Abstract
Recent reports reveal that there is increasing incidence of infections of multidrug-resistant bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE). Flavonoids and related compounds have been shown to possess potent antimicrobial activities. Most of the flavonoids are considered as constitutive antimicrobial substances recently termed as “Phytoanticipins,” especially those belonging to prenylated flavonoids and isoflavones. The current review highlights the structure prerequisites for isoflavones as antibacterial agents. Structure–activity relationship (SAR) conclusions have been drawn by comparing the reported minimum inhibitory concentration values for the various isoflavones against S. aureus and MRSA. There exists a significant co-relationship between the presence of certain functional groups (prenyl group, phenolic hydroxyl) at particular positions and antibacterial activity of the compounds. These trends have been postulated with a view of assisting better drug designing of future next-generation antiinfectives, particularly against the bothersome multidrug-resistant microbes. The SAR of these isoflavones has also proved to be a basis to explore the mechanism of antibacterial action. Thus, the study would prove extremely useful to synthesize antibacterial isoflavones in future, which would eventually be beneficial for optimizing the lead molecule for the antibacterial action
Keywords: Antibacterial activity, Isoflavones, SAR of isoflavones
INTRODUCTION
In recent times, microbial infections have once emerged as the leading cause of concern globally. The increasing prevalence of multidrug-resistant strains of bacteria and the recent appearance of strains with reduced susceptibility to antibiotics raises the specter of untreatable bacterial infections, which adds urgency to the search for novel infection-fighting strategies.
Infections caused by methicillin-resistant S. aureus (MRSA) in compromised hosts pose a serious problem all over the world, because MRSA strains are resistant to numerous antibiotics and can be transmitted from patient to patient via transiently colonized hands of hospital personnel. Although vancomycin is the most effective antibiotic for MRSA infections, clinical use often results in unexpected side effects and development of infections by vancomycin -resistant enterococci (VRE).[1] Rational drug design does not always yield effective antimicrobials. In the past, potent enzyme inhibitors have been successfully designed and synthesized but they had only modest antibacterial activity, probably owing to the complex issue of drug uptake by cells. Broad empirical screening of a chemical entity for antimicrobial activity represents an alternative strategy for the development of novel drugs.
Natural products have been a particularly rich source of antiinfective agents, yielding, for example, the penicillins in 1940, the tetracyclines in 1948, and the glycopeptides in 1955.[2] Naturally occurring products from plants have played an important role in the discovery of new therapeutic agents since ancient times, for example, quinine obtained from Cinchona has been successfully used to treat malaria. Substantial attention has been focused on exploration and utilization of secondary metabolites of plants (phytochemicals) as an alternative to and/or in combination with traditional antibiotics for treating MRSA infections.[3–5] Among the phytochemicals, flavonoids, isoflavonoids, and related compounds seem to be the most potentially useful candidates because they are widely distributed in edible plants and possess broad pharmacologic activity.[6] The antimicrobial properties showed by various plants could be due to the presence of mixtures of active constituents (mostly prenylated flavonoids), which show a broad spectrum of biological and pharmacologic activities. Most of the flavonoids [Figures 1–2] are considered as constitutive antimicrobial substances recently termed as “Phytoanticipins,” especially those belonging to prenylated flavonoids and isoflavones, but not excluding other classes of compounds.[7]
Figure 1.
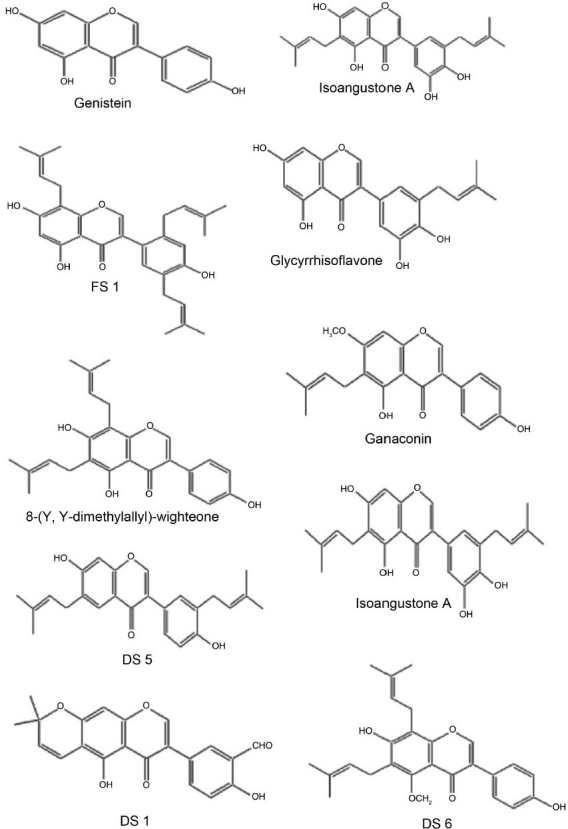
Some of the isolated flavonoids
Figure 2.
Some of the isolated flavonoids
The current report highlights the structural peculiarities of isoflavones in various plant species. Special focus lies on the antibacterial activity of isoflavones and an attempt to draw the Structure–Activity Relationships are made.
ANTIBACTERIAL ACTIVITY OF ISOFLAVONES
Plants have an almost limitless ability to synthesize aromatic substances, most of which are phenols or their oxygen-substituted secondary metabolites. In many cases, these substances serve as plant defense mechanisms against predation by microorganisms, insects, and herbivores. Thus, keeping in mind the possibility of these secondary metabolites having an inherent capability to be antimicrobial or antibacterial agents, the current focus is on identification and screening of such secondary metabolites.
Isoflavonoids are a subclass of the more ubiquitous flavonoids, which are naturally occurring polyphenols. Isoflavonoids differ from other classes of flavonoids by their greater structural variability, their frequent presence in plants in their free form, rather than as a glycoside, and by the greater frequency of isoprenoid substitution. They are divided into subclasses depending on the oxidation level of the central pyran ring. Isoflavonoids constitute a specific branch of flavonoid metabolism, differing from the other flavonoids by the position of the phenolic ring B.[8]
STRUCTURE–ACTIVITY RELATIONSHIPS OF ISOFLAVONES WITH ANTIBACTERIAL ACTIVITY
An exhaustive literature survey was done and isoflavones possessing antibacterial activity were identified from various plants.[9–17] The antibacterial activities of these different isoflavones were compared with respect to their minimum inhibitory concentration (MIC) values. There existed a significant co-relationship between the presence of certain functional groups (prenyl group, phenolic hydroxyl) at particular positions and antibacterial activity of the compounds. These trends have been studied keeping in mind the activity of the isoflavones against S. aureus and MRSA. Thus, the present study draws an attempt to predict the structure–activity relationship (SAR) of isoflavones with good antibacterial activity against the susceptible as well as resistant forms of bacteria.
Following conclusions with respect to structural prerequisites of isoflavones with lethal activity against the susceptible and resistant forms of bacteria have been drawn.
Prenyl group
Presence of the Prenyl group at C-6 produces isoflavones with potent activity. For example, Genistein with no prenyl group has very less activity (MIC values against S. aureus and MRSA: >128 μg/mL).[17]
Removal of Prenyl group or movement to C-8 decreases activity. For example, Isoangustone (MIC values against S. aureus and MRSA: 16 μg/mL),[17] FS 1(MIC values against S. aureus and MRSA: 28 and 36 μg/mL, respectively)[16] to Glycyrrhizoflavone (MIC values against S. aureus and MRSA: 32 and 32–64 μg/mL, respectively).[17]
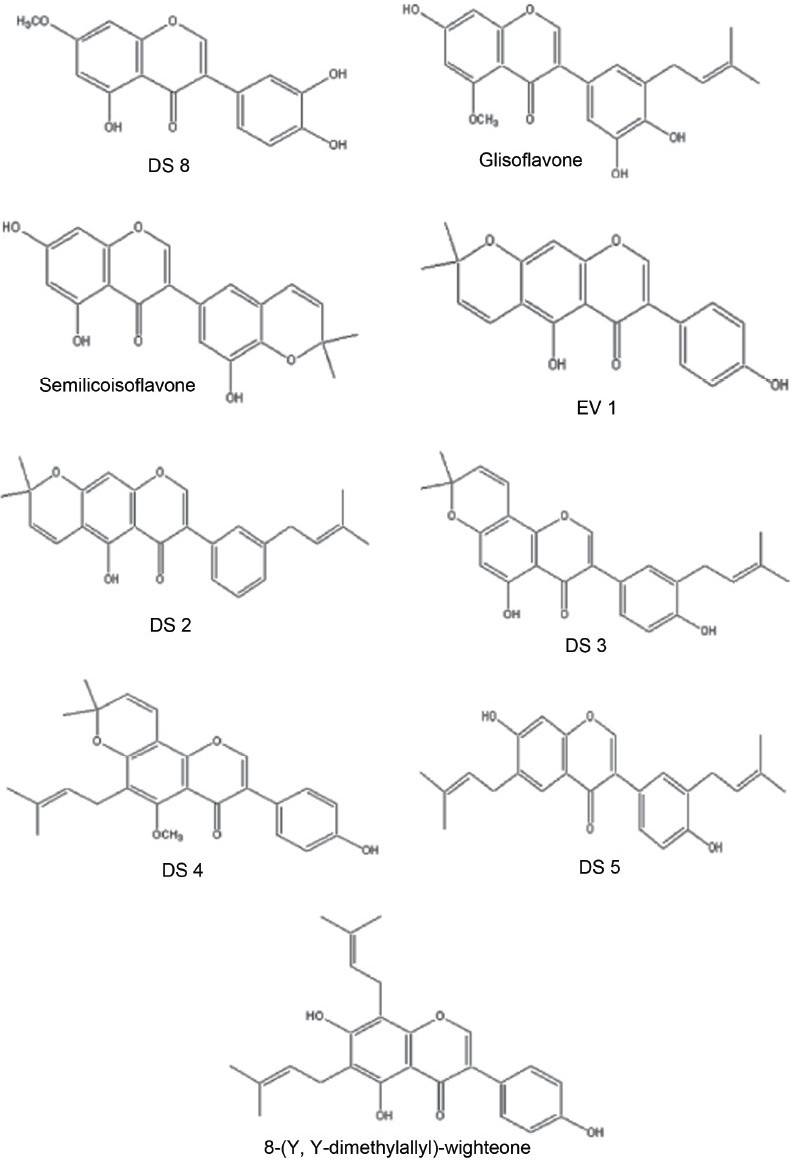
Prenyl group on the fused pyran ring system governs activity. There is no mandatory requirement of presence of the prenyl group on aryl substitution. For example, 8-(γ,γ-dimethylallyl)-wighteone (MIC values against S. aureus and MRSA: 8 μg/mL),[17] and Ganaconin (MIC values against S. aureus and MRSA: 16 μg/mL)[17] show potent activity, although there are no prenyl groups on aryl substitution.
Phenolic hydroxyl groups
Hydroxyl group on fused pyran ring system as well as the aryl substitution is very important for biological activity. Hydroxyl group should be present at positions C-7 and/or C-5 position. For example, DS 5 (MIC values against S. aureus and MRSA: 2 and 4 μg/mL, respectively),[10] and Isoangustone A (MIC values against S. aureus and MRSA: 16 μg/mL, respectively).[17]
Removal of hydroxyl group or conversion of hydroxyl group to –CHO abolishes activity. For example, DS 1.[10]
Methylation of fused pyran ring hydroxyl group decreases anti-S. aureus activity, but sharply increases anti-MRSA activity. For example, DS 6 (MIC values against S. aureus and MRSA: 128 and 2 μg/mL, respectively), and DS 8 (MIC values against S. aureus and MRSA: 128 and 2 μg/mL, respectively).[10]
Methylation of flavonoid hydroxyl group coupled with absence of prenyl group further decreases antibacterial activity. For example, Glisoflavone (MIC values against S. aureus and MRSA: 64 μg/mL).[17]
Cyclization between prenyl and hydroxyl groups
Cyclization between prenyl and hydroxyl groups on the aryl substitution decreases activity, but not greatly. For example, Semilicoisoflavone (MIC values against S. aureus and MRSA: 32 and 32–64 μg/mL, respectively).[17]
Cyclization between C-6 prenyl and C-7 hydroxyl group drastically decreases activity. For example, EV 1 (reported to have “No significant activity”),[12] but presence of a prenyl group at 3′ position of aryl substitution with this cyclization retains activity. For example, DS 2 (MIC values against S. aureus and MRSA: 128 and 16 μg/mL, respectively).[10]
Cyclization between C-8 prenyl and C-7 hydroxyl group abolishes activity, but presence of a prenyl group at C-6 or 3′ position with this cyclization also does not retain much activity. For example, DS 3 and DS 4.[10]
The antimicrobial effect of isoflavones is also attributed to the presence of phenolic hydroxyl groups that have affinity for proteins and therefore act as inhibitors of microbial enzymes as well as their biosynthetic pathways. In addition, substitution of the flavonoid ring system with prenyl groups is thought to increase their lipophilicity and consequently, enhances their antibacterial activity through interaction with cellular membranes.[18]
The lipophilicity of the aliphatic groups (prenyl) seems to be required to disturb the bacterial cell membrane or its function, in a way analogous to the supposed mechanisms related to the antibacterial activities of catechin derivatives and alkyl gallates. The requirements of the phenolic hydroxyl groups may be correlated with the damage on the bacterial tissues through some pro-oxidant effects in the participation of the phenolic hydroxyl groups, in accordance with the reported role of hydroxyl groups in the antibacterial effects of catechins.[19]
Possible mechanisms of antibacterial activity
Antibacterial activity of many of the synthetic and natural agents has been thought to be mediated via interference with the bacterial topoisomerase activity. Two bacterial type II topoisomerases have been identified: topoisomerase IV (topo IV) and DNA gyrase. Topo IV catalyzes the separation of two double-stranded covalently closed circular DNA molecules that are intertwined in a chain. DNA gyrase is a type II topoisomerase that introduces negative supercoils (or relaxes positive supercoils) into DNA by looping the template so as to form a crossing, then cutting one of the double helices and passing the other through it before releasing the break, changing the linking number by two in each enzymatic step. This process occurs in prokaryotes (particularly in bacteria), whose single circular DNA is cut by DNA gyrase and the two ends are then twisted around each other to form supercoils. The unique ability of gyrase to introduce negative supercoils into DNA is what allows bacterial DNA to have free negative supercoils. The ability of gyrase to relax positive supercoils comes into play during DNA replication. Several inhibitors of DNA gyrase and/or topo IV have been developed for clinical use as antimicrobials. Synthetic antibiotics target both DNA gyrase and topo IV by stabilizing the topo II-DNA reaction intermediate, known as the cleavable complex. By trapping this complex, they introduce lesions into the intracellular DNA. Isoflavone genistein inhibits the catalytic activity of the enzyme by stabilizing the covalent topoisomerase II-DNA cleavage complex. Genistein inhibits bacterial growth in vitro by the inhibition of topo IV, based on several lines of evidence. First, genistein inhibits the activity of topo IV rather than that of DNA gyrase. Second, topo IV in S. aureus is the primary target of topo II-targeted drugs, whereas in Escherichia coli the primary target is DNA gyrase. Our results demonstrate genistein-mediated growth inhibition of S. aureus but not of E. coli.[11]
Many of the isoflavones have potent activity against gram-positive bacteria and have no activity or negligible activity against gram-negative bacteria. It has been postulated that the protoplasmic membrane of the bacterial cell is the operative target of isoflavones and related compounds, and thus they exhibit antibacterial activity by interfering with the incorporation of metabolites and nutrients into bacterial cells. As stated earlier, these compounds show a broad spectrum of antibacterial activity on gram-positive bacteria, including Staphylococci, Streptococci, Actinomyces, and Lactobacillus species, however, they fail to inhibit gram-negative bacteria, such as E. coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae even at relatively high concentrations, suggesting that the outer membrane of gram-negative bacteria interferes with the accessibility of the compounds to the protoplasmic membrane. The second possibility is that the nucleic acids in MRSA cells get affected. In addition to complete inhibition of metabolite incorporation of bacterial cells, the observed intense bactericidal action of these compounds bears some resemblance to that induced by 4-quinolone antimicrobial agents, which inhibit DNA synthesis.[1] This is in agreement with the fact that isoflavones bear some structural resemblance with 4-quinolone antibacterial agents.
CONCLUSIONS
Newer antiinfectives are being continuously designed and synthesized by researchers world over, but the problem of antibiotic resistance is on the rise. Thus, there is a dire need for the discovery of new compounds with antibiotic activity against the resistant forms of bacteria. The current study is an attempt to evaluate isoflavones as potential antibacterial agents. The SAR of isoflavones with antibacterial activity revealed a co-relationship between the presence of prenyl group and hydroxyl group at certain positions of the isoflavone ring structure and anti-S. aureus and anti-MRSA activity. Of the compounds included in this study, DS 5 and 8-(γ,γ-dimethylallyl)-wighteone were found to be the most active compounds. DS 5 has the C-6 prenyl group as well as the C-7 hydroxyl group. On the other hand, 8-(γ,γ-dimethylallyl)-wighteone has C-6 and C-8 prenyl group and C-5 and C-7 hydroxyl group. These structural peculiarities possessed by these two compounds are very important for anti-S. aureus and anti-MRSA activity as revealed by this review. Thus, these two compounds can act as future candidates to develop newer synthetic antibacterials in due course.
The current study would be useful to design and synthesize newer antibacterial agents. These agents can either be used alone or in combination with other antibiotics to combat infections of S. aureus and MRSA. Use of these agents in combination would reduce the effective dose to be administered and also reduce the incidence of resistance to antibiotics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tanaka H, Sato M, Oh-Uchi T, Yamaguchi R, Etoh H, Shimizu H, et al. Antibacterial properties of a new isoflavonoid from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus. Phytomedicine. 2004;11:331–7. doi: 10.1078/0944711041495137. [DOI] [PubMed] [Google Scholar]
- 2.Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–56. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iinuma M, Tsuchiya H, Sato M, Yokoyama J, Ohyama M, Ohkawa Y, et al. Flavanones with potent antibacterial activity against methicillin- resistant Staphylococcus aureus. J Pharm Pharmacol. 1994;46:892–5. doi: 10.1111/j.2042-7158.1994.tb05709.x. [DOI] [PubMed] [Google Scholar]
- 4.Sato M, Tsuchiya H, Miyazaki T, Fujiwara S, Yamaguchi R, Kureshiro H, et al. Antibacterial activity of hydroxychalcone against methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 1996;6:227–31. doi: 10.1016/0924-8579(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka H, Sato M, Fujiwara S, Hirata M, Etoh H, Takeuchi H. Antibacterial activity of isoflavonoids isolated from Erythrina variegata against methicillin-resistant Staphylococcus aureus. Lett Appl Microbiol. 2002;35:494–8. doi: 10.1046/j.1472-765x.2002.01222.x. [DOI] [PubMed] [Google Scholar]
- 6.Pathak D, Pathak K, Singla AK. Flavonoids as medicinal agent-recent advances. Fitoterapia. 1991;62:371–89. [Google Scholar]
- 7.Barron D, Ibrahim RK. Isoprenylated Flavonoids: a review. Phytochemistry. 1996;43:921–82. [Google Scholar]
- 8.Albulescu M, Popovici M. Isoflavones – biochemistry, pharmacology and therapeutic use. Revue Roumaine De Chimie. 2007;52:537–50. [Google Scholar]
- 9.Jian He, Li Chen, Heber D, Wenyuan Shi, Qing-Yi Lu. Antibacterial compounds from Glycyrrhiza uralensis. J Nat Pro. 2006;69:121–4. doi: 10.1021/np058069d. [DOI] [PubMed] [Google Scholar]
- 10.Mahabusarakam W, Deachathai S, Phongpaichit S, Jansakul C, Taylor WC. A benzil and isoflavone derivatives from Derris scandens Benth. Phytochemistry. 2004;65:1185–91. doi: 10.1016/j.phytochem.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Verdrengh M, Collins LV, Bergin P, Tarkowski A. Phytoestrogen genistein as an anti-staphylococcal agent. Microbes Infect. 2004;6:86–92. doi: 10.1016/j.micinf.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed RZ, Shirin SJ, Chowdhury FF, Faisol F, Mohammad RS, Mohammad S, et al. Phytochemical and Biological Investigations of Erythrina variegata. Saudi Pharm J. 2007;15:140–5. [Google Scholar]
- 13.Rahman MM, Gibbons S, Gray AI. Isoflavanones from Uraria picta and their antimicrobial activity. Phytochemistry. 2007;68:1692–7. doi: 10.1016/j.phytochem.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Chacha M, Bojase-Moleta G, Majinda RR. Antimicrobial and radical scavenging flavonoids from the stem wood of Erythrina latissima. Phytochemistry. 2005;66:99–104. doi: 10.1016/j.phytochem.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Yao-Kouassi PA, Magid AA, Richard B, Martinez A, Jacquier MJ, Caron C, et al. Isoflavonoid glycosides from the roots of Baphia bancoensis. J Nat Prod. 2008;71:2073–6. doi: 10.1021/np8005138. [DOI] [PubMed] [Google Scholar]
- 16.Madan S, Singh GN, Kohli K, Ali M, Kumar Y, Singh RM, et al. Isoflavonoids from Flemingia strobilifera (L) R. Br. Roots. Acta Pol Pharm. 2009;66:297–303. [PubMed] [Google Scholar]
- 17.Hatano T, Shintani Y, Aga Y, Shiota S, Tsuchiya T, Yoshida T. Phenolic constituents of licorice: structures of glicophenone and glicoisoflavanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem Pharm Bull. 2000;48:1286–92. doi: 10.1248/cpb.48.1286. [DOI] [PubMed] [Google Scholar]
- 18.Pethakamsetty L, Seru G, Kandula L. Antimicrobial activity of the root extracts of T. pumila and T. tinctoria on clinical and phytopathogens. J Pharm Res. 2009;2:1694–6. [Google Scholar]
- 19.Hatano T, Kusuda M, Tsugawa M, Taniguchi S, Shiota S, Tsuchiya T. Matsumoto T, et al., editors. Effects of Natural Polyphenols on Methicillin-Resistant Staphylococcus aureus in relation to their chemical and physical properties in phytochemistry research progress. 2008:31–47. [Google Scholar]


