Abstract
Propofol infusion syndrome (PRIS) is a rare but often fatal complication as a result of large doses of propofol infusion (4–5 mg/kg/hr) for a prolonged period (>48 h). It has been reported in both children and adults. Besides large doses of propofol infusion, the risk factors include young age, acute neurological injury, low carbohydrate and high fat intake, exogenous administration of corticosteroid and catecholamine, critical illness, and inborn errors of mitochondrial fatty acid oxidation. PRIS manifestation include presence of metabolic acidosis with a base deficit of more than 10 mmol/l at least on one occasion, rhabdomyolysis or myoglobinuria, acute renal failure, sudden onset of bradycardia resistant to treatment, myocardial failure, and lipemic plasma. The pathophysiology of PRIS may be either direct mitochondrial respiratory chain inhibition or impaired mitochondrial fatty acid metabolism mediated by propofol. We report a case of supermorbidly obese patient who received propofol infusion by total body weight instead of actual body weight and developed PRIS.
Keywords: Complication, obesity, propofol infusion syndrome, propofol
INTRODUCTION
Propofol is a potent short-acting intravenous sedative-hypnotic agent used to induce and maintain anesthesia and provide continuous sedation in the ICU. After its introduction in 1989 in USA, it became instantly popular among anesthesia providers and intensive care specialists because of its rapid onset along with rapid recovery even after prolonged infusion.[1] Propofol sedation in the ICU allows frequent and ongoing neurological evaluations especially in neurological ICU and it does not accumulate in patients with renal or hepatic disease.[1] Propofol infusion syndrome (PRIS) is a potentially fatal event following administration of large doses (4–5 mg/kg/h) of propofol over prolonged period (>48 h). We report a case of PRIS in a supermorbidly obese patient who recovered from the acute event; however, she died of other causes.
CASE REPORT
A 42-year-old female was scheduled to undergo an elective parathyroidectomy. Initially she presented with stag horn calculus which had led to the diagnosis of hyperparathyroidism. Her background medical history was significant for morbid obesity with BMI of 75 (weight 192 kg and height 160 cm) with limited exercise tolerance, obstructive sleep apnea (OSA) on BiPAP, hypertension, diabetes, acid reflux disease, and hepatitis C. She was a smoker with history of postoperative nausea and vomiting (PONV) and no known drug allergies. She was taking insulin, lisinopril, hydrochlorthiazide, atorvastatin, aspirin, venlafaxine, trazadone, and rosuvastatin. Her preoperative examination revealed the following: heart rate, 77/min; BP, 93/53 mmHg; respiration, 16/min; SpO2, 93% on room air; and BMI 75. Her airway examination was significant for decreased thyromental distance (<4 cm) and interincisor distance of 4 cm. Her laboratory work was within normal limits except for elevated parathyroid hormone and calcium.
On arrival to preoperative area, she was premedicated with 30 ml of 0.3 molar sodium citrate and 50 mg of IV ranitidine. A 20 G intravenous canula was placed on her right foot and an arterial line in her right radial artery. In addition to invasive blood pressure monitoring, we planned to use arterial line for frequent blood sampling to measure parathyroid hormone levels during surgery. She was transferred to the operating room, placed on a rapid airway management positioner (RAMP), and standard ASA monitoring. She was preoxygenated for 3 min and anesthesia was induced using rapid sequence induction with propofol (300 mg) and suxamethonium (160 mg). Her trachea was intubated with size 7.5 endotracheal using MAC 4 blade with a grade I view. Anesthesia was maintained with oxygen/air, and sevoflurane and intermittent doses of rocuronium were given for muscle relaxation. Her neck was explored and multiple frozen sections were sent to the laboratory for identification of parathyroid tissue, which all came back as fat tissue. After four-and-a-half hours of surgery, surgeons were able to resect left inferior parathyroid adenoma. Following the resection of the adenoma, her parathyroid hormone levels came back to normal in 15 min. As a result of extensive neck dissection and resultant edema, we planned to keep her intubated in the ICU and extubate on the following day.
She was transferred to ICU intubated and ventilated with propofol infusion for sedation. Her propofol was started at 20 μg/kg/min. However she was not tolerating the endotracheal tube and her propofol dose was escalated to 80 μg/kg/min (4 mg/kg/h) within hours upon arrival in the ICU. Even though our plan was to extubate on the following day, she failed spontaneous breathing test (SBT) and her oxygen requirements were increasing. She developed respiratory failure secondary to basal atelectasis and ventilator-associated pneumonia. On postoperative day 3, it was noted that her creatinine kinase (66900 IU/l) and myoglobin (19470 ng/ml) levels started to climb leading to the diagnosis of rhabdomyolysis [Figure 1]. On the same day, we noticed that her urine output was declining along with increasing creatinine (3.1 mg/dl) and BUN (41 mg/dl) [Table 1] levels with the development of acute renal failure. Her postoperative course was also complicated by septic shock secondary to urinary tract infection and ventilator-associated pneumonia, requiring inotropic support with norepinephrine to maintain her hemodynamics. She developed metabolic acidosis with the base deficit of more than 10 mmols/l. As there was no obvious cause for rhabdomyolysis, acute renal failure, and metabolic acidosis, and she received large doses (4 mg/kg/h) of propofol for 65 h, we suspected that she may have developed PRIS. Propofol infusion was stopped and replaced with titrating doses of lorazepam and fentanyl for sedation, and she was dialyzed for acute renal failure. On postoperative day 10, both her rhabdomyolysis and renal failure were resolved. After multiple attempts of extubation failure, she underwent tracheostomy on postoperative day 16. Subsequently she developed large decubitus ulcer requiring frequent debridement. While she was awaiting for palliative care facility location, she died on postoperative day 65 following a fall from her bed, and occlusion of her tracheostomy in prone position.
Figure 1.
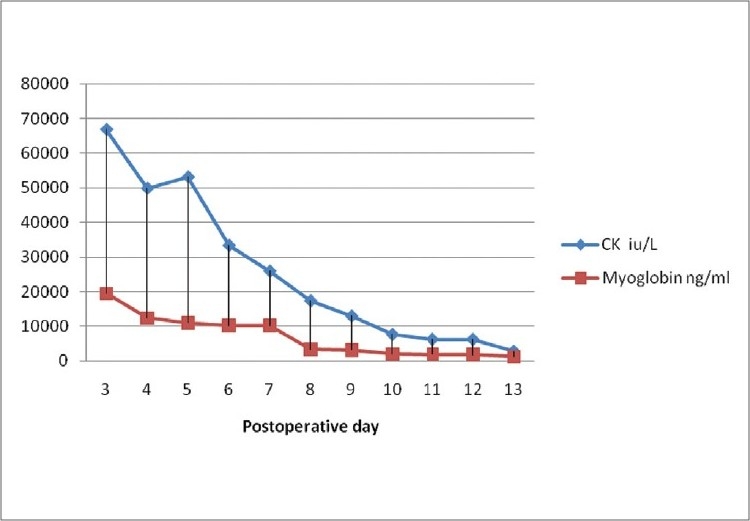
Postoperative lab results of CK and Myoglobin
Table 1.
Postoperative lab results

DISCUSSION
Propofol has been marketed in USA since 1989; it became popular among both intensivists and anesthesiologists because of its favorable pharmacokinetic profile. Numerous cases of adverse reactions and death following administration of propofol have been reported in both children and adults. However, PRIS is a more life-threatening adverse event initially described in children in 1990s,[2,3] resulting from prolonged (more than 48 h) administration of propofol at high dose (4–5 mg/kg/h).
The incidence of PRIS is unknown; however an attempt was made in a recent multicenter prospective observational study.[4] They estimate that the incidence of PRIS is approximately 1% in a heterogeneous population of critically ill adult patients receiving propofol infusion for more than 24 h. PRIS is characterized by the presence of metabolic acidosis with a base deficit of more than 10 mmol/l at least on one occasion, rhabdomyolysis or myoglobinuria, acute renal failure, sudden onset of bradycardia resistant to treatment, myocardial failure, and lipemic plasma.[5] Proposed predisposing factors for the development of PRIS include large cumulative doses of propofol, young age, acute neurological injury, low carbohydrate and high fat intake, exogenous administration of corticosteroid and catecholamine,[6] critical illness, and inborn errors of mitochondrial fatty acid oxidation.[7]
Development of PRIS in susceptible patients may be due to the impairment of free fatty acid utilization and mitochondrial activity. This causes an imbalance between energy demand and utilization, leading to cardiac and peripheral muscle necrosis.[8] In a retrospective data analysis, the mortality of PRIS in suspected patients who were receiving propofol is estimated to be 30%.[9] The death is more likely if patients were young, male, received vasopressors, or had the following clinical manifestations: cardiac dysfunction, metabolic acidosis, renal failure, hypotension, rhabdomyolysis, or dyslipidemia.
We report a case of PRIS in adult supermorbid obese patient with the BMI of 75, who received high dose of propofol for 65 h. Her increased susceptibility to PRIS could be due to contribution from large dose of propofol, exogenous catecholamines (norepinephrine), sepsis, excess body fat, and prior use of simvastatin. There are some case reports that prior administration of statins may trigger rhabdomyolysis in patients who have received propofol infusion.[10] She initially had metabolic acidosis and developed rhabdomyolysis and acute renal failure requiring hemodialysis. Her PRIS symptoms were resolved on postoperative day 10. Unfortunately she died on postoperative day 65 following a fall from her bed, resulting in occlusion of her tracheostomy tube in prone position. Our patient received propofol according to the total body weight instead of actual body weight; as a result she received large dose of propofol which was one of the leading risk factor for the development of PRIS. One of the limitations in our case report is that we did not measure the plasma concentrations of propofol or the lipid load. To our knowledge, this is the first case of PRIS in adult morbidly obese patient, who recovered from PRIS but died from other causes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Marik PE. Propofol: Therapeutic indications and side-effects. Curr Pharm Des. 2004;10:3639–49. doi: 10.2174/1381612043382846. [DOI] [PubMed] [Google Scholar]
- 2.Bray RJchildren. Propofol infusion syndrome in children. Paediatr Anaesth. 1998;8:491–9. doi: 10.1046/j.1460-9592.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 3.Parke T. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: Five case reports. BMJ. 1992;305:613–6. doi: 10.1136/bmj.305.6854.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts RJ, Barletta JF, Fong JJ, Schumaker G, Kuper PJ, Papadopoulos S, et al. Incidence of propofol-related infusion syndrome in critically ill adults: A prospective, multicenter study. Crit Care. 2009;13:R169. doi: 10.1186/cc8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holzki J, Aring C, Gillor A. Death after re-exposure to propofol in a 3-year-old child: A case report. Paediatr Anaesth. 2004;14:265–70. doi: 10.1046/j.1460-9592.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith H, Sinson G, Varelas P. Vasopressors and propofol infusion syndrome in severe head trauma. Neurocrit Care. 2009;10:1662–72. doi: 10.1007/s12028-008-9163-y. [DOI] [PubMed] [Google Scholar]
- 7.Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62:690–701. doi: 10.1111/j.1365-2044.2007.05055.x. [DOI] [PubMed] [Google Scholar]
- 8.Vasile B, Rasulo F, Candiani A, Latronico N. The pathophysiology of propofol infusion syndrome: A simple name for a complex syndrome. Intensive Care Med. 2003;29:1417–25. doi: 10.1007/s00134-003-1905-x. [DOI] [PubMed] [Google Scholar]
- 9.Fong JJ, Sylvia L, Ruthazer R, Schumaker G, Kcomt M, Devlin JW. Predictors of mortality in patients with suspected propofol infusion syndrome. Crit Care Med. 2008;36:2281–7. doi: 10.1097/CCM.0b013e318180c1eb. [DOI] [PubMed] [Google Scholar]
- 10.Francis L, Bonilla E, Soforo E, Neupane H, Nakhla H, Fuller C, et al. Fatal toxic myopathy attributed to propofol, methylprednisolone, and cyclosporine after prior exposure to colchicine and simvastatin. Clin Rheumatol. 2008;27:129–31. doi: 10.1007/s10067-007-0696-9. [DOI] [PubMed] [Google Scholar]


