Abstract
Background:
Aminoglycosides are commonly used antibiotics in the intensive care unit (ICU), but are associated with nephrotoxicity. This study evaluated the development of aminoglycoside-associated nephrotoxicity (AAN) in a single surgical intensive care unit.
Materials and Methods:
Adult patients in our surgical ICU who received more than two doses of aminoglycosides were retrospectively reviewed for demographics, serum creatinine, receipt of nephrotoxins [angiotensin converting enzyme (ACE) inhibitors, angiotensin-II receptor blockers, diuretics, non-steroidal anti-inflammatory drugs, cyclosporine, tacrolimus, vasopressors, vancomycin and intravenous iodinated contrast] and the need for dialysis. AAN was defined as an increase in serum creatinine >0.5 mg/dL on at least 2 consecutive days. Univariate and multiple regression analyses were performed.
Results:
Sixty-one patients (43 males) receiving aminoglycoside were evaluated. Mean age, weight, initial serum creatinine, and duration of aminoglycoside therapy were 58.7 (±15) years, 83.3 (±24.4) kg, 0.9 (±0.5) mg/dL, and 4 (±2.3) days, respectively. Thirty-one (51%) aminoglycoside recipients also received additional nephrotoxins. Seven aminoglycoside recipients (11.5%) developed AAN, four of whom required dialysis and all had received additional nephrotoxins. Only concurrent use of vasopressors (P = 0.041) and vancomycin (P = 0.002) were statistically associated with AAN. Receipt of vasopressors or vancomycin were independent predictors of acute kidney insufficiency (AKI) with odds ratios of 19.9 (95% CI: 1.6–245, P = 0.019) and 49.8 (95% CI: 4.1–602, P = 0.002), respectively. Four patients (6.6%) required dialysis.
Conclusions:
In critically ill surgical patients receiving aminoglycosides, AAN occurred in 11.5% of the patients. Concurrent use of aminoglycosides with other nephrotoxins increased the risk of AAN.
Keywords: Aminoglycosides, anntibiotics, intensive care unit, nephrotoxicity
INTRODUCTION
The incidence of drug-resistant infections is increasing throughout the world, leading the World Health Organization to declare antibiotic resistance as one of the top three greatest threats to humans.[1] Unfortunately, there are few pharmaceuticals in the pipeline that will effectively treat infections caused by pathogens responsible for the majority of hospital-acquired infections in the US.[2–3] Studies have demonstrated that appropriate empiric antibiotic therapy is associated with improved outcomes in critically ill patients.[4–6] In the intensive care unit (ICU), it is recommended that empiric antibiotic choices should be determined based on numerous factors including local antibiotic susceptibilities and prevalence of resistant organisms.[7–9] Consequently, aminoglycosides are commonly used in many ICUs, especially if resistant gram-negative infections are suspected.[10–13]
One the most deleterious adverse effects of aminoglycosides is nephrotoxicity.[14] The classic presentation of aminoglycoside-associated nephrotoxicity (AAN) is nonoliguric acute kidney insufficiency (AKI) occurring between 7 and 10 days after the initiation of therapy.[14,15] Known risk factors for the development of AAN include intravascular volume depletion, sepsis, pre-existing renal insufficiency, length of therapy, advanced age, liver dysfunction and concomitant use of other nephrotoxic drugs.[14,16] A recent study of 360 critically ill patients who received 4 or more days of antibiotics was conducted to assess the prevalence of aminoglycoside-associated AKI and determine the risk factors. AAN was defined as a decrease in calculated glomerular filtration rate (GFR) of 20% or more using the abbreviated modification of diet in renal insufficiency formula and excluding patients with impaired (<30 mL/min/1.73 m2) baseline GFR.[14] The overall incidence of AAN in that study was 58% and it developed after a mean of 6.7 days of aminoglycoside therapy. The authors concluded that diabetes mellitus, hypotension, concurrent use of other nephrotoxins or intravenous contrast were independent risk factors for AAN, while baseline renal insufficiency (estimated GFR) between 60 and 30 mL/min/1.73 m2 seemed to be protective. This article has been criticized because the inclusion criteria of 4 or more days of aminoglycosides may not accurately reflect aminoglycoside utilization, as patients typically receive empiric treatment for a short duration until culture data are collected.[12]
In our surgical ICU (SICU), aminoglycosides are often used empirically to treat suspected healthcare-acquired infections based on local susceptibility patterns. These broad-spectrum, multi-antibiotic, aminoglycoside-containing regimens are then quickly de-escalated to minimize the risks of AAN. The purpose of this study was to assess the prevalence of AAN in our SICU and to help identify pharmaceutical agents that can potentially exacerbate AAN when co-administered with aminoglycosides.
MATERIALS AND METHODS
A retrospective review was performed after obtaining approval from the Institutional Review Board. Adult patients (age > 18 years) admitted to our 44-bed SICU at The Ohio State University Medical Center, receiving aminoglycosides (amikacin, gentamicin or tobramycin) between July 2004 and June 2008, were identified from an existing creatinine clearance database.[17] Patients with steady-state aminoglycoside concentration measurements using 24-hour urine collection (±2 days of serum aminoglycoside concentrations) and albumin assessment (±7 days of serum aminoglycoside concentration) were included. Pregnant women, prisoners and patients receiving dialysis at the time of initiation of aminoglycoside therapy were excluded.
Other clinical data collected included demographics (age, gender, weight, height), laboratory values (blood urea nitrogen or BUN, serum creatinine, albumin, aminoglycoside concentrations), concurrent use of known nephrotoxic medications [amphotericin B, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers, cyclosporine, diuretic agents, non-steroidal anti-inflammatory drugs (NSAIDs), tacrolimus, vancomycin, and vasopressors], intravenous iodinated contrast exposure within 5 days of aminoglycoside therapy and need for new-onset dialysis (intermittent hemodialysis or continuous renal replacement therapy). AAN was defined using the recently published vancomycin therapeutic guidelines as an increase in serum creatinine by more than 0.5 mg/dL on two consecutive days from baseline or more than a 50% increase in serum creatinine.[18] Those who developed AAN were classified by the Acute Kidney Injury Network criteria.[19–20]
Data were presented as mean ± standard deviation. Univariate and multiple logistic regression analyses were performed using SPSS for Windows (SPSS, Inc., Chicago, IL, USA) to determine the risk for AAN. Stastistical significance was set at α < 0.05 and pertinent statistical results were presented in the format of odds ratio (OR) with 95% confidence interval (CI).
RESULTS
A total of sixty-one patients (43 or 70.5% males, mean age 58.7 ± 15.7 years) were analyzed. The patients had a mean weight of 83.3 ± 24.2 kg, mean body surface area of 1.95 ± 0.26 m2, baseline serum creatinine of 0.90 ± 0.50 mg/dL and mean albumin of 1.7 ± 0.4 mg/dL. Aminoglycosides administered included tobramycin (96.7% patients) and amikacin (3.3% patients), with a mean duration of 4.0 ± 2.3 days. The GFR estimated by steady-state aminoglycoside pharmacokinetics (52.5 ± 30.8 mL/min/1.73 m2) was significantly lower than the GFR estimated by the modification of diet in renal disease formula (112.1 ± 65.3 mL/min/1.73 m2, P < 0.001) or the modified Crockroft-Gualt formula (96.6 ± 57.0 mL/min/1.73 m 2, P < 0.001).
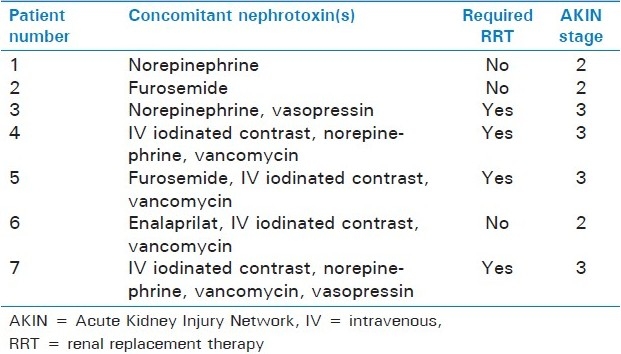
Overall, 31/61 patients (51%) received at least one concomitant known nephrotoxin. Among these patients, 20 received one concomitant nephrotoxin, 6 received two concomitant nephrotoxins, 4 received three, and 1 patient received four concomitant nephrotoxins. Among the 31 patients who received nephrotoxins, 7 (22.6%) developed AAN, with 4/31 patients (12.9%) ultimately requiring dialysis.
The most commonly used concomitant nephrotoxins included intravenous iodinated contrast (17/31 patients), followed by vasopressors (9/31), intravenous vancomycin (7/31) and diuretics (6/31). Table 1 for more detailed information on nephrotoxin administration patterns. When compared to patients without AAN, all patients who developed AAN were on at least one concomitant nephrotoxin (22.8% in AAN group vs. 0% in non-AAN group, P = 0.011). Of note, the incidence of AAN increased as the number of concomitant nephrotoxins increased [Figure 1 and Table 2]. In univariate analysis, patients who developed AAN were more likely to have received concurrent vasopressors (42.9% vs. 9.3%, P = 0.042) and intravenous vancomycin (57.1% vs. 5.6%, P = 0.020). Subsequent multivariate analyses confirmed these observations, with concurrent use of vasopressors (OR 19.9, 95% CI: 1.6–245, P = 0.019) or vancomycin (OR 49.8, 95% CI: 4.1–602, P = 0.002), both being independent predictors of AAN.
Table 1.
Concomitant nephrotoxin and acute kidney injury
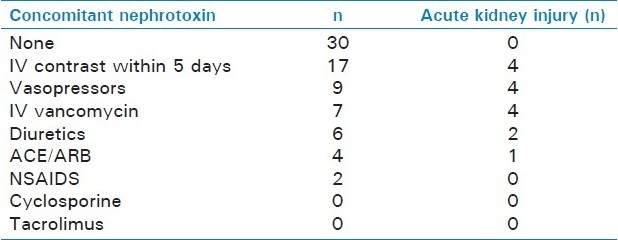
Figure 1.
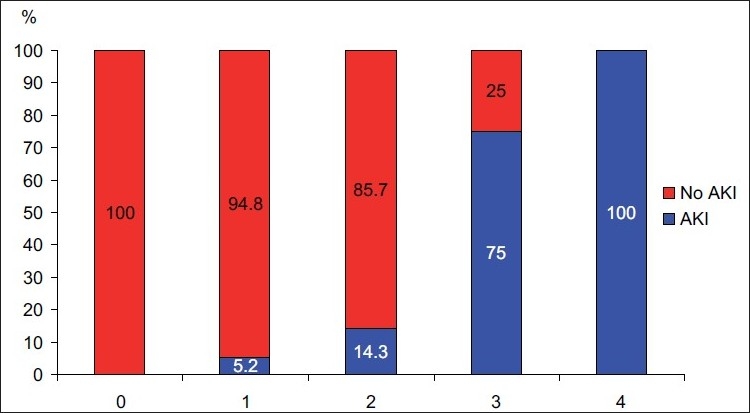
Number of Concomitant Nephrotoxins and Incidence of Acute Kidney Injury (AKI=Acute Kidney Injury)
Table 2.
Patients with acute kidney injury
DISCUSSION
In this study, the rate of AAN was 11.5%, but the majority of these patients required dialysis with an overall rate of 6.6%. The rate of AAN reported in clinical studies varies widely depending of the characteristics of the patient population and the definition for AAN, with an incidence between 10 and 25% in most studies.[14] The incidence of AAN in critically ill patients has been reported to be higher between 29 and 58%.[14,21] The rate of AAN may by higher in critically ill patients since they are more likely to experience ischemic episodes which enhance aminoglycoside accumulation in the proximal tubes and in turn interfere with energy production, increasing the risk of AKI during the ischemic episode.[14,22,23] However, in the current study, the rate of AAN was lower than that previously reported in ICU patients.[14,21]
There are currently no uniform criteria for the definition of AAN. Some studies have used increases in serum creatinine, while others use decreases in creatinine clearance estimated by various formulas to define AAN. The definition of nephrotoxicity in the Schentag study was an increase in serum creatinine by 0.5 mg/dL or more over baseline.[21] In the Oliveira study, the definition of was a 20% decrease in estimated GFR using the abbreviated modification of diet in renal formula from baseline.[14] The authors admit that this formula as well as other commonly used estimations of GFR (i.e., Crockroft–Gault or modification of diet in renal disease) have not been validated in the critically ill, and these formulae may not apply to patients with oliguria or anuria.[14,19] In fact, more patients with a baseline estimated GFR >60 mL/min/1.73 m2developed AKI (61%) than those with an estimated GFR between 60 and 30 mL/min/1.73 m2(49.5%, P = 0.044) which seems counterintuitive.[14] Estimation of GFR by aminoglycoside concentrations or Crockroft–Gault in critically ill patients has been demonstrated to have less error than other methods.[24,25] In our study, the estimated GFR was significantly lower when calculated by aminoglycoside pharmacokinetics compared with the modification of diet in renal disease formula or Crockroft-Gault. We used the recently published vancomycin therapeutic guidelines definition of an increase in serum creatinine by more than 0.5 mg/dL or a greater than 50% increase in serum creatinine for at least two consecutive days as our definition for AAN.[18] To us this seems more concrete and may explain why Oliveira and colleagues found baseline renal insufficiency as protective.
It is very interesting that only aminoglycoside recipients receiving concomitant nephrotoxins developed AAN, and further that only vancomycin and vasopressors were independent predictors of AAN. This was despite the fact that over half of them received nephrotoxins. Importantly, it appears that as the number of concomitant nephrotoxins increased, so did the rates of AAN [Table 2 and Figure 1]. All patients sustaining AAN while receiving aminoglycosides and vancomycin also received intravenous iodinated contrast, making it difficult to determine if the combination of vancomycin and aminoglycoside is a particular risk.
A possible reason for why the rate of AAN was low in this study may be the fact that aminoglycosides were used for a relatively short duration. As is the practice in our ICU, aminoglycosides are used initially as empiric therapy due to sensitivity patterns but typically discontinued when culture results are available due to the risk of AKI. In our study, the mean duration of aminoglycosides was 4.0 ± 2.3 days. The Oliveira study only included patients who received aminoglycosides for at least 4 days and the mean duration of aminoglycoside was much longer (9.4 ± 4.6 days) than in our study for those who developed AAN and was 9.9 ± 4.4 days for those who did not develop AAN.[12,14] Schentag and colleagues stated that shorter duration of treatment with aminoglycosides (less than 5 days) was less frequently nephrotoxic.[21] The longer duration of therapy may have contributed to the increased incidence in AAN and suggests that early de-escalation of aminoglycosides may minimize the risk for AAN.
This study has several limitations. Because of its retrospective nature and small sample size, it may be insufficiently powered to detect minor (but important) differences between groups, especially the influence of multiple concomitant nephrotoxic agents. During the time of this study, aminoglycosides were being dosed by conventional dosing, not as extended interval dosing and the rate of AAN may be influenced by this practice. Finally, this study was conducted in a single SICU and the results may not be applicable to other critically ill patients.
CONCLUSIONS
In this study of critically ill surgical patients, empiric use of aminoglycosides was associated with low rates of AAN (11.5%). When used without other nephrotoxins, the rate of AAN was negligible; but when aminoglycosides were combined with other nephrotoxins, the incidence of AAN increased with the number of concomitant nephrotoxins. The risk-benefit profile of concurrent aminoglycoside and nephrotoxin use must be carefully weighed before institution of such therapeutic combinations and continuously re-evaluated during aminoglycoside therapy. Further studies are needed to determine the rate of AKI with increasing numbers of concurrent nephrotoxins.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.The 10 × ‘20 Initiative: Pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis. 2010;50:1081–3. doi: 10.1086/652237. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J Infect Dis. 2008;197:1079–81. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 4.Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: A risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462–74. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 5.Micek ST, Isakow W, Shannon W, Kollef MH. Predictors of hospital mortality for patients with severe sepsis treated with Drotrecogin alfa (activated) Pharmacotherapy. 2005;25:26–34. doi: 10.1592/phco.25.1.26.55615. [DOI] [PubMed] [Google Scholar]
- 6.Zaragoza R, Artero A, Camarena JJ, Sancho S, Gonzalez R, Nogueira JM. The influence of inadequate empirical antimicrobial treatment on patients with bloodstream infections in an intensive care unit. Clin Microbiol Infect. 2003;9:412–8. doi: 10.1046/j.1469-0691.2003.00656.x. [DOI] [PubMed] [Google Scholar]
- 7.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 8.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 9.Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 10.Falagas ME, Kopterides P. Old antibiotics for infections in critically ill patients. Curr Opin Crit Care. 2007;13:592–7. doi: 10.1097/MCC.0b013e32827851d7. [DOI] [PubMed] [Google Scholar]
- 11.Taccone FS, Laterre PF, Spapen H, Dugernier T, Delattre I, Layeux B. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care. 2010;14:53. doi: 10.1186/cc8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pogue JM, Potoski BA, Kaye KS. Aminoglycoside use in intensive care units and aminoglycoside nephrotoxicity.Comment letter 1. Antimicrob Agents Chemother. 2010;54:2750. doi: 10.1128/AAC.00892-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namias N, Meizoso JP, Livingston DH. Survey of surgical infections currently known (SOSICK): A multicenter examination of antimicrobial use from the surgical infection society scientific studies committee. Surg Infect (Larchmt) 2008;9:509–14. doi: 10.1089/sur.2007.078. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira JF, Silva CA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009;53:2887–91. doi: 10.1128/AAC.01430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appel GB. Aminoglycoside nephrotoxicity. Am J Med. 1990;88:16S–20. doi: 10.1016/0002-9343(90)90082-o. [DOI] [PubMed] [Google Scholar]
- 16.Bertino JS, Jr, Booker LA, Franck PA, Jenkins PL, Franck KR, Nafziger AN. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis. 1993;167:173–9. doi: 10.1093/infdis/167.1.173. [DOI] [PubMed] [Google Scholar]
- 17.Gerlach AT, Murphy CV, Stawicki PS, Cook CH. Modification of diet in renal disease, modified Cockcroft-Gault and 24-urine formulas are poor predictors of aminoglycosides in critically ill surgical patients. Pharmacotherapy [Abstracr] 2009;29:93–4e. [Google Scholar]
- 18.Rybak MJ, Lomaestro BM, Rotscahfer JC, Moellering RC, Craig WA, Billeter M. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325–7. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 19.Brochard L, Abroug F, Brenner M, Broccard AF, Danner RL, Ferrer M. An Official ATS/ERS/ESICM/SCCM/SRLF Statement: Prevention and Management of Acute Renal Failure in the ICU Patient: An international consensus conference in intensive care medicine. Am J Respir Crit Care Med. 2010;181:1128–55. doi: 10.1164/rccm.200711-1664ST. [DOI] [PubMed] [Google Scholar]
- 20.Bagshaw SM, George C, Bellomo R. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569–74. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 21.Schentag JJ, Cerra FB, Plaut ME. Clinical and pharmacokinetic characteristics of aminoglycoside nephrotoxicity in 201 critically ill patients. Antimicrob Agents Chemother. 1982;21:721–6. doi: 10.1128/aac.21.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molitoris BA, Meyer C, Dahl R, Geerdes A. Mechanism of ischemia-enhanced aminoglycoside binding and uptake by proximal tubule cells. Am J Physiol. 1993;264:F907–16. doi: 10.1152/ajprenal.1993.264.5.F907. [DOI] [PubMed] [Google Scholar]
- 23.Zager RA. Gentamicin effects on renal ischemia/reperfusion injury. Circ Res. 1992;70:20–8. doi: 10.1161/01.res.70.1.20. [DOI] [PubMed] [Google Scholar]
- 24.Zarowitz Robert S, Peterson EL. Prediction of glomerular filtration rate using aminoglycoside clearance in critically ill medical patients. Ann Pharmacother. 1992;26:1205–10. doi: 10.1177/106002809202601001. [DOI] [PubMed] [Google Scholar]
- 25.Robert S, Zarowitz BJ, Peterson EL, Dumler F. Predictability of creatinine clearance estimates in critically ill patients. Crit Care Med. 1993;21:1487–95. doi: 10.1097/00003246-199310000-00016. [DOI] [PubMed] [Google Scholar]


