Abstract
H2O2 is a widespread molecule in many biological systems. It is created enzymatically in living cells during various oxidation reactions and by leakage of electrons from the electron transport chains. Depending on the concentration H2O2 can induce cell protective responses, programmed cell death, or necrosis. Here we provide evidence that H2O2 may function as a developmental signal in the differentiation of secondary walls in cotton (Gossypium hirsutum) fibers. Three lines of evidence support this conclusion: (a) the period of H2O2 generation coincided with the onset of secondary wall deposition, (b) inhibition of H2O2 production or scavenging the available H2O2 from the system prevented the wall differentiation process, and (c) exogenous addition of H2O2 prematurely promoted secondary wall formation in young fibers. Furthermore, we provide support for the concept that H2O2 generation could be mediated by the expression of the small GTPase Rac, the accumulation of which was shown previously to be strongly induced during the onset of secondary wall differentiation. In support of Rac's role in the activation of NADPH oxidase and the generation of reactive oxygen species, we transformed soybean (Glycine max) and Arabidopsis cells with mutated Rac genes. Transformation with a dominantly activated cotton Rac13 gene resulted in constitutively higher levels of H2O2, whereas transformation with the antisense and especially with dominant-negative Rac constructs decreased the levels of H2O2.
The signals that control the developmental switch from primary to secondary wall synthesis in higher plants are not known. This transition is characterized by a cessation of synthesis of polymers unique to the primary cell wall accompanied by enhanced rates of cellulose deposition and induction of synthesis of specific secondary wall matrix polysaccharides and lignin. The developing fibers of cotton (Gossypium hirsutum) and tracheary elements differentiated from isolated mesophyll cells of zinnia have emerged as two good model systems for studying this transition. In the zinnia system the mesophyll cells are cultured in noninductive medium and differentiation is induced by changes in hormonal balance. Specialized, localized regions of secondary wall formation unique to these tracheary elements have been shown to contain large amounts of cellulose, xylan, and lignin (for reviews, see Fukuda, 1991, 1996). The developing cotton fiber is unique in that the secondary wall consists of nearly pure cellulose and is devoid of hemicellulose and lignin (for reviews of cotton fiber development, see Basra and Malik, 1984; Ryser, 1985). Furthermore, development occurs synchronously for nearly all fibers within a boll, with the transition to secondary wall formation beginning abruptly in varieties of cotton at about 14 to 16 DPA, which is a few days prior to the cessation of fiber elongation (Meinert and Delmer, 1977).
Rates of secondary wall cellulose synthesis peak at about 24 DPA; the fibers mature and die sometime after 40 DPA, presumably by a process of programmed cell death. During the transition the rate of cellulose synthesis increases abruptly to about 100-fold (Meinert and Delmer, 1977), and recent evidence indicates that genes that most likely encode the catalytic subunit of cellulose synthase are also strongly induced at this time (Pear et al., 1996). During xylogenesis there is also evidence that expression of genes involved in synthesis of hemicellulose (Bolwell and Northcote, 1981) and lignin (Fukuda, 1991) also undergo induction. The transition in both cotton fibers and tracheary elements is also characterized by a reorganization of the cytoskeleton that directs the specific patterns of cellulose deposition (Seagull, 1990; Fukuda, 1991, 1996).
Our interest in the events regulating this transition was stimulated by our recent characterization of genes that encode two small GTPases of the Rho subfamily in cotton, named Rac13 and Rac9. Rac13 in particular shows highly induced expression at the transition from primary to secondary wall synthesis (Delmer et al., 1995). In animals Rac proteins function in several possibly related signal transduction pathways. Rac has been shown to be involved in regulation of reorganization of the actin cytoskeleton (Symons, 1996) and it plays another role in leukocytes as a specific activator of the plasma membrane NADPH oxidase (Freeman et al., 1996). Activation of the NADPH oxidase leads to generation of the oxidative burst, which serves as a defense against pathogen invasion (Abo et al., 1994; Diekmann et al., 1994). This enzyme is the major superoxide-producing enzyme in these cells (Segal and Abo, 1993).
In higher plants ROS can probably be generated by several alternative pathways (Allan and Fluhr, 1997; Bestwick et al., 1997). Such pathways include the possibility of a cell-wall-localized peroxidase (Bolwell et al., 1995), amine oxidases (Allan and Fluhr, 1997), non-flavin NADPH oxidases (Van Gestelen et al., 1997), or NADPH oxidases that resemble the flavin-containing type that is activated by Rac in leukocytes (Xing et al., 1997). A plant homolog of the leukocyte NADPH oxidase has been identified through analysis of rice and Arabidopsis expressed sequence tags (Groom et al., 1996; Keller et al., 1998). The activity of this enzyme has been implicated in plant responses to UV light and in pathogen-induced oxidative stress in soybean (Glycine max; Tenhaken et al., 1995; Rao et al., 1996). The amino acid sequence of the Arabidopsis homolog of the human NADPH oxidase has an additional calcium-binding domain, suggesting a possible role for calcium influx in ROS production (Jabs et al., 1997; Keller et al., 1998). In a recent report, Xing et al. (1997) have shown that a tomato or a tobacco Rac, along with soluble NADPH oxidase subunits, becomes translocated to the plasma membrane in response to elicitor treatment, suggesting a second function for Rac analogous to that observed in leukocytes (Diekmann et al., 1994; Freeman et al., 1996). The involvement of calcium is also intriguing in view of its role in callose and cellulose deposition (Maltby et al., 1979; Hayashi et al., 1987).
Production of .O2, H2O2, or other ROS over an extended time creates an oxidative stress within the cell, eventually reaching the threshold that triggers the programmed cell death response. This is true for animal as well as plant cell systems (Levine et al., 1994; Korsmeyer et al., 1995; Jabs et al., 1996). The precise mechanism of the signal transduction, from the generation of ROS to cell death, is not fully elucidated, but the downstream events involve calcium influx and activation of specific proteases and kinases (Levine et al., 1996). In plants prolonged periods of ROS generation that result in oxidative stress have been reported during abiotic stress, such as chilling (Prasad, 1996), ozone (Schraudner et al., 1997), or UV exposure (Hideg and Vass, 1996), and following an attack by avirulent pathogens (Baker et al., 1991; Levine et al., 1994). In all of these cases programmed cell death follows.
In this report we have explored the question of whether an oxidative burst coincides with high expression of the Rac genes in cotton and whether the generated H2O2 affects secondary wall differentiation. We present evidence that the production of H2O2 coincides with the onset of both Rac gene expression and secondary wall synthesis in cotton fibers. Our results suggest that at a low dosage the produced H2O2 functions as a developmental signal for the onset of secondary wall differentiation. We also show that constitutive expression of the dominantly activated form of the cotton Rac13 gene induced the production of ROS in cultured soybean and Arabidopsis cells, whereas reducing the expression of Rac mRNA by transformation with antisense mRNA or with the dominant-negative form of cotton Rac had the opposite effect. These data support a role for Rac in the signaling pathway of H2O2 production.
MATERIALS AND METHODS
Chemicals
Tempo, 4-hydroxy-tempo, DPI, SHAM, and Evans blue were from Sigma. DCFDA and dihydrorhodamine-123 were from Molecular Probes (Eugene, OR), and pyranine was from Eastman Kodak.
Plant Material and H2O2 Measurements
Cotton (Gossypium hirsutum var Acala SJ-2) was used for both field and cultured fiber experiments. For some of the experiments with cultured ovules, similar results were also obtained using var Coker 130. Cotton was grown in irrigated fields in Israel in the summer of 1996 or 1997. Flowers were marked at anthesis and at indicated times the bolls were removed and immediately assayed for H2O2. Locules were excised from the bolls with a scalpel and detached fibers (without seeds) were used for total H2O2 determination by a method based on titanium oxidation (Snell and Snell, 1949). H2O2 concentration was determined by a standard graph from 5 μm to 1 mm H2O2 constructed by direct addition of H2O2 to the titanium solution. H2O2 generation was also measured in whole locules that were washed with sterile water to remove possible ROS released by cutting and incubated in 0.5 mg/mL 2,7-DCFDA. ROS-dependent conversion of DCFDA to 2′,7′-dichlorofluorescein was measured for 60 min in a fluorometer (model FL500, BioTek Instruments, Winooski, VT) with excitation/emission wavelengths set to 485/525, respectively. Selected locules were incubated in the presence of 2.5 mg/mL thymol-free catalase (Sigma). For microscopic examination fibers (still attached to the locules) were stained with 0.005% dihydrorhodamine-123 or pyranine (Apostol et al., 1989) for 5 min and observed as described below.
Fertilized ovules of cotton cv Coker 130 were cultured in 12-well plates in vitro, as described by Meinert and Delmer (1977). Compounds were added to medium at indicated concentrations dissolved in DMSO. The final concentration of DMSO was 0.1% in all of the samples.
Microscopy
Fluorescence microscopy was performed with an inverted microscope (model IX-70, Olympus). Fluorescence of dihydrorhodamine-123 was observed with a filter set (excitation = 485/22 and emission = 535/35, respectively; model X-23, Omega, Brattleboro, VT). To enable the comparison of differences in signal intensity, photographs were taken using identical exposure conditions (in manual setup) for all samples. Experiments were repeated at least four times with similar results. The selected pictures are parts of a microscope field, which is representative of the entire field. Photographs were taken with Pan 100 black and white (Ilford, Paramus, NJ) or Kodak Gold film. Birefringence was monitored using an inverted microscope (Zeiss Axiophot) equipped with a polarization reflector at the light source and a crossed polarizer and analyzer, as described by Potikha and Delmer (1995). Birefringence was verified in all cases by confirmation that patterns reversed when samples were rotated by 90o.
For determination of cellulose content, cultured fibers, with their associated ovules, were washed free of medium, frozen, and lyophilized and the dry weight was determined from the isolated fibers. Whole fibers were then subjected to digestion by acetic-nitric reagent and the residual crystalline cellulose not digested was quantified by use of the anthrone reagent, as described by Updegraff (1969).
Rac Constructs
Site-directed mutagenesis was performed with the Mutagen kit (Bio-Rad). The starting templates were uracil containing single-stranded DNA isolated from Escherichia coli CJ236 cells containing pBluescript SK− (Rac13). The nucleotide sequence of the G15V mutagenic primer was GGTCGGTGATCTAGCTGTGG (underlined T is G in the wild-type sequence). The nucleotide sequence of the T20N mutagenic primer was GCTGTGGGGAAAAATTGTATGCTC (underlined A is C in the wild-type sequence). For both mutant genes, the presence of the desired mutations was confirmed by sequencing the entire coding region of the gene using automated dideoxy sequencing (Vermont Cancer Center DNA Sequencing Facility, Burlington, VT). The mutated Rac constructs were excised from the pBluescript vector with EcoRV plus SmaI and recloned into a blunted NotI site of pBluescript SK. Orientation of the inserted genes was tested by digestion with BamHI, which cleaves 0.3 kb from the 5′ end. Constructs with correct gene orientation (for sense or antisense) were digested with SacI plus XbaI and ligated into the XbaI/SacI sites of the binary vector EL103 (provided by Dr. Eric Lam, Rutgers University-Cook College, New Brunswick, NJ). After the correct insertion in E. coli XL-1-Blue was verified, the final plasmids were transformed into Agrobacterium tumefaciens strain EHA105.
Transient Expression of Rac in Suspension-Cultured Cells
Soybeans (Glycine max cv Williams 82) were grown as described previously (Levine et al., 1994). Arabidopsis (cv Columbia) were obtained from Dr. Doerner (Salk Institute, La Jolla, CA) and were cultured in medium supplemented with Murashige and Skoog vitamins, 3% Suc, and 0.5 μg/L 2,4-D. Both cultures were diluted 1:6 once a week, and all experiments were performed 2 d after transfer. Soybean and Arabidopsis transformations were done by coinoculation of 3 × 108 cells of A. tumefaciens strain EHA105, carrying the appropriate plasmids per milliliter of plant suspension culture, and incubated in 24-well plates for 50 h. Cells were extensively washed over Miracloth (Calbiochem) and resuspended in the original volume of fresh medium that contained 300 mg/L claforan antibiotic to suppress bacterial growth. GUS activity was assayed by staining the cells with 5-bromo-4-chloro-3-indoyl-β-d-glucuronic acid and microscopic examination of the blue cells. H2O2 was assayed over a period of 3 d with DCFDA, as described for the cotton locules.
RESULTS
Generation of ROS in Differentiating Cotton Fibers
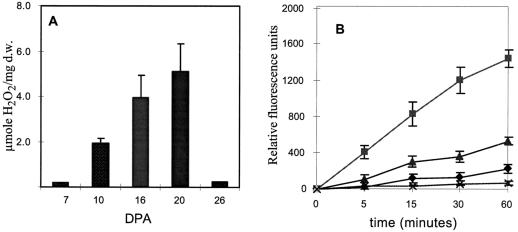
To study the possible relationships between the induction of Rac accumulation, the generation of ROS, and differentiation of secondary walls, we tagged field-grown flowers of cotton var Acala SJ-2 at anthesis and collected cotton bolls from variously aged plants that spanned the time of primary wall synthesis, the transition, and the later times when secondary wall synthesis was highly active. The concentration of H2O2 was assessed in fibers quantitatively and qualitatively immediately following the opening of bolls with a scalpel. For the quantitative measurements of H2O2, locules from bolls of different aged plants were excised and detached fibers were placed in titanium sulfate, according to the method of Snell and Snell (1949; Fig. 1A). The levels of ROS in young cotton bolls were very low but increased strongly at 16 to 20 DPA, a time that coincides with the peak time of increased Rac expression (see figure 4 in Delmer et al., 1995). H2O2 accumulation corresponded to the time of secondary wall biosynthesis and declined afterward. It is probable that the decline was caused by elevated levels of ROS, which induced antioxidant enzymes, although this was not tested.
Figure 1.
Measurements of H2O2 levels during development of field-grown cotton fibers. Flowers of field-grown cotton were tagged at anthesis and bolls were collected at the indicated times postanthesis. A, H2O2 was determined in detached fibers according to the method of Snell and Snell (1949). B, H2O2 production was followed in whole locules by incubating them in DCFDA for 60 min. 2′,7′-Dichlorofluorescein, the H2O2-dependent oxidation product, was measured at indicated times at 485-nm excitation and 525-nm emission wavelengths. Catalase (x) was added to the 19-DPA sample. 9 DPA (♦), 19 DPA (▴), and 26 DPA (▪). d.w., Dry weight.
We also measured the rate of H2O2 production in whole locules by a more-sensitive, cell-permeable fluorescent dye, DCFDA (Fig. 1B; Ubezio and Civoli, 1994; Trayner et al., 1995; Allan and Fluhr, 1997). Oxidation of this dye by O2− or by H2O2 catalyzed by peroxidase produces a fluorescent and cell-permeable product, 2′,7′-dichlorofluorescein, that can be detected in the medium (Ubezio and Civoli, 1994). The amount of fluorescence accumulation in the incubation medium can therefore serve as a measure of ROS production in the boll. This was verified by addition of catalase to the medium, which almost completely inhibited the fluorescence accumulation. The inhibition of DCFDA oxidation by catalase implicates the H2O2 as the ROS present in the locule. Locules were washed to remove possible peroxides released by the cutting and placed in a culture dish in the presence of the DCFDA. The increase in fluorescence during the 60 min of the incubation corresponded to the measurements of total H2O2, as determined by titanium oxidation (Fig. 1A). Since the oxidation of DCFDA is catalyzed by endogenous peroxidases, it is possible that insufficient concentration of the enzyme affected the H2O2 estimation. We checked that the amount of peroxidase in young bolls was not limiting by parallel measurements in the presence of horseradish peroxidase and obtained similar results (data not shown).
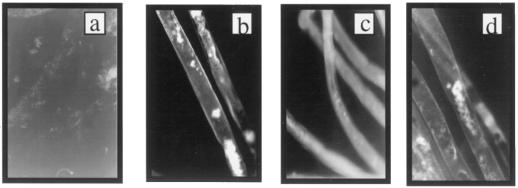
We also followed the production of H2O2 during fiber development in a qualitative way by direct microscopic observation of differentiating cotton fibers using another chemically unrelated fluorescent dye, dihydrorhodamine-123. This dye penetrates the cells in its reduced (and nonfluorescent) form but becomes trapped inside following oxidation by H2O2 (Royall and Ischiropoulos, 1993). To observe the redox state of individual fiber cells, locules were incubated in the dye solution for 5 min and parts of the locule were placed under the microscope, with the fibers still fully attached to the ovule. Consistent with the results shown in Figure 1, increased dye oxidation was detected in fibers during the period of secondary wall differentiation (Fig. 2). Dihydrorhodamine fluorescence was observed both in localized regions within the fiber and as a uniform layer localized to the cell surface (Fig. 2, b and c). The increase in fluorescence with age occurred in both areas but was especially pronounced at the cell surface. The H2O2 production that was uniformly distributed at the cell surface was first seen in fibers 15 to 18 DPA (Fig. 2b) and increased further after 20 DPA (Fig. 2c). This onset of H2O2 production coincides with the onset of Rac13 gene expression (Delmer et al., 1995), as well as the onset of deposition of the highly ordered secondary wall microfibrils of cellulose (Meinert and Delmer, 1977).
Figure 2.
Microscopic observation of H2O2 generation during development of field-grown cotton fibers. Bolls were opened and placed in a solution of dihydrorhodamine-123, as described in Methods. Fibers that were still attached to ovules were analyzed by fluorescence microscopy. a, Primary walls (10 DPA); b, transition period (15 DPA); c, massive secondary wall synthesis (20 DPA); and d, mature fibers (26 DPA).
To exclude the possibility that the observed fluorescence was due to the formation of autofluorescent compounds during wall differentiation and to further validate the results of H2O2 generation, the fibers were treated with either water or pyranine and observed with the microscope. No fluorescence was seen in the water-treated samples, indicating lack of autofluorescence and confirming that the fluorescent signal shown in Figures 1 and 2 originated from the dye (data not shown). This observation is consistent with the absence of lignification in fibers, a process that is associated with production of autofluorescent compounds in many plant species. The staining of fibers with pyranine, a fluorescent dye that emits light in its reduced form but becomes bleached after reaction with H2O2 and peroxidase, resulting in a nonfluorescent compound (Apostol et al., 1989), produced a pattern of fluorescence that was opposite to that observed with dihydrorhodamine-123, i.e. no fluorescence was seen within the fibers, on a fluorescent background of pyranine in the surrounding medium. Also, the intensity decreased as a function of fiber age, consistent with the measurements in Figure 1 (data not shown). These controls, together with the measurements in the presence of catalase, further support the validity of the results with the dihydrorhodamine-123 and DCFDA dyes for estimating H2O2.
H2O2 as a Signal for Differentiation of the Secondary Wall
To test whether the observed H2O2 generation had a signaling function in the process of secondary wall generation, we carried out experiments to (a) block the generation of H2O2, (b) scavenge generated H2O2 from the system, or (c) test the effect of premature addition of H2O2 to the system. Since it is technically difficult to introduce these compounds into fiber cells of field-grown cotton, we used a system with which fibers are cultured with their associated ovules (Beasley, 1992), conditions that Meinert and Delmer (1977) have shown allow fiber development that closely mimics that which occurs in planta. Compounds dissolved in DMSO (with same level of DMSO in controls) could then be directly added to the culture solution. The effects on secondary wall synthesis were assessed by the birefringence of fibers using polarized light microscopy (Fig. 3) and also by direct quantitative analysis of cellulose content (Fig. 4A).
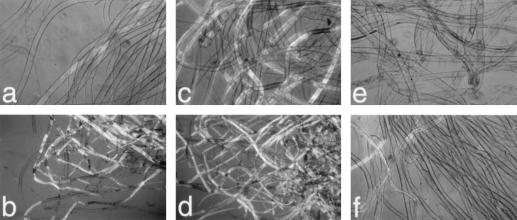
Figure 3.
Birefringence of cultured cotton fibers following either addition of exogenous H2O2 or inhibition of H2O2 generation by DPI. Cotton fibers were cultured with their associated fertilized ovules in 24-well plates at 30°C. a and b, Controls 15 DPA (a) or 16 DPA (b) to which only 0.1% DMSO was added 12 DPA. c and d, Fibers 15 DPA (c) or 16 DPA (d) to which 50 μm H2O2 was added once daily 13 and 14 DPA. e and f, Fibers 15 DPA (e) or 16 DPA (f) that had been cultured in 1 μm DPI/0.1% DMSO since 12 DPA. a, c, e, and f were viewed at ×250 magnification, whereas b and d were at ×100.
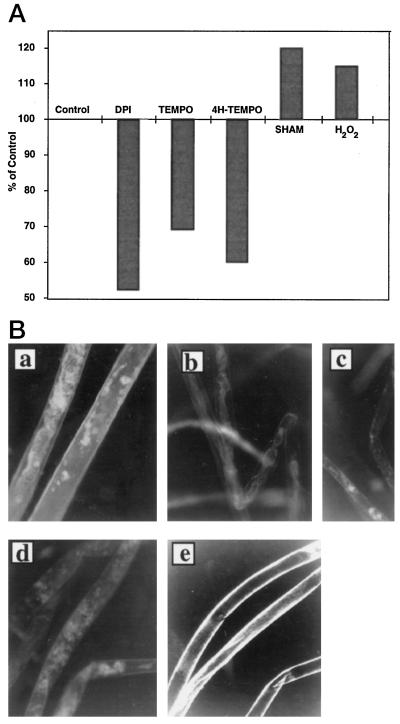
Figure 4.
The effects of H2O2-regulating compounds on cellulose content and the amount of H2O2 in cultured fibers. A, Total cellulose content was measured 16 DPA after the following compounds were added 12 DPA: Control (no additions) 1 μm DPI, 0.5 mm Tempo, 1 mm 4-hydroxy-tempo, 5 mm SHAM, and 50 μm H2O2. The control value was 282 ± 6 μg cellulose/mg dry weight of fibers. The sd of samples was <7%. B, Dihydrorhodamine-123 staining of 15-DPA cotton fibers cultured from 12 DPA in the presence of 0.1% DMSO as control (a), 1 mm 4-hydroxy-tempo (4H-TEMPO; b), 0.5 mm Tempo (c), 1 μm DPI (d), and 5 mm SHAM (e).
With ovules/fibers cultured at 30°C, we observed, 15 DPA, the first faint signs of birefringence of fibers when viewed under polarized light, which is characteristic of the onset of secondary wall deposition (Fig. 3a). After 1 additional d, many more of the control fibers were clearly birefringent, indicating the beginning of the abrupt transition to secondary wall synthesis. Cellulose content increased from a level of about 170 to 280 μg/mg dry weight of fibers over this 1-d period.
DPI is known to be a potent inhibitor of the leukocyte NADPH oxidase and other flavin-containing oxidases and has been used extensively to inhibit production of ROS in both animal and plant systems (O'Donnell et al., 1993; Dwyer et al., 1996; Murphy and Auh, 1996; Jabs et al., 1997; Mithoefer et al., 1997). We found that addition of 1 μm DPI to the cultured fiber system 12 DPA substantially blocked the subsequent development of birefringence (Fig. 3, e and f). In addition, the level of cellulose in the fibers, when expressed as a percentage of the control value at 16 DPA (control value = 282 ± 6 μg/mg dry weight of fibers), was much lower, further confirming that the DPI inhibited the onset of secondary wall deposition.
An alternative way to decrease the concentration of H2O2 is to scavenge the generated ROS by addition of synthetic scavengers. In a similar experimental design, we used the ROS scavenger 4-hydroxy-tempo (Weiss et al., 1993; Charloux et al., 1995). The addition of 1 mm of this antioxidant showed a similar effect on inhibition of cellulose deposition but was somewhat less effective than DPI (Fig. 4A). We also tested the nonhydroxylated analog of the scavenger, Tempo, which produced a similar although weaker effect (Fig. 4A).
As predicted, the presence of DPI or scavengers strongly reduced the production or accumulation of H2O2, respectively, when judged qualitatively by fluorescence microscopy using the technique described above (Fig. 4B). These data support the suggestion that the developmentally generated H2O2 was produced enzymatically (most probably by NADPH oxidase or a related oxidase) and was not a result of electron leakage caused by membrane damage. None of these compounds appeared to exert overall toxicity, because the fibers remained viable as judged by their appearance and ability to exclude Evans blue dye. Also noteworthy is that the inhibitory activity of the scavenger and the inhibitor molecules on the birefringence was reversible, since removal of the inhibitor/scavengers from the culture medium led to the appearance of birefringence at a later stage (data not shown).
These results suggest that H2O2 may serve as a signal for the onset of secondary wall deposition. The DPI inhibitor results also implicate the NADPH oxidase (or a similar enzyme) in the generation of the H2O2. If this is so, then we predicted that bypassing the oxidase by direct addition of H2O2 to younger fibers might cause a premature initiation of secondary wall deposition. When 50 μm H2O2 was added once a day to the culture solution on d 13 and 14, by 15 DPA appearance of birefringence much stronger than in the controls was observed (compare Fig. 3, a and b, with 3, c and d). This enhancement of the timing of onset of deposition was further reflected in the increased level of cellulose measured in the fibers 16 DPA (Fig. 4A). We also observed enhanced levels of cellulose 16 DPA (Fig. 4A) by addition on 12 DPA of 5 mm SHAM, a compound known to elevate H2O2 levels (Pantoja and Willmer, 1988).
Ectopic Expression of Rac13 Induces the Production of H2O2
A natural question that arises from the above experiments is what causes the elevated levels of H2O2 in cotton fibers. The effective inhibition of the H2O2 generation in ovule cultures by DPI (Figs. 3 and 4) supports the possible involvement of NADPH oxidase in the generation of ROS, an enzyme that in leukocytes is activated by Rac. Our finding that cotton Rac13 expression (Delmer et al., 1995) coincides with the onset of oxidative stress and formation of secondary wall led us to probe the role of cotton Rac in the production of ROS in plants. Because the activity of small GTPases, such as the human Rac1 and the cotton Rac13, depends on the type of the bound nucleotide, GTP or GDP, the regulation of Racs can be achieved by modifying the rate of GTP hydrolysis.
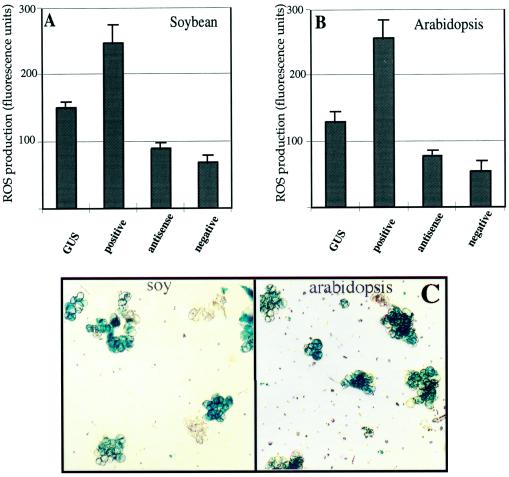
We took advantage of knowledge gained from the studies of mutant animal Racs, regarding the critical amino acids that govern the GTP hydrolysis, to perform site-directed mutagenesis on the cotton Rac13 cDNA to create constructs that encode constitutively active (“dominant-activated”) or constitutively negative (“dominant-negative”) forms of Rac. Cotton Rac13 possesses the conserved sequences at and surrounding Gly-15 and Thr-20 that are analogous to Gly-12 and Thr-17 found in animal Racs. When the cDNAs are mutated so that Gly is converted to Val (termed V15-Rac13), a dominant-activated mutation results, whereas conversion of the Ser residue to Asn (termed N20-Rac13) results in a dominant-negative phenotype (Ridley et al., 1992). These, as well as an anti-sense construct of the dominant-activated Rac13, were cloned into a binary plant vector under the control of constitutively expressed 35S cauliflower mosaic virus promoter to introduce the mutated genes into plant cells via A. tumefaciens-mediated transformation. Since such experiments would be technically very complex, if not impossible, to carry out with cotton fibers, we chose to use cultured cells for these studies. Two types of suspension-cultured cells were selected: soybean cv Williams 82 and Arabidopsis var Columbia. Both systems are well characterized with respect to oxidative burst and yield a high percentage of cells that transiently express the transgenes. The use of heterologous systems also avoids possible cosuppression effects that may interfere with the results. As a control, the soybean and Arabidopsis cells were transformed with an identical vector carrying the bacterial GUS gene driven by the same 35S promoter. The efficiency of transgene expression was estimated by staining a sample of the GUS-transformed cells for GUS activity 48 h after coinoculation, after removing the majority of A. tumefaciens from the medium. As shown in Figure 5C, the procedure yielded more than 65% of transformed soybean and more than 75% of Arabidopsis cells. The high efficiency enabled us to analyze the possible function of the introduced genes. The influence of the introduced Rac constructs on the redox status of the cultures was determined 16 h after removal of the agrobacteria by measuring the conversion of DCFDA to 2′,7′-dichlorofluorescein in the transformed cells. Results of the fluorescence measurements clearly showed that the dominant-activated form of Rac induced higher levels of H2O2 in both soybean and Arabidopsis cells. Conversely, expression of the dominant-negative Rac construct or inhibition of expression of the endogenous Rac by expression of the antisense construct reduced the constitutive ROS production by the soybean and Arabidopsis cells. The influence of the transiently expressed genes was sustained for at least 3 d after removal of agrobacteria because similar results were obtained on the consecutive days. Taken together, these results support the regulatory role of the Rac gene in the regulation of H2O2 levels in plant cells. Because of the transient nature of the gene expression and also because cultured cells do not usually undergo secondary wall differentiation, we have not examined any effects of Rac on cellulose synthesis in this system.
Figure 5.
The effects of transient Rac expression on H2O2 production in soybean and Arabidopsis cells. Soybean (A) and Arabidopsis (B) cells were transformed with A. tumefaciens carrying the specific Rac constructs cloned into a binary vector. H2O2 generation was measured with the DCFDA dye, added directly to the medium of cultured cells, for 10 min as described in the Figure 1 legend. Fluorescence emitted from cells transformed in parallel with the GUS gene served as the control. C, Representative field of GUS-transformed (control) cells stained with 5-bromo-4-chloro-3-indoyl-β-d-glucuronic acid. soy, Soybean.
DISCUSSION
Taken together, our results allow us to propose a partial sequence of events that lead to differentiation of cotton fibers. At present, unknown developmental signal(s) induce both the expression and activation of Rac, which in turn activates an oxidase that leads to a sustained generation of H2O2. The constant production of low levels of H2O2 stimulated the onset of secondary wall cellulose biosynthesis and secondary wall differentiation (perhaps by inducing expression of CelA and other necessary genes). The continuously elevated level of H2O2 would result in the accumulation of H2O2-dependent reaction products, many of which are destructive to vital cellular components, such as membranes, proteins, and nucleic acids. Eventually, after reaching a certain threshold, this could trigger the activation of a cell-death process, which for the cotton fiber cells constitutes a form of terminal differentiation.
The concept that H2O2 might serve as a signal for the initiation of such a developmental sequence is novel, but it has a striking parallel to recent results by Altamura et al. (1998), who have shown in tobacco leaf explants that oligogalacturonides stimulate pericycle cell wall thickening due to stimulation of cellulose deposition. Since such oligogalacturonides have also been shown to elicit production of H2O2 (Legendre et al., 1993; Svalheim and Robertsen, 1993), the same signaling pathways may be involved in the tobacco system and the cotton fibers studied by us. ROS, in general, and H2O2, in particular, have been previously implicated as second messengers in the regulation of the plant hypersensitive response (Mehdy, 1994; Low and Merida, 1996). The results presented here are novel in that they indicate that H2O2 may also serve as a signal for a major developmental process in plants: the differentiation of secondary walls. The following results support this conclusion: (a) H2O2 generation coincides with secondary wall deposition, (b) inhibition of either H2O2 production or scavenging it from the system prevented the differentiation process, and (c) exogenous addition of H2O2 to young fibers prematurely promoted secondary wall formation. To our knowledge, H2O2 is not required for any direct metabolic process that is associated with cellulose biosynthesis and secondary wall formation in nonlignified walls. Therefore, the latter two results support the hypothesis that the production of H2O2 is not just correlated with the process but may actually serve as a regulatory molecule in the process of cellulose biosynthesis. Moreover, the effective blocking of H2O2 production through the inhibition of an endogenous cotton oxidase by DPI argues that the generation of the oxidative stress is a programmed event, regulated by developmental cues, and was not a result of electron leakage because of nonspecific membrane damage. Since cotton fibers do not produce lignin during the formation of secondary walls, one cannot argue that the production of H2O2 is related to this process. This may also explain why others studying secondary wall development in lignifying systems would not have thought to consider a signaling role for H2O2 production in addition to its potential role in lignin synthesis.
DPI is a known inhibitor of NADPH oxidases (O'Donnell et al., 1993) but it can also inhibit other flavohemoproteins. Thus, one cannot be certain that the generation of ROS observed here is initiated by an enzyme analogous to the leukocyte NADPH oxidase, although our experiments with the mutant Rac genes and previous studies of patterns of Rac gene expression (Delmer et al., 1995) and other studies relating Rac to elicitor-induced oxidative bursts in plants (Kieffer et al., 1997; Xing et al., 1997) do argue for such an involvement. We have yet to explore whether the theoretically proposed initial product of this enzyme, superoxide, is produced and directly converted to H2O2 in fibers, either spontaneously or by the action of superoxide dismutase.
It is also noteworthy that in intact bean plants H2O2-dependent oxidative protein cross-linking occurs during the later stages of hypocotyl growth (Bradley et al., 1992; Schopfer, 1994). The cross-linking of the bean cell wall proteins does not occur in the young cells despite the presence of peroxidase and the specific cell wall proteins (Bradley et al., 1992); it happens only later, when H2O2 is being produced (Schopfer, 1994). The role of H2O2 in development, particularly with respect to developmental changes in arabinogalactan proteins and Hyp-rich glycoproteins at the plant cell surface, was recently discussed by Knox (1995). These results further support the notion that H2O2 generation in plants is developmentally regulated. Some interesting parallels exist between the H2O2-driven events that occur during the transition from primary to secondary wall synthesis and those that occur following oxidative burst during the pathogenesis response. For example, there is an influx of Ca2+ into the cytoplasm and induction of transient callose deposition (shown for cotton fibers by Maltby et al. [1979] and described in pathogenesis by Bestwick et al. [1995] and in lignification [common to many secondary cell walls but not cotton fibers] by Fukuda [1991]). Furthermore, one might expect that cross-linking of cell wall polymers via the action of H2O2 and wall-associated peroxidases that also characterizes the hypersensitive response (Bradley et al., 1992; Iiyama et al., 1994) occurs at the cessation of cell expansion and at the onset of secondary wall formation.
In the developmentally induced cell wall differentiation, we observed a continuous production of H2O2, which appears to be necessary to sustain the signaling process, since the process could be halted by the addition of DPI or 4-hydroxy-tempo. Such a continually elevated concentration of H2O2 within the cell is likely to produce an accumulation of damage to vital cellular components and therefore may result in the activation of programmed cell death. H2O2 was indeed shown to act as a self-generated programmed cell death trigger during the plant response to pathogens (Levine et al., 1994). The main differences between the developmentally and the pathogen-induced oxidative bursts lies in the duration of the burst and in the concentration of the H2O2. During the pathogenesis response the oxidative burst lasts about 4 to 6 h and may reach millimolar concentrations (Legendre et al., 1993). The rate of H2O2 production during fiber differentiation is much lower; however, the duration of the developmentally produced oxidative burst is longer, lasting several days. For this reason, it may be more appropriate to call it an oxidative “phase” in development rather than a “burst.” Nevertheless, it is highly probable that programmed cell death is triggered by the gradual accumulation of H2O2-dependent oxidation products in both cases: the short but high dose that induces cell death, as happens during the hypersensitive response, and the prolonged but low dose characteristic of the developmentally regulated processes. In support of programmed cell death induction by a constant low level of ROS, transient transfection experiments of animal cell cultures with small GTPases, Rac or Ras, showed a significant increase in the levels of ROS production and in subsequent cell death (Sundaresan et al., 1996; Irani et al., 1997). Our preliminary results showed the same trend in the suspension-cultured plant cells (data not shown), but the conclusive proof for the role of the developmentally produced H2O2 in the cotton fiber cell death needs further experimentation.
The terminal differentiation of many cells with thick secondary walls, such as tracheary elements, sclereids, and presumably also cotton fibers, culminates in autolysis, which can be considered programmed cell death (Fukuda, 1996; Groover et al., 1997). Consistent with the role of H2O2 in secondary wall synthesis in other plants and different cell types, we observed H2O2 generation during secondary wall formation in trichomes of young Arabidopsis leaves and developing tracheary elements in cultured mesophyll cells of zinnia, although a detailed time course of production has not yet been determined (data not shown). Schopfer (1994) has detected developmentally regulated distribution of H2O2 in bean hypocotyls, which was most pronounced in differentiating xylem cells. Groover et al. (1997), working with the tracheary element differentiation system in zinnia, did not detect an oxidative burst during the wall differentiation and the programmed cell death processes, although they did find a small but constant increase in H2O2 during this period. However, this production was not correlated with conditions that induce tracheary element differentiation. Whether this is due to true differences between the zinnia and cotton systems is not clear. It should be noted that the two systems differ in the type of secondary walls with respect to lignification. Moreover, the addition of H2O2 to zinnia cultures induced a necrotic type of death response, thus arguing against a role for H2O2 in signaling for a programmed process of cell death. However, the doses of H2O2 used in the zinnia studies were in the range of 0.25 and 4 mm, whereas for the cotton fiber differentiation was induced by only 50 μm.
What triggers this regulated oxidative phase of development is not known. The correlation of the onset of H2O2 production with the onset of expression of the Rac genes in cotton suggested to us that changes in the expression levels of the Rac protein (as well as its activation state) may be the developmentally regulated step that controls the production of ROS. The role of small GTPases, Rac1 and Ras, in the induction of ROS generation through the activation of NADPH oxidase has been shown in animal systems (Sundaresan et al., 1996). In plants, the involvement of Rac in the stimulation of NADPH oxidase was suggested recently by studies that showed changes in the localization of Rac upon elicitation (Kieffer et al., 1997; Xing et al., 1997). Our own experiments involving the transient manipulation of Rac gene expression in cultured soybean and Arabidopsis cells further support the suggestion that this GTPase has a causative role in signaling the generation of oxidative stress in plant cells.
It is also interesting to note that Meinert and Delmer (1977) observed a net loss of uronic acid from the fiber wall when fibers enter the stage of secondary wall synthesis. In view of the study by Legendre et al. (1993) of the H2O2 activation by polygalacturonic acid oligomers, this offers an appealing tool for constitutive induction of ROS production during wall differentiation. Future work will explore the possible epistatic relationship between generation of pectic fragments and activation of Rac. In view of the studies by Legendre et al. (1993) and Svalheim and Robertsen (1993) of the elicitation of H2O2 production by oligogalacturonides, as well as the recent work showing their effects on stimulation of cellulose deposition in pericycle cells of tobacco (Altamura et al., 1998), this offers an attractive mechanism for induction of ROS production during wall differentiation.
Abbreviations:
- DCFDA
dichlorodihydrofluorescein diacetate
- DPA
days postanthesis
- DPI
diphenyleneiodonium
- ROS
reactive oxygen species
- SHAM
salicylhydroxamic acid
Footnotes
This work was supported by grants from the U.S.-Israel Binational Agricultural Research and Development Fund and the Israel Academy of Sciences and by a fellowship to T.S.P. from the Giladi Foundation.
LITERATURE CITED
- Abo A, Webb MR, Grogan A, Segal AW. Activation of NADPH oxidase involves the dissociation of p21-rac from its inhibitory GDP-GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochem J. 1994;298:585–591. doi: 10.1042/bj2980585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura MM, Zaghi D, Salvi G, De Lorenzo G, Bellincampi D. Oligogalacturonides stimulate pericycle cell wall thickening and cell divisions leading to stoma formation in tobacco leaf explants. Planta. 1998;204:429–436. [Google Scholar]
- Apostol I, Heinstein PF, Low PS. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Role in defense and signal transduction. Plant Physiol. 1989;90:109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CJ, O'Neill NR, Keppler LD, Orlandi EW. Early responses during plant-bacteria interactions in tobacco cell suspensions. Phytopathology. 1991;81:1504–1507. [Google Scholar]
- Basra AS, Malik CP. Development of the cotton fiber. Int Rev Cytol. 1984;89:65–113. [Google Scholar]
- Beasley CA (1992) In vitro cotton ovule culture: a review. In Cotton Fiber Cellulose: Structure, Function and Utilization Conference. National Cotton Council, Savannah, GA, pp 55–90
- Bestwick CS, Bennett MH, Mansfield JW. Hrp mutant of Pseudomonas syringae pv phaseolicola induces cell wall alterations but not membrane damage leading to the hypersensitive reaction in lettuce. Plant Physiol. 1995;108:503–516. doi: 10.1104/pp.108.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennett MHR, Mansfield JW. Plant Cell. 1997;9:209–221. doi: 10.1105/tpc.9.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Butt VS, Davies DR, Zimmerlin A. The origin of the oxidative burst in plants. Free Radical Res. 1995;23:517–532. doi: 10.3109/10715769509065273. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Northcote DH. Control of hemicellulose and pectin synthesis during differentiation of vascular tissue in bean (Phaseolus vulgaris) callus and in bean hypocotyl. Planta. 1981;152:225–233. doi: 10.1007/BF00385148. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Charloux C, Paul M, Loisance D, Astier A. Inhibition of hydroxyl radical production by lactobionate, adenine, and tempol. Free Radical Biol Med. 1995;19:699–704. doi: 10.1016/0891-5849(95)00079-d. [DOI] [PubMed] [Google Scholar]
- Delmer DP, Pear JR, Andrawis A, Stalker DM. Genes encoding small GTP-binding proteins analogous to mammalian rac are preferentially expressed in developing cotton fibers. Mol Gen Genet. 1995;248:43–51. doi: 10.1007/BF02456612. [DOI] [PubMed] [Google Scholar]
- Diekmann D, Abo A, Johnston C, Segal AW, Hall A. Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science. 1994;265:531–533. doi: 10.1126/science.8036496. [DOI] [PubMed] [Google Scholar]
- Dwyer SC, Legendre L, Low PS, Leto TL. Plant and human neutrophil oxidative burst complexes contain immunologically related proteins. Biochim Biophys Acta. 1996;1289:231–237. doi: 10.1016/0304-4165(95)00156-5. [DOI] [PubMed] [Google Scholar]
- Freeman JL, Abo A, Lambeth JD. Rac “insert region” is a novel effector region that is implicated in the activation of NADPH oxidase. J Biol Chem. 1996;271:19794–19801. doi: 10.1074/jbc.271.33.19794. [DOI] [PubMed] [Google Scholar]
- Fukuda H. Tracheary element formation as a model system of cell differentiation. Int Rev Cytol. 1991;136:289–332. [Google Scholar]
- Fukuda H. Xylogenesis—initiation, progression, and cell death. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:299–325. doi: 10.1146/annurev.arplant.47.1.299. [DOI] [PubMed] [Google Scholar]
- Groom QJ, Torres MA, Fordham-Skelton AP, Hammond-Kosack KE, Robinson NJ, Jones JDG. RbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. Plant J. 1996;10:515–522. doi: 10.1046/j.1365-313x.1996.10030515.x. [DOI] [PubMed] [Google Scholar]
- Groover A, Dewitt N, Heidel A, Jones A. Programmed cell death of plant tracheary elements differentiating in vitro. Protoplasma. 1997;196:197–211. [Google Scholar]
- Hayashi T, Read SM, Bussell J, Thelen M, Lin FC, Brown RMJ, Delmer DP. UDPglucose:(1,3)-glucan synthases from mung bean and cotton: differential effects of Ca2+ and Mg2+ on enzyme properties and on macromolecular structure of the glucan product. Plant Physiol. 1987;83:1054–1062. doi: 10.1104/pp.83.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideg E, Vass I. UV-B induced free radical production in plant leaves and isolated thylakoid membranes. Plant Sci. 1996;115:251–260. [Google Scholar]
- Iiyama K, Lam TBT, Stone BA. Covalent cross-links in the cell wall. Plant Physiol. 1994;104:315–320. doi: 10.1104/pp.104.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science. 1996;273:1853–1856. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- Jabs T, Tschoepe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. A plant homolog of the neutrophil NADPH oxidase gp91-phox subunit gene encodes a plasma membrane protein with Ca2+-binding motifs. Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer F, Simon-Plas F, Maume BF, Blein JP. Tobacco cells contain a protein, immunologically related to the neutrophil small G protein Rac2 and involved in elicitor-induced oxidative burst. FEBS Lett. 1997;403:149–153. doi: 10.1016/s0014-5793(97)00038-0. [DOI] [PubMed] [Google Scholar]
- Knox JP. The extracellular matrix in higher plants. 4. Developmentally regulated proteoglycans and glycoproteins of the plant cell surface. FASEB J. 1995;9:1004–1012. doi: 10.1096/fasebj.9.11.7544308. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Yin XM, Oltvai ZN, Veis-Novack DJ, Linette GP. Reactive oxygen species and the regulation of cell death by the Bcl-2 gene family. Biochim Biophys Acta. 1995;1271:63–66. doi: 10.1016/0925-4439(95)00011-r. [DOI] [PubMed] [Google Scholar]
- Legendre L, Rueter S, Heinstein PF, Low PS. Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol. 1993;102:233–240. doi: 10.1104/pp.102.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Pennell R, Alvarez M, Palmer R, Lamb CJ. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;6:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Low PS, Merida JR. The oxidative burst in plant defense–function and signal transduction. Physiol Plant. 1996;96:533–542. [Google Scholar]
- Maltby D, Carpita NC, Montezinos D, Kulow C, Delmer DP. Glucan in developing cotton fibers: Structure, localization, and relationship of synthesis to that of secondary wall cellulose. Plant Physiol. 1979;63:1159–1164. doi: 10.1104/pp.63.6.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert M, Delmer DP. Changes in the biochemical composition of the cell wall of the cotton fiber during development. Plant Physiol. 1977;59:1088–1097. doi: 10.1104/pp.59.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer A, Daxberger A, Fromhold-Treu D, Ebel J. Involvement of an NAD(P)H oxidase in the elicitor-inducible oxidative burst of soybean. Phytochemistry. 1997;45:1101–1107. [Google Scholar]
- Murphy TM, Auh CK. The superoxide synthases of plasma membrane preparations from cultured rose cells. Plant Physiol. 1996;110:621–629. doi: 10.1104/pp.110.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja O, Willmer CM. Redox activity and peroxidase activity associated with the plasma membrane of guard-cell protoplasts. Planta. 1988;174:44–50. doi: 10.1007/BF00394872. [DOI] [PubMed] [Google Scholar]
- Pear JR, Kawagoe Y, Schreckengost WE, Delmer DP, Stalker DM. Higher plants contain homologs of the bacterial celA genes encoding the catalytic subunit of cellulose synthase. Proc Natl Acad Sci USA. 1996;93:12637–12642. doi: 10.1073/pnas.93.22.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potikha T, Delmer DP. A mutant of Arabidopsis thaliana displaying altered patterns of cellulose deposition. Plant J. 1995;7:453–460. [Google Scholar]
- Prasad TK. Mechanisms of chilling-induced oxidative stress injury and tolerance in developing maize seedlings: changes in antioxidant system, oxidation of proteins and lipids, and protease activities. Plant J. 1996;10:1017–1026. [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP. UV-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996;110:125–136. doi: 10.1104/pp.110.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Royall JA, Ischiropoulos H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- Ryser U. Cell wall biosynthesis in differentiating cotton fibers. Eur J Cell Biol. 1985;39:236–265. [Google Scholar]
- Schopfer P. Histochemical demonstration and localization of H2O2 in organs of higher plants by tissue printing on nitrocellulose paper. Plant Physiol. 1994;104:1269–1275. doi: 10.1104/pp.104.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraudner M, Langebartels C, Sandermann H. Changes in the biochemical status of plant cells induced by the environmental pollutant ozone. Physiol Plant. 1997;100:274–280. [Google Scholar]
- Seagull RW. The effects of microtubule and microfilament disrupting agents on cytoskeletal arrays and wall deposition in developing cotton fibers. Protoplasma. 1990;159:44–59. [Google Scholar]
- Segal AW, Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci. 1993;18:43–47. doi: 10.1016/0968-0004(93)90051-n. [DOI] [PubMed] [Google Scholar]
- Snell FD, Snell CT (1949) Colorimetric methods of analysis. In Inorganic, Vol 2, Ed 3. Van Nostrano Company, New York, pp 882–883
- Sundaresan M, Yu ZX, Ferrans VJ, Sulciner DJ, Gutkind JS, Irani K, Goldschmidt-Clermont PJ, Finkel T. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem J. 1996;318:379–382. doi: 10.1042/bj3180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svalheim O, Robertsen B. Elicitation of hydrogen peroxide production in cucumber hypocotyl segments by oligo-14-alpha-d-galacturonides and an oligo-beta-glucan preparation from cell walls of Phytophthora megasperma f. sp. glycinea. Physiol Plant. 1993;88:675–681. doi: 10.1111/j.1399-3054.1993.tb01388.x. [DOI] [PubMed] [Google Scholar]
- Symons M. Rho family GTPases: the cytoskeleton and beyond. Trends Biochem Sci. 1996;21:178–181. [PubMed] [Google Scholar]
- Tenhaken R, Levine A, Brisson LF, Dixon RA, Lamb C. Function of the oxidative burst in hypersensitive disease resistance. Proc Natl Acad Sci USA. 1995;92:4158–4163. doi: 10.1073/pnas.92.10.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayner ID, Rayner AP, Freeman GE, Farzaneh F. Quantitative multiwell myeloid differentiation assay using dichlorodihydrofluorescein diacetate (H-2DCF-DA) or dihydrorhodamine 123 (H-2R123) J Immunol Metheds. 1995;186:275–284. doi: 10.1016/0022-1759(95)00152-z. [DOI] [PubMed] [Google Scholar]
- Ubezio P, Civoli F. Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells. Free Radical Biol Med. 1994;16:509–516. doi: 10.1016/0891-5849(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Updegraff DM. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969;32:420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Van Gestelen P, Asard H, Caubergs RJ. Solubilization and separation of a plant plasma membrane NADPH-O2 synthase from other NAD(P)H oxidoreductases. Plant Physiol. 1997;115:543–550. doi: 10.1104/pp.115.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RH, Flickinger AG, Rivers WJ, Hardy MM, Aston KW, Ryan US, Riley DP. Evaluation of activity of putative superoxide dismutase mimics: direct analysis by stopped-flow kinetics. J Biol Chem. 1993;268:23049–23054. [PubMed] [Google Scholar]
- Xing T, Higgins VJ, Blumwald E. Race-specific elicitors of Cladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to the plasma membrane of tomato cells. Plant Cell. 1997;9:249–259. doi: 10.1105/tpc.9.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]