Abstract
Trauma is the leading cause of death in young adults and acute blood loss contributes to a large portion of mortality in the early post-trauma period. The recognition of lethal triad of coagulopathy, hypothermia and acidosis has led to the concepts of damage control surgery and resuscitation. Recent experience with managing polytrauma victims from the Iraq and Afghanistan wars has led to a few significant changes in clinical practice. Simultaneously, transfusion practices in the civilian settings have also been extensively studied retrospectively and prospectively in the last decade. Early treatment of coagulopathy with a high ratio of fresh frozen plasma and platelets to packed red blood cells (FFP:platelet:RBC), prevention and early correction of hypothermia and acidosis, monitoring of hemostasis using point of care tests like thromoboelastometry, use of recombinant activated factor VII, antifibrinolytic drugs like tranexamic acid are just some of the emerging trends. Further studies, especially in the civilian trauma centers, are needed to confirm the lessons learned in the military environment. Identification of patients likely to need massive transfusion followed by immediate preventive and therapeutic interventions to prevent the development of coagulopathy could help in reducing the morbidity and mortality associated with uncontrolled hemorrhage in trauma patients.
Keywords: Coagulopathy, hemorrhage, massive transfusion, trauma, transfusion, thromboelastometry
INTRODUCTION
An estimated 5 million people worldwide died from injuries in 2000 – a mortality rate of 83.7 per 100,000 population. The World Health Organization (WHO) estimates that the burden of disease related to injuries, particularly road traffic injuries, interpersonal violence, war and self-inflicted injuries is expected to rise dramatically by the year 2020.[1] Trauma is the leading cause of death in all ages from 1 to 44 years.[2] Up to 40% of polytrauma patients die as a result of circulatory shock from acute blood loss.[3] Besides surgical control of hemorrhage, adequate volume resuscitation with blood products and fluids is crucial for the survival of these victims. In this article, we review the trends in transfusion practices in trauma patients.
TRANSFUSION TRIGGERS IN TRAUMA
The basis of RBC transfusion is to augment the oxygen delivery to tissues. The guidelines from Advanced Trauma Life Support (ATLS) suggest that in patients with hypovolemic shock, failure to restore normal vital signs after infusion of 40–60 mL/kg of crystalloids should trigger the need for transfusion of blood products. Where laboratory tests are available, clinicians have traditionally used a hemoblogin (Hb) level of 10 g% or a hematocrit (Hct) of 30% as the lowest acceptable level. Several other physiologic measures such as mixed venous oxygen saturation, oxygen extraction ratio,[4] near infra-red spectroscopic estimation of regional cerebral oxyhemoglobin concentration, and brain tissue oxygenation[5] have been suggested as potential transfusion triggers in polytrauma. Recently, survival has been reported in a patient with Hb level of 0.7 g% (Hct 2.2%).[6] The risks of allogenic blood transfusion with all the attendant risks should be carefully balanced with the benefits of optimizing oxygen delivery to the tissues. Blood transfusion is an independent risk factor for infection and increased resource utilization in combat trauma.[7] One should exercise caution in allowing the Hb level to drop below 6 g%, especially in the presence of ongoing blood loss and coagulopathy. Recent guidelines for perioperative transfusion from the American Society of Anesthesiologists,[8] and for trauma transfusion from the UK and Europe are excellent resources[2,9] for further reading on this topic.
MASSIVE TRANSFUSION
Massive transfusion (MT) refers to the transfusion of 10 or more RBC products in 24 hours or to replacement of one or more RBC blood volumes in pediatric patients.[10,11] When there is no time to do blood typing and crossmatching, group O RBCs and AB plasma products should be used till the patient's blood type is known. Women of child bearing potential should receive group O, D-negative RBCs till type-specific and cross-matched blood is available.[12] Table 1 shows the approximate times required for the various products at our level I trauma center. Institutions that handle polytrauma patients must have their own MT protocol (MTP) that is developed by trauma team and blood bank services. Table 2 shows one such protocol. MT using preset packages of various blood components in a predetermined sequence is commonly used till the laboratory tests can guide further transfusion. These transfusion packs and the protocols help to reduce the delay in ordering, preparing and transfusing the products and also to reduce the amount of crystalloids infused and thus reduce the dilutional coagulopathy.[13] Figure 1shows the conventional triggers for administration of various fluids and blood products based on laboratory testing.
Table 1.
Sequence for transfusing and approximate time needed for availability

Table 2.
Predetermined blood product administration in massive transfusion in trauma victims. A sample protocol
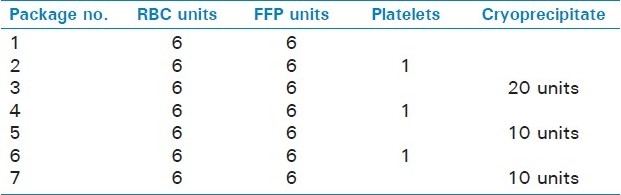
Figure 1.
Laboratory-based blood product administration. Fluid and blood component treatment in major bleeding. Values of various parameters represent trigger points at which relevant blood components are presently transfused in a non-massive transfusion. RBC = red blood cells; FFP = fresh frozen plasma; PCC = prothrombin complex concentrate; Fg = fi brinogen; Plt = platelets; Hct = hematocrit; PT = prothrombin time; aPTT = activated partial thromboplastin time (Reproduced with permission from Br J Anaesth)[12,44]
MONITORING HEMOSTASIS IN TRAUMA PATIENTS
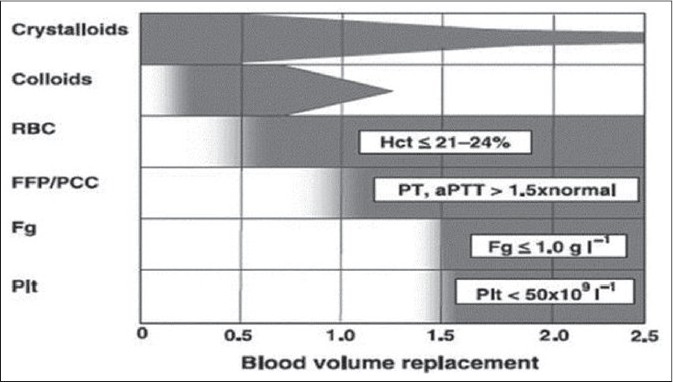
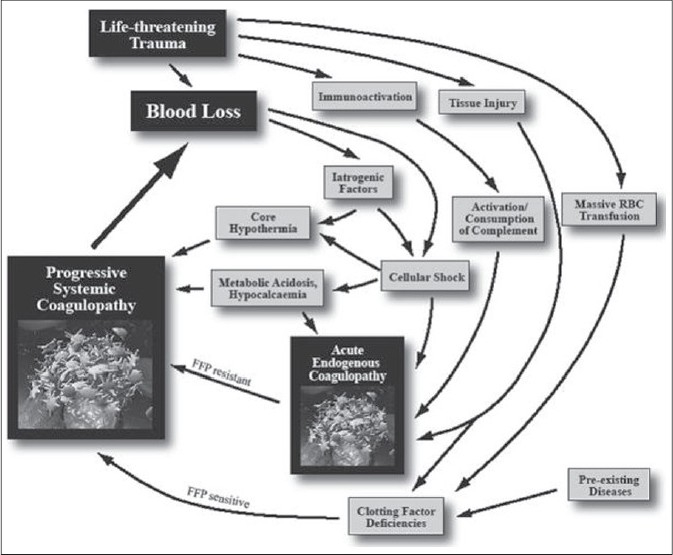
Thus far, prothrombin time (PT) and activated partial thromboplastin time (aPTT) have been used to guide the need for procoagulant factor administration during blood transfusion.[14] However, these in vitro tests are not reliable in the rapidly changing hemostatic circumstances where hemodilution, consumption, hypothermia, and acidosis constantly alter the levels and functioning of clotting factors. Also, these tests cannot provide information on the platelet function.[15] Thromboelastography (TEG), which can quantify initiation of clotting, propagation kinetics, fibrin-platelet interactions, clot strength and fibrinolysis may be a more suitable test in the hemorrhaging trauma victim.[16] The benefits of goal-directed transfusion therapy by real-time assessment of coagulation function via point of care rapid thromboelastography and rotational thromboelastometry (ROTEM) are being reported by several investigators.[17–19] Figure 2 shows the development of acute endogenous coagulopathy of trauma which progressively becomes worse with dilution and consumption of procoagulant factors, acidosis and, hypothermia. Figures 3 and 4 show the principle/technique of TEG, the normal thrombolestogram and the impact of various types of coagulation abnormalities on the thromboelastogram.
Figure 2.
Acute endogenous coagulopathy occurs very early after injury and is present even before signifi cant blood loss occurs. Subsequently, dilution and consumption of procoagulant factors, acidosis and, hypothermia lead to progressive systemic coagulopathy (Reproduced with permission from [17])
Figure 3.
Technique of thrombelastography (reprinted with permission from Hemoscope Corporation, Niles, IL, USA) (Reproduced with permission from 17). (A) A torsion wire suspending a pin is immersed in a cuvette filled with blood. A clot forms while the cuvette is rotated 45°, causing the pin to rotate depending on the clot strength. A signal is then discharged to the transducer that refl ects the continuity of the clotting process. The subsequent tracing (B) corresponds to the entire coagulation process from thrombin generation to fi brinolysis. The R value, which is recorded as TEG-ACT in the rapid TEG specimen, is a reflection of enzymatic clotting factor activation. The K value is the interval from the TEG-ACT to a fixed level of clot firmness, refl ecting thrombin's cleavage of soluble fibrinogen. α is the angle between the tangent line drawn from the horizontal base line to the beginning of the cross-linking process. The MA, or maximum amplitude, measures the end result of maximal platelet– fibrin interaction, and Ly30 is the percent lysis which occurs at 30 minutes from the initiation of the process, which is also calculated as the EPL, or estimated percent lysis
Figure 4.
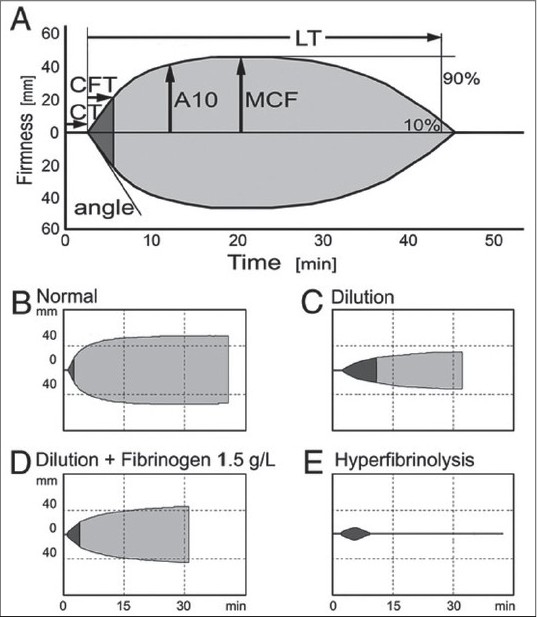
Thromboelastometry after dilution. Thromboelastometry assesses the kinetics of clot formation and stability or lysis of the formed clot. (A) Thromboelastometric parameters are defi ned as follows: Initiation of coagulation measured as coagulation time (CT) shows initial thrombin and fibrin formation. Propagation of clot formation is a function of the interactions of fibrin(ogen) with platelets. It is measured as α angle or clot formation time (CFT), which is defined as the time needed to achieve a clot firmness of 20 mm. Maximal clot firmness (MCF) represents the final clot strength and results from firm aggregation of platelets and formation of a stable fibrin network. A10 represents the amplitude 10 min after the onset of clot formation. Clinically relevant fibrinolysis can be diagnosed by shortened lysis time (LT), which is defi ned by the time to diminish the clot fi rmness to 10% of maximal clot firmness. (B–E) Thromboelastometric patterns in normal whole blood (B), after severe dilution (C), after severe dilution and supplementation with 1.5 g/L fibrinogen (D), and in hyperfibrinolysis (E). (Data are adapted from Bolliger D, Szlam F, Molinaro RJ, Rahe-Meyer N, Levy JH, Tanaka KA: Finding the optimal concentration range for fibrinogen replacement after severe haemodilution: An in vitro model. Br J Anaesth 2009; 102:793-9, used by permission of Oxford University Press. Reproduced with permission from [34])
DAMAGE CONTROL RESUSCITATION
Damage control resuscitation (DCR) differs from conventional resuscitation in that it attempts an earlier and more aggressive correction of coagulopathy and metabolic derangements.[20] Though beyond the scope of this article, the key components of DCR include permissive hypotension, preferential use of blood products over isotonic fluids, and early correction of coagulopathy with goal-directed component therapy.[21] DCR has been shown to be associated with more blood products and less crystalloid solution in the perioperative period, and when used in combination with damage control surgery, offers a survival advantage and shorter trauma intensive care unit length of stay in patients with severe hemorrhage.[22]
CELL SAVER
In a retrospective study comparing trauma surgical patients who received intraoperative cell salvage versus those who did not, Brown et al. reported that intraoperative use of cell saver reduces the need for allogenic blood products and results in reduced cost.[23] Most relevant contraindication for the use of cell saver is the contamination of collected blood by other body fluids such as gut contents.
FRESH FROZEN PLASMA
In the absence of laboratory tests to guide the administration of procoagulant factors in fresh frozen plasma (FFP), the optimal ratio for administration of Packed red blood cells (PRBC): FFP remains debatable. Reports from military[24] and civilian[10] settings indicate that early use of FFP in a ratio of 1:1–1.5:1 (RBC:FFP) is associated with lower mortality in massively transfused trauma victims. Kashuk et al. reported that 1:1 ratio of FFP:RBC did reduce coagulopathy but did not reduce mortality. They suggest that a ratio of 1:2–1:3 (FFP:RBC) may be more Appropriate.[25] In patients undergoing MT, a meta-analysis reported that plasma infusion at high plasma:RBC ratios is associated with a significant reduction in the risk of death [odds ratio (OR), 0.38; 95% confidence interval (CI), 0.24–0.60] and multiorgan failure (OR, 0.40; 95% CI, 0.26–0.60).[26] However, in patients undergoing surgery without MT, FFP infusion was associated with a trend toward increased mortality (OR, 1.22; 95% CI, 0.73–2.03) and increased risk of developing acute lung injury (OR, 2.92; 95% CI, 1.99–4.29).[26] A survey of American trauma centers indicated that most centers have an MT protocol. Protocols are variable and new, and only about half of these have a 1:1 FFP:RBC ratio.[27]
PLATELET TRANSFUSIONS
Following fluid resuscitation in massive trauma, platelet count is often higher than predicted by the extent of dilution, due to the release of sequestered platelets into the circulation from the spleen, lungs and bone marrow. Most current transfusion guidelines recommend that the platelet count should be maintained at or above 50 × 103/μL.[8,9] Holcomb et al. analyzed 467 massively transfused patients and observed that the 30-day survival was higher (59.9%) in patients with high platelet:RBC ratio (≥1:2) compared to those who received low platelet:RBC ratio (<1:2) (40.1%). Furthermore, the combination of high FFP and high platelet to RBC ratios were associated with decreased truncal hemorrhage, increased 6-hour, 24-hour, and 30-day survival, and increased intensive care unit, ventilator, and hospital-free days, with no change in multiple organ failure deaths.[10] Similar improvement in survival with increasing apheresis platelet-to-PRBC ratio was also reported by Inaba.[28] Administration of fresh whole blood is also an excellent source of viable platelets. However, experience from Iraq war suggests that survival for massively transfused trauma patients receiving fresh whole blood appears to be similar to patients resuscitated with apheresis platelets.[29]
FIBRINOGEN
Among all the clotting factors, fibrinogen concentration is the highest in plasma (1.5–2.5 g/L). Polymerization of fibrin is probably one of the most important steps in hemostasis. Fibrinogen is most often administered in the form of cryoprecipitate. There is no consensus on the minimum levels of fibrinogen required to stop ongoing blood loss. While most guidelines prior to 2008 suggested fibrinogen levels of 0.8–1 g/L,[8,30] it is now believed that the levels should be maintained between 1.5 and 2 g/L.[9]
RECOMBINANT ACTIVATED FACTOR VII
Factor VIIa is an initiator of thrombin generation. Factor VIIa acts primarily via two pathways to activate factor Xa. One pathway is at the site of tissue injury complexed with tissue factor (factor III), and the other is on the surface of platelets, independent of tissue factor. It is supplied in 1.2- and 4.8-mg vials and has a half-life of 2–3 h. Correction of acidosis (pH ≥ 7.2) and thrombocytopenia (platelet count ≥ 100 Χ 10 3/ μL) are essential for optimal functioning of recombinant activated factor VII (rFVII). Rizoli et al. observed improved early survival with the use of rFVII in MT.[31] Spinella et al. reported that the early use of rFVIIa was associated with reduced 30-day mortality (31% vs. 51% who did not receive rFVII) in severely injured combat casualties requiring MT, and was not associated with increased risk of severe thrombotic events.[32] Two prospective randomized trials of rFVII in MT in trauma patients observed no differences in PRBC transfusion within 48 h between the patients who received rFVII (400 μg/kg in three divided doses) and those who received a placebo.[33]
ANTIFIBRINOLYTIC DRUGS
Bolliger and colleagues have demonstrated in vitro and in vivo that progressive hemodilution decreases endogenous antifibrinolytic proteins, including alpha(2)-antiplasmin and thrombin-activatable fibrinolysis inhibitor, resulting in increased fibrinolytic tendency.[34] It is conceivable that antifibrinolytic agents may help to reduce bleeding and hence the transfusion requirements in these patients. In a randomized, placebo-controlled trial involving over 20,000 trauma patients, Shakur and other CRASH-2 trial workers reported that tranexamic acid safely reduced the risk of death in bleeding trauma patients.[35]
PREDICTING THE NEED FOR MT
Cotton et al. validated the Assessment of Blood Consumption (ABC) score at three level I trauma centers.[36] The score is calculated by assigning a value (0 or 1) to each of the four parameters: Penetrating mechanism, positive focused assessment with sonography for trauma for fluid, arrival blood pressure <90 mm Hg, and arrival pulse >120 bpm. A score of 2 was used as "positive" to predict MT. Sensitivity and specificity for the ABC score predicting MT ranged from 75 to 90% and from 67 to 88%, respectively. They concluded that the ABC score is a valid instrument to predict MT early in the patient's care and across various demographically diverse trauma centers.[36]
COMMON COMPLICATIONS OF MT
These include hypothermia, acid/base derangements, electrolyte abnormalities such as hypocalcemia, hypo/hyperkalemia, citrate toxicity, and transfusion-associated acute lung injury.[37] Transfusion of blood products in trauma has been identified as an independent predictor of multiple organ failure,[38] acute respiratory distress syndrome (ARDS), increased infection, and increased mortality in many studies.[39] Once definitive control of hemorrhage has been established, a restrictive approach to blood transfusion should be implemented to minimize additional morbidity/mortality.
POTENTIAL PROBLEMS IN USING HIGH FFP:RBC RATIOS
Current available evidence suggests that only massively transfused patients could potentially benefit from a higher FFP:RBC ratio. Increased transfusion of FFP to non-massively transfused patients would be potentially wasteful, probably provides no survival benefit, and is potentially dangerous as plasma transfusion can cause complications.[40] Transfusion related acute lung injury (TRALI) is currently the leading cause of transfusion-related death in the United States.[41] Patients receiving higher FFP:PRBC ratio have been observed to have a twofold higher incidence of ARDS in massive[42] and other trauma transfusions.[43] Resource utilization issues associated with more aggressive use of FFP and platelets in MT should always be considered when using a high FFP ratio. The preparation and storage of plasma and platelets are expensive. Group AB plasma is scarce, and maintaining a supply of pre-thawed AB plasma that is readily available for MT is both expensive and a huge burden on the blood bank.
CONCLUSION
Hemorrhage and subsequent hemodilution during fluid resuscitation induce complex hemostatic changes to the coagulation and fibrinolysis pathways, the end result of which is a coagulopathy of trauma. This is worsened by hypothermia and acidosis, which often accompany polytrauma. The ideal ratio between PRBCs, FFP and platelet transfusion is still under investigation. There is some evidence that use of PRBCs:FFP:platelets in the 1:1:1 ratio helps control hemorrhage, reduces coagulopathy, and improves survival. However, this ratio has been challenged by studies which have found the optimal ratio of plasma to be in the 1:2–1:3 range. Providers caring for trauma victims should differentiate between hemorrhage requiring MT and one that does not, as these are entirely different physiologic states, especially with respect to coagulation profiles. Routine use of thromoboelastometry to guide the transfusion of procoagulant blood components may optimize the utilization of blood components. The use of tranexamic acid and rFVII (after correction of acidosis, fibrinogen levels and platelet count) should be considered in cases of uncontrolled hemorrhage following trauma.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Peden MK, McGee, Sharma G. The injury chart book: A graphical overview of the global burden of injuries. 2002. Available from: http://whqlibdoc.who.int/publications/924156220x.pdf .
- 2.Thomas D, Wee M, Clyburn P, Walker I, Brohi K, Collins P, et al. Blood transfusion and the anaesthetist: Management of massive haemorrhage. Anaesthesia. 2010;65:1153–61. doi: 10.1111/j.1365-2044.2010.06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keel M, Trentz o. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Sehgal LR, Zebala LP, Takagi I, Curran RD, Votapka TV, Caprini JA. Evaluation of oxygen extraction ratio as a physiologic transfusion trigger in coronary artery bypass graft surgery patients. Transfusion. 2001;41:591–5. doi: 10.1046/j.1537-2995.2001.41050591.x. [DOI] [PubMed] [Google Scholar]
- 5.Sharma D, Vavilala MS. Should brain tissue oxygenation be the transfusion trigger in traumatic brain injury? Pediatr Crit Care Med. 2010;11:420–1. doi: 10.1097/PCC.0b013e3181c315c4. [DOI] [PubMed] [Google Scholar]
- 6.Dai J, Tu W, Yang Z, Lin R. Case report: Intraoperative management of extreme hemodilution in a patient with a severed axillary artery. Anesth Analg. 2010;111:1204–6. doi: 10.1213/ANE.0b013e3181e668b8. [DOI] [PubMed] [Google Scholar]
- 7.Dunne JR, Riddle MS, Danko J, Hayden R, Petersen K. Blood transfusion is associated with infection and increased resource utilization in combat casualties. Am Surg. 2006;72:619. [PubMed] [Google Scholar]
- 8.Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Th erapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. Management of bleeding following major trauma: An updated European guideline. Crit Care. 2010;14:R52. doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 11.Sihler KC, Napolitano LM. Massive transfusion: New insights. Chest. 2009;136:1654–67. doi: 10.1378/chest.09-0251. [DOI] [PubMed] [Google Scholar]
- 12.Shaz BH, Dente CJ, Harris RS, MacLeod JB, Hillyer CD. Transfusion management of trauma patients. Anesth Analg. 2009;108:1760–8. doi: 10.1213/ane.0b013e3181a0b6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: Mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680–5. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 14.Yuan S, Ferrell C, Chandler WL. Comparing the prothrombin time INR versus the APTT to evaluate the coagulopathy of acute trauma. Thromb Res. 2007;120:29–37. doi: 10.1016/j.thromres.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Bolliger D, Görlinger K, Tanaka KA. Tanaka, Pathophysiology and treatment of coagulopathy in massive hemorrhage and hemodilution. Anesthesiology. 2010;113:1205–19. doi: 10.1097/ALN.0b013e3181f22b5a. [DOI] [PubMed] [Google Scholar]
- 16.Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5:289–95. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 17.Kashuk JL, Moore EE, Sawyer M, Le T, Johnson J, Biffl WL, et al. Postinjury coagulopathy management: Goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251:604–14. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 18.Schöchl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14:R55. doi: 10.1186/cc8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenni M, Worn M, Brüesch M, Spahn DR, Ganter MT. Successful rotational thromboelastometry-guided treatment of traumatic haemorrhage, hyperfibrinolysis and coagulopathy. Acta Anaesthesiol Scand. 2010;54:111–7. doi: 10.1111/j.1399-6576.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 20.Duchesne JC, McSwain NE, Jr, Cotton BA, Hunt JP, Dellavolpe J, Lafaro K, et al. Damage control resuscitation: The new face of damage control. J Trauma. 2010;69:976–90. doi: 10.1097/TA.0b013e3181f2abc9. [DOI] [PubMed] [Google Scholar]
- 21.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, et al. Damage control resuscitation: Directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–10. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 22.Duchesne JC, Kimonis K, Marr AB, Rennie KV, Wahl G, Wells JE, et al. Damage control resuscitation in combination with damage control laparotomy: A survival advantage. J Trauma. 2010;69:46–52. doi: 10.1097/TA.0b013e3181df91fa. [DOI] [PubMed] [Google Scholar]
- 23.Brown CV, Foulkrod KH, Sadler HT, Richards EK, Biggan DP, Czysz C, et al. Autologous blood transfusion during emergency trauma operations. Arch Surg. 2010;145:690–4. doi: 10.1001/archsurg.2010.113. [DOI] [PubMed] [Google Scholar]
- 24.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 25.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, et al. Postinjury life threatening coagulopathy: Is 1:1 fresh frozen plasma: packed red blood cells the answer? J Trauma. 2008;65:261. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 26.Murad MH, Stubbs JR, Gandhi MJ, Wang AT, Paul A, Erwin PJ, et al. The effect of plasma transfusion on morbidity and mortality: A systematic review and meta-analysis. Transfusion. 2010;50:1370–83. doi: 10.1111/j.1537-2995.2010.02630.x. [DOI] [PubMed] [Google Scholar]
- 27.Schuster KM, Davis KA, Lui FY, Maerz LL, Kaplan LJ. The status of massive transfusion protocols in United States trauma centers: Massive transfusion or massive confusion? Transfusion. 2010;50:1545–51. doi: 10.1111/j.1537-2995.2010.02587.x. [DOI] [PubMed] [Google Scholar]
- 28.Inaba K, Lustenberger T, Rhee P, Holcomb JB, Blackbourne LH, Shulman I, et al. The Impact of Platelet Transfusion in Massively Transfused Trauma Patients. J Am Coll Surg. 2010;211:573–9. doi: 10.1016/j.jamcollsurg.2010.06.392. [DOI] [PubMed] [Google Scholar]
- 29.Perkins JG, Cap AP, Spinella PC, Shorr AF, Beekley AC, Grathwohl KW, et al. Comparison of platelet transfusion as fresh whole blood versus apheresis platelets for massively transfused combat trauma patients. Transfusion. 2011;51:242–252. doi: 10.1111/j.1537-2995.2010.02818.x. [DOI] [PubMed] [Google Scholar]
- 30.Spahn DR, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Gordini G, et al. Management of bleeding following major trauma: A European guideline. Crit Care. 2007;11:R17. doi: 10.1186/cc5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizoli SB, Nascimento B, Jr, Osman F, Netto FS, Kiss A, Callum J, et al. Recombinant activated coagulation factor VII and bleeding trauma patients. J Trauma. 2006;61:1419–25. doi: 10.1097/01.ta.0000243045.56579.74. [DOI] [PubMed] [Google Scholar]
- 32.Spinella PC, Perkins JG, McLaughlin DF, Niles SE, Grathwohl KW, Beekley AC, et al. The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J Trauma. 2008;64:286. doi: 10.1097/TA.0b013e318162759f. [DOI] [PubMed] [Google Scholar]
- 33.Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: Two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59:8–15. doi: 10.1097/01.ta.0000171453.37949.b7. discussion 15-8. [DOI] [PubMed] [Google Scholar]
- 34.Bolliger D, Szlam F, Levy JH, Molinaro RJ, Tanaka KA. Haemodilution-induced profibrinolytic state is mitigated by fresh-frozen plasma: Implications for early haemostatic intervention in massive haemorrhage. Br J Anaesth. 2010;104:318–25. doi: 10.1093/bja/aeq001. [DOI] [PubMed] [Google Scholar]
- 35.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 36.Cotton BA, Dossett LA, Haut ER, Shafi S, Nunez TC, Au BK, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010;69:S33–9. doi: 10.1097/TA.0b013e3181e42411. [DOI] [PubMed] [Google Scholar]
- 37.Sihler KC, Napolitano LM. Complications of massive transfusion. Chest. 2010;137:209–20. doi: 10.1378/chest.09-0252. [DOI] [PubMed] [Google Scholar]
- 38.Johnson JL, Moore EE, Kashuk JL, Banerjee A, Cothren CC, Biffl WL, et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg. 2010;145:973–7. doi: 10.1001/archsurg.2010.216. [DOI] [PubMed] [Google Scholar]
- 39.Inaba K, Branco BC, Rhee P, Holcomb JB, Blackbourne LH, Shulman I, et al. Impact of ABO-identical vs ABO-compatible nonidentical plasma transfusion in trauma patients. Arch Surg. 2010;145:899–906. doi: 10.1001/archsurg.2010.175. [DOI] [PubMed] [Google Scholar]
- 40.Phan HH, Wisner DH. Should we increase the ratio of plasma/platelets to red blood cells in massive transfusion: What is the evidence? Vox Sang. 2010;98:395–402. doi: 10.1111/j.1423-0410.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- 41.Triulzi DJ. Transfusion-related acute lung injury: Current concepts for the clinician. Anesth Analg. 2009;108:770–6. doi: 10.1213/ane.0b013e31819029b2. [DOI] [PubMed] [Google Scholar]
- 42.Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, et al. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–93. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 43.Watson GA, Sperry JL, Rosengart MR, Minei JP, Harbrecht BG, Moore EE, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67:221–7. doi: 10.1097/TA.0b013e3181ad5957. discussion 228-30. [DOI] [PubMed] [Google Scholar]


