. [Google Scholar]
- 38.Mumenthaler MS, Taylor JL, O'Hara R, et al. Influence of nicotine on simulator flight performance in non-smokers. Psychopharmacology. 1998;140:38–41. doi: 10.1007/s002130050736. [DOI] [PubMed] [Google Scholar]
- 39.Wesnes K, Warburton DM, Matz B. Effects of nicotine on stimulus sensitivity and response bias in a visual vigilance task. Neuropsychobiology. 1983;9:41–44. doi: 10.1159/000117935. [DOI] [PubMed] [Google Scholar]
- 40.Kumari V, Gray JA, ffytche DH, et al. Cognitive effects of nicotine in humans: an fMRI study. Neuroimage. 2003;19:1002–1013. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- 41.Heishman SJ, Henningfield JE. Tolerance to repeated nicotine administration on performance, subjective, and physiological responses in nonsmokers. Psychopharmacology. 2000;152:321–333. doi: 10.1007/s002130000541. [DOI] [PubMed] [Google Scholar]
- 42.Kleykamp BA, Jennings JM, Blank MD, et al. The effects of nicotine on attention and working memory in never-smokers. Psych Addict Behav. 2005;19:433–438. doi: 10.1037/0893-164X.19.4.433. [DOI] [PubMed] [Google Scholar]
- 43.Ernst M, Heishman SJ, Spurgeon L, et al. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- 44.Foulds J, Stapleton J, Swettenham J, et al. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology. 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- 45.Fine BJ, Kobrick JL, Lieberman HR, et al. Effects of caffeine or diphenhydramine on visual vigilance. Psychopharmacology. 1994;114:233–238. doi: 10.1007/BF02244842. [DOI] [PubMed] [Google Scholar]
- 46.Smit HJ, Rogers PJ. Effects of low doses of caffeine on cognitive performance, mood, and thirst in low and higher caffeine users. Psychopharmacology. 2000;152:167–173. doi: 10.1007/s002130000506. [DOI] [PubMed] [Google Scholar]
- 47.Hasenfratz M, Battig K. Action profiles of smoking and caffeine: Stroop effect, EEG, and peripheral physiology. Pharmacol Biochem Behav. 1992;42:155–161. doi: 10.1016/0091-3057(92)90459-s. [DOI] [PubMed] [Google Scholar]
- 48.Marshall WR, Epstein LH, Green SB. Coffee drinking and cigarette smoking: I. Coffee, caffeine, and cigarette smoking behavior. Addict Behav. 1980;5:389–394. doi: 10.1016/0306-4603(80)90012-x. [DOI] [PubMed] [Google Scholar]
- 49.Kozlowski LT. Effects of caffeine consumption on nicotine consumption. Psychopharmacologia. 1976;47:165–168. doi: 10.1007/BF00735816. [DOI] [PubMed] [Google Scholar]
- 50.Griffiths RR, Evans SM, Heishman SJ, et al. Low-dose caffeine discrimination in humans. J Pharmacol Exp Therap. 1990;252:970–978. [PubMed] [Google Scholar]