Abstract
Natural product market has seen tremendous growth in the last few years. It results in the formulation of a number of proprietary herbal products, majority of them being multi-component formulations. With the advancement of herbal drug treatments, it has now been observed that many of the constituents present in the drug may react with each other, raising the serious concern about the stability of such formulations which is an important issue in the field of phytochemistry and natural medicines. Natural products are often prone to deterioration, especially during storage, leading to loss of active component, production of metabolites with no activity and, in extreme cases, production of toxic metabolites. This area needs to be addressed in order to determine the efficacy of the formulation. Understanding the problems related to natural product stability can give the idea of dealing with the stability issues. Modifications of the conventional herbal formulations can deal with the stability problems to a large extent. This article deals with the stability problems and is aimed to provide some tools and techniques to increase stability of natural medicines and herbal formulations.
Keywords: Herbal formulation, instability, natural products, phytoformulations, shelf-life, stability
INTRODUCTION
There has been an alarming increase in the number of diseases and disorders caused by synthetic drugs prompting a switch over to traditional natural medicine. Herbal medicines are being used by nearly about 80% of the world population, primarily in developing countries for primary health care.[1] Presently, India contributes less than 1% to the global herbal market. However, it is fast emerging as a key supplier of medicinal plant formulations across the globe.[2] Plants, as a whole or their parts or exudates, are subjected to certain treatments such as extraction, distillation, purification, concentration or fermentation to obtain herbal preparations. Stability is defined as the capacity of a drug substance or drug product to remain within established specifications to maintain its identity, strength, quality, and purity throughout the retest or expiration dating periods. Currently, the usage of standardized extract in natural medicines has become common and popular. However, during manufacturing and the extraction process for natural products, the drug molecules or active components are exposed to oxidation, hydrolysis, microbial attack and other environmental degradation which poses a problem of stability to the products.[3] The monitoring of the presence and concentration of the bioactive constituents is extremely vital as it also affects the quality, efficacy and shelf-life of the natural medicines. In case of a herbal medicinal product containing a natural product or herbal drug preparation (HDP) with constituents of known therapeutic activity, the variation in content during the proposed shelf-life should not exceed ±5% of the initial assay value, unless justified.[4]
WORLD HEALTH ORGANIZATION GUIDELINES FOR HERBAL DRUG STABILITY
The physical and chemical stability of a product in the container in which it is to be marketed should be tested under defined storage conditions and the shelf-life should be established.[5] Quality of crude drug material, plant preparations and finished products depend upon the content variation and stability during storage. The active and characteristic constituents should be specified and, if possible, content limits should be defined. Foreign matter, impurities and microbial content should be defined or limited in the crude natural product.[6] In 1998, Food and Drug Administration (FDA) draft guidance for stability testing.[7]
STABILITY STUDY OF HERBAL DRUG
Determination of stability of herbal drug in formulations is important. The stability is aimed at assuring that the drug/drug product remains within the specifications established to ensure its identity, strength, quality and purity. It can be interpreted as the length of time under specific conditions and storage that a product will remain within the pre-defined limits for all its important characteristics. Each ingredient, whether therapeutically active or inactive, in a dosage form can affect stability. Environmental factors such as temperature, light, air (specifically oxygen, carbon dioxide and water vapors) and humidity can affect stability. Similarly, factors such as particle size, pH, the properties of water and other solvents employed, the nature of container and the presence of other chemicals resulting from contamination or from the intentional mixing of different products can influence stability [Figure 1].[8] Stability testing on typical natural extracts such as flavonoid containing herbal drugs has been reported by researchers to understand the stability criteria for natural products.[9] A report on stability testing of herbal medicinal products and problematic cases from practice with discussion of possible resolution approaches has been established.[10]
Figure 1.
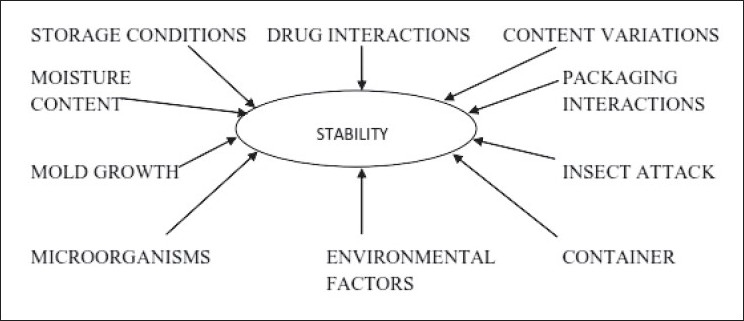
Factors affecting stability of natural medicines
PROBLEMS RELATED TO THE HERBAL PRODUCT STABILITY[11]
Physical instability
Natural medicines often suffer the problem of the physical instability due to the presence of impurities and reaction with the container. Conditions like growth of the microorganisms and insect feeding affect the secondary metabolites and chemical composition of plants. Volatile active components of natural medicine have the problem of volatility and decreasing activity during storage for a long time.
Environmental conditions
Environmental conditions such as rainfall, altitude, temperature, soil, storage conditions as well as different harvesting procedures, time and method of collection, manufacturing processes such as selecting, drying, purifying, extracting, and genetic variability can create substantial variability in the product quality, stability and in the concentration of plant chemicals within different products. Light is also a prominent factor affecting phytoformulations by generation of free radicals.
Chemical instability
Phytoformulations often suffer degradation during storage by oxidation, hydrolysis, crystallization, emulsion breakdown, enzymatic deterioration and chemical reactions with the additives and excipients. Temperature and moisture are the two major factors that affect quality and stability of a herbal product. A chemical reaction increases by a factor of between two- and three fold for every 10°C rise in temperature. Moisture absorbed on to the surface of solid drug often increases the rate of decomposition if it is susceptible to hydrolysis. Presence of enzymes in the product also increases the rate of chemical degradation.
Complex mixtures, variability and inconsistency
Herbal formulations are complex mixtures of different components obtained during extraction process. Each component has variable shelf-life, activity, concentration and consistency. It creates a problem during storage condition determination as it is not easy to determine the stability of final herbal preparation based on the activity and stability profile of a single component.
Drug interactions, deterioration, decomposition and storage[12]
Moisture content above the critical value and mold growth in natural products can cause the interaction of the active components with the packaging material. Also, interactions of active component with the other ingredients of formulations used such as additives cause alteration in the novel drug activity. Herbal formulations have many active constituents as alkaloids, glycosides, tannins, flavonoids etc. and each component is having different stability conditions. Hence actual stability condition for the herbal formulation is different than its individual component.
DIFFERENT TOOLS AND TECHNIQUES TO DEAL WITH PROBLEMS RELATED TO THE NATURAL MEDICINES′ STABILITY
Tools for dealing with natural product instability
Determination of the physical parameters
Determination of the impurity profile
Nonbiased identification and quantification of all metabolites
Controlled storage conditions
Different techniques to deal with instability related to natural medicines
Nanoparticle coating for enhancing the shelf-life of a natural product
Semisolid preparations based on supercritical carbon dioxide
Novel approach for natural products
Topical herbal formulation
Liquid preparation coated with water-soluble cellulose derivative
Polymeric plant-derived excipients in drug delivery
Long chain fatty acid derivative
Protein coating substance and use of the plant chaperonin-like Sp1 protein to stabilize therapeutic protein in pharmaceutical use
Chelating agents for stabilization of the aqueous plant extracts
Suspension form of the herbal products
Emulsion form of herbal products
Continuous multi-microencapsulation process for biologically active ingredients
Antioxidants and liquid formulations
Linctuses for herbal preparations
Transgenic plants and immunoprotective compounds
Tablet formulation for volatile liquids
Formulations without the use of the stabilizer
Plant pigments
Powders with oil composition containing aqueous active substance
Vitamin solutions
Preventing precipitation with improved storage stability by using β-cyclodextrin as a component
Herbal hair dye
Herbal compositions with high contents of herb medicine extracts
Use of colloidal silicon dioxide in the enhancement of the drying of herbal preparations
Tools for dealing with natural product instability
Determination of the physical parameters
The availability, stability and quality of the raw materials are frequently problematic, the active principles are frequently unknown; and standardization, stability, and quality control are feasible but not easy. Depending on the type of preparation, sensory properties, physical constants, moisture, ash content, solvent residues, and adulterations have to be checked to prove identity and purity. Microbiological contamination and foreign materials such as heavy metals, pesticide residues, alfatoxins, and radioactivity also need to be tested for. To prove the constant composition of herbal preparations, appropriate analytical methods have to be applied and different concepts have to be used in order to establish relevant criteria for uniformity.[13]
Determination of the impurity profile
This technique helps in the identification of impurities, especially of a new drug or a chemical. The substance in question is subjected to a known degradation process and the products thus obtained are identified. As we know the degradation process (may be oxidation or hydrolysis), we can have an idea of what could be the degradation products. These can be listed and kept as a reference library of degradation products. For routinely doing an impurity profile, one can take help from this library and trace the nature and structure of the impurity. Since impurities decrease the stability of the natural medicines, it is important to note the type of impurities. It can be done by the analytical methods as high performance liquid chromatography (HPLC), capillary electrophoresis, spectrophotometry, gas chromatography-mass spectrometry (GC-MS), thin layer chromatography (TLC), etc.[14]
Nonbiased identification and quantification of all metabolites
Nonbiased identification and quantification of all metabolites in herbal or other natural products is vital to determine the status and stability of the complex mixtures. IR spectroscopy in combination with chemometric data processing could provide total metabolic fingerprint profile of phytoformulations. The technique enables on-site inspection and ACCEPT and REJECT classification of material.[15]
Controlled storage conditions
Control measure to protect against deterioration includes the use of airtight container made of materials that will not interact physically or chemically with the material being stored. Storage in ventilated cool, dry area and periodic spraying of the stored area with insecticides will help to prevent the spread of infestation. Influence of environmental factors such as temperature, light, oxygen, moisture, other ingredient or excipients in the dosage form, particle size of drug, microbial contamination, trace metal contamination, leaching from the container, etc. should be established to recommend proper storage conditions.[16]
Different techniques to deal with instability problems related to natural medicines
Nanoparticle coating for enhancing shelf-life of a natural product
Nanocoating of active components of herbal formulation is effective in protecting the active drug molecule from oxidative, hydrolytic and environmental degradation processes and hence enhances the shelf-life of the herbal products. In phytoformulation research, developing nano dosage forms [polymeric nanoparticles (nanospheres and nanocapsules), liposomes (protection from enzymatic degradations), proliposomes, solid lipid nanoparticles, nanoemulsion, etc.] has a large number of advantages for herbal drugs, including enhancement of solubility and bioavailability, protection from toxicity, enhancement of pharmacological activity and stability.[17] Liquid dosage compositions of stable nanoparticulate drugs have improved stability than the conventional dosage forms.[18]
Semisolid preparations based on supercritical carbon dioxide
Recently, use of supercritical carbon dioxide technique has been found to effectively increase the stability of the herbal preparation with high amount of active principles. It is observed that conventional HDPs based on Arnica montana L. have a low content of the active principles, sesquiterpene lactones, which show poor stability and low physical compatibility in semisolid formulations. However, supercritical carbon dioxide extracts have high sesquiterpene content and show increase in stability and compatibility in formulation.[19]
Novel approach for natural products
Formulation of chewable tablet has advantages of good dispersion, short disintegrating time, quick dissolution, quick absorption by human body, high bioavailability and stability, good taste and convenient administration as compared with existent dosage forms of the same component.[20] As an example, chewable tablet which is a mixture of Herba artemisiae Scopariae, Ardenia jasminoides fruit, and Flos lonicerae is formulated to have better stability and bioavailability than liquid and injection formulation of the same.[21]
Topical herbal formulation
A nanoemulsion formulation comprises a therapeutically active aqueous phase entrapped in oil phase selected from one or more essential oils, for the topical treatment of acne and other skin disorders like eczema, psoriasis, aging scarring and shows increased efficacy, improved percutaneous penetration, excellent thermodynamic stability ensuring long shelf-life, low skin irritation and reservoir effect that promotes drug localization in the skin, enabling controlled delivery of the said therapeutic agents.[22]
Liquid preparation coated with water-soluble cellulose derivative
A stable liquid preparation contains a solution which has water content from 10% to 80% and an active ingredient coated with a material containing a water soluble cellulose derivative. The liquid preparation can be made into a hard capsule and stably retain the water-unstable active ingredient in it and at the same time mask unpleasant taste or smell.[23]
Polymeric plant-derived excipients in drug delivery
Plant polysaccharides comply with many requirements expected of pharmaceutical excipients such as non-toxicity, stability, availability and hence investigated for use in the development of solid oral dosage forms. Some of the polysaccharides have the characteristic of gelation and hence can also be used for the controlled delivery of the herbal formulations with increased storage stability.[24]
Long chain fatty acid derivative
Plant derived long chain fatty acids are incorporated in many of the formulations by using a low-linolenic acid base compound to enhance the stability of the product as it reduces the need for the hydrogenation of the oil based formulations and enhances the shelf-life of product.[25]
Protein coating substance and use of the plant chaperonin-like Sp1 protein to stabilize therapeutic protein in pharmaceutical use
Formulation of therapeutic protein with improved thermal stability, which comprises a core and a coating layer, and the core containing at least one biologically active protein and the coating layer containing a micronized product from leguminous plants, can be done by forming a fusion product and complex with the chaperonin-like Sp1 protein.[26]
Chelating agents for stabilization of the aqueous plant extracts
Plant extract which is obtained by aqueous extraction can be stabilized by using a water soluble agent capable of chelating the extract. As an example, water soluble plant extract can be stabilized by the use of the polyvinylpyrrolidone (PVP) and other water soluble chelating agents.[27]
Suspension form of the herbal products
The stability of the suspensions depends on the viscosity and other factors. Viscosity enhancers such as xanthan gum can enhance the viscosity and hence the stability of the formulation. In comparison to the xanthan gum, cellulose derivatives have lesser viscosity and hence lead to decreased stability than xanthan gum. Insoluble or sparingly insoluble or sparingly soluble plant extracts are stabilized in solution by the use of suspending agent and nonionic surfactant with or without propylene glycol.[28]
Emulsion form of herbal products
A recent study on the storage stability of oil-in-water emulsions and number and size of globules during the storage at room temperature of herbal extract prepared by dispersing ethoxypolysiloxane in water containing emulsifiers such as methyl cellulose, oleinol 7 (heptaethylene) glycol mono-oleate, and Tween 20 shows that there was a reduction in viscosity of the emulsions containing methyl cellulose, indicating chain degradation. After storage, the emulsions containing Tween 20 had increased viscosity but the globule size and number remained unchanged. The least stable was the emulsion containing oleinol 7 (heptaethylene) glycol mono-oleate. The ability of the emulsion to suppress foaming of aqueous solutions containing plant extract was retained throughout the storage period.[29]
Continuous multi-microencapsulation process for biologically active ingredients
For the production of nutraceuticals as well as cosmetic products such as anti-wrinkle creams, spray microcapsule preparations are adequate to stabilize compounds of natural origin. Microcapsules are obtained by a continuous water-in-oil-in-water microencapsulation process. A formulation comprises a continuous water phase having dispersion of microcapsules which contain oil drops, and in the inside of each oil phase drop, there is a dispersion of water, or aqueous extract or water-dispersible material or water-soluble material. The oil drops are encapsulated with a polymerizable material of natural origin. Such microcapsules are appropriate for spray-drying to be used as a dry powder, lyophilized, self-emulsifiable powder, gel, cream, and any liquid form. The active compounds included in the microcapsules are beneficial to health and other biological purposes.[30]
Antioxidants and liquid formulations
Use of the antioxidants such as the Glycyrrhiza, Ginkgo biloba, polyphenols, esters of flavanol and fatty acids is important to reduce the oxidation. Generation of the free radicals damages the formulation more readily. Antioxidants act as free radical scavengers and hence increase the stability of liquid herbal formulations and other products.[31]
Linctuses for herbal preparations
Physical properties of the linctuses preparation such as the pH, viscosity, and shelf-life are determined using the glycerin based formulation. Specific gravity and the viscosity and properties of the formulation are stable on storage with glycerin based formulation and have higher values.[32]
Transgenic plants and immunoprotective compounds
Studies have shown that disrupted plant cells are genetically transformed to express immunogens or other polypeptides to produce immunoprotective compounds, biologically active proteins which display stability and robustness and are useful in vaccine and pharmacological preparations. The antigens or functional proteins accumulate in the cytoplasmic cell wall and membrane areas of the plant cell and can be released in the form of particles, by mechanical or physical disruption or some other means which produce immunoprotective compounds.[33]
Tablet formulation for volatile liquids
A visually stable tablet containing volatile liquid medicine is prepared by damp proof-coating after including and moisturizing the volatile liquid active medicine. The process comprises the following steps: making the volatile liquid active medicine to be included in β-cyclodextrin and moisturizing with colloidal silicon dioxide, mixing with the other additives, pressing out and pulverizing into fixed size to prepare dried granules, mixing again with disintegrating agents, lubricants, and re-pressing out to get a tablet, then damp proof-coating using isopropane of polyvinylacetaldimethylaminoacetate as a coating material, and coating sugar on it.[34]
Formulations without the use of the stabilizer
Stable pharmaceutical natural preparations without the use of a stabilizer are also formulated now-a-days. The active ingredient is sealed away from excipients that can adversely affect stability by sealing the excipients rather than the active ingredient. The sealing components are water-soluble polymers such as cellulose ethers. The preparations are substantially unaffected by exposure to storage conditions of elevated temperature and elevated relative humidity.[35]
Plant pigments
Light sensitive insoluble drugs are stabilized in solid formulations by the use of the plant pigments such as chlorophyll, caramel, saffron yellow and red beet pigment. The pigments reduce the light dependent degradation of the herbal products. Free radical scavenging activity is the accepted mode of action followed by the plant pigments for protection against light.[36]
Powders with oil composition containing aqueous active substance
The powders comprise base powders and oil compounds. The oil compounds are manufactured by drying water-in-oil emulsions that have aqueous phase containing water-soluble and/or dispersible active substances and oil phase containing emulsifiers. The active substances such as flavors and plant extracts are stabilized in the powders. A powder was prepared from gum arabic, pinedex 3 (starch hydrolyzate), etc. The taste of the extract was masked in the powder.[37]
Vitamin solutions
Improved compositions and methods for producing individual dosages of nutritional supplements containing a large dose of stable ascorbic acid, vitamins and herbal extracts having extended shelf-life without substantial degradation for mammals are formulated. The process involves heating a mixture of ascorbic acid and humectants to elevated temperature with agitation to stabilize ascorbic acid at selected water activity. Thus, a vitamin C solution containing vitamin C, glycerin, citric acid, and water was prepared. In order to achieve a low level of water activity, it was concluded that high levels of glycerin (about 46%) and temperature above the detrimental temperature (135°F) were required. To achieve complete solubilization at that temperature, about 6 min of heat exposure was needed, which in a conventional solution may severely damage vitamin C.[38]
Preventing precipitation with improved storage stability by β-cyclodextrin in the compound
A method for preparing a herbal liquid compound has been proved to inhibit the precipitation of the herbal liquid compound and improve the storage stability, by adding, 0.05–1.00% wt. /vol. of β-cyclodextrin to the compound. The method for preparing a herbal liquid compound containing β-cyclodextrin consists of the following steps: (a) adding purified water or solvent, extracting it at a temperature of 80-100°C (b) obtaining an extract filtrate (c) adding β-cyclodextrin and then dissolving it at a temperature of 80–100°C and (d) sterilizing the mixture and then adding purified water to obtain a final liquid compound.[39]
Herbal hair dye
New method and formula for stable and safe hair dye has been formulated recently. The preparation method consists of preparing oil phase and water phase, adding oil phase into water phase, homogenizing, defoaming, cooling, adding alkalizer, and further homogenizing. The hair dye has the advantages of good safety, low allergy rate, mild nature, no damage to hair, no contamination to scalp and skin, good dyeing effect and good glossiness. The vegetable proteins in the dye have good effects for hair care.[40]
Herbal compositions with high contents of herb medicine extracts
Stable pharmaceutical compositions with high contents of herb medicine extracts are formulated with porous calcium silicate. The compositions containing herb medicine extracts mainly contain anthrones and porous calcium silicate and are adjusted to a relative humidity of 40%. Tablets contain licorice extract, sodium dioctyl sulfosuccinate, crystalline cellulose, croscarmellose sodium and magnesium stearate.[41]
Use of colloidal silicon dioxide in the enhancement of the drying of herbal preparations
Several problems arise during the bed drying of herbal extracts, such as bed instability, product accumulation, particle agglomeration, and bed collapse, mainly due to the complex composition. The addition of drying carriers, like colloidal silicon dioxide, to the extractive solution can minimize these unwanted effects. Higher concentration of the drying carrier significantly improves the bed drying performance in herbal extracts.[42]
CONCLUSIONS
Natural medicines are continuously gaining attention as the therapy for many of the ailments in the modern era. Hence, it becomes the prime responsibility of the herbal drug manufacturer to provide adequate stability for long-term storage and safety for consumption by the patients. As the phytoformulation is a mixture of more than one active ingredient, care should be taken to the determination of the stability profile for natural medicines. A stable formulation will gain confidence in the modern patient compliance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Goldberg B. Washington: Puyallup; 1994. Alternative medicine: The definitive guide future medicine; p. 257. [Google Scholar]
- 2.Majumdar S. Global herbal market where India actually stands. [cited in 2009]. Available from: http://www.pharmainfo.net/majumdarshiv/global-herbal-market-where-India-actually-stands .
- 3.Rangari VD. Pharmacognosy and phytochemistry. Career Pub. (2nd ed) 2008;1:78–100. [Google Scholar]
- 4.Willmar SG, Lang F, Vortrag BM. Entwurf für eine Leitlinie zur Stabilitätsprüfung von Phytopharmaka. Pharm Ind. 2002:20–23. [Google Scholar]
- 5.WHO Expert Committee on Specifications for Pharmaceutical Preparations. 34th report. Geneva: WHO Technical Report Series No. 863; 1996. World Health Organization; pp. 178–84. [PubMed] [Google Scholar]
- 6.Natural Health Product Directorate, Health Canada. Evidence for Quality of Finished Natural Health Products. 2007:19–22. [Google Scholar]
- 7.Rockville MD. FDA draft guidance for industry, stability testing of drug substances and drug products. Glossary FDA. 1998 [Google Scholar]
- 8.Pingale SS, Pokharkar RD, Pingale MS. Stability study of a herbal drug.Pharmacologyonline. University of Salerno. 2008:20–3. [Google Scholar]
- 9.Heigl D, Franz G. Stability testing on typical flavonoid containing herbal drugs. Pharmazie Govi-Verlag Pharmazeutischer Verlag GmbH. 2003;58:881–5. [PubMed] [Google Scholar]
- 10.Poetsch FA, Steinhoff B. Cantor Verlag., editor. Stability testing of herbal medicinal products: A report on problematic cases from practice with discussion of possible resolution approache. Pharmazeutische Industrie. 2006;68:476–83. [Google Scholar]
- 11.Shinde VM, Dhalwal K, Potdar M, Mahadik KR. Application of quality control principles to herbal drugs. Int J Phytomed. 2009;1:4–8. [Google Scholar]
- 12.Peter JH, Raman A. Laboratory Handbook for the Fractionation of Natural Extracts. Chapman and Hall, Thomson Science. 1988:17–8. [Google Scholar]
- 13.Natural Health Product Directorate, Health Canada. Evidence for quality of finished natural health products. 2007:22–33. [Google Scholar]
- 14.Mukherjee PK. Quality control of herbal drugs: An approach to evaluation of botanicals. Business Horizons. 2007 [Google Scholar]
- 15.Ahmad I, Aquil F, Owais M. Modern phytomedicine: Turning medicinal plants into drugs. WILEY-VCH Verlag GmbH and Co KGaA, Weinheim. 2006:29–36. [Google Scholar]
- 16.Thakur AK, Prasad NA, Laddha KS. Stability testing of herbal products. The Pharma Review. KONGPOSH Publications. 2008 [Google Scholar]
- 17.Musthaba SM, Ahmad S, Ahuja A, Ali J, Baboota S. Nano approaches to enhance pharmacokinetic and pharmacodynamic activity of plant origin drugs. Curr Nanosci. 2009;5:344–52. [Google Scholar]
- 18.Bosch WH, Hilborn MR, Hovey DC, Kline LJ, Lee RW, Pruitt JD, et al. Liquid dosage compositions of stable nanoparticulate drugs have the improved stability than conventional dosage forms. PCT Int Appl. 2004:68. [Google Scholar]
- 19.Anna RB, Bergonzi CM, Giovanni M, Franco FV. Development and stability of semisolid preparations based on a supercritical CO 2 Arnica extract. J Pharmaceut Biomed Anal. 2006;41:449–54. doi: 10.1016/j.jpba.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Salunkhe V, Bhise SB. Formulation and stability studies of chewable herbal tablets. Indian Pharma. 2009;8:53–8. [Google Scholar]
- 21.Xiangwu Y, Fan J, Yubin Z, Ting P, Kun Y, Wenchao Y. New chewable tablet formulation of mixture of Herba Artemisiae Scopariae, Gardenia jasminoides, and Flos Lonicerae. Zhuanli Shenqing Gongkai Shuomingshu. 2007:25. [Google Scholar]
- 22.Chaudhary M, Naithani V. Novel herbal topical formulations for treatment of acne and skin disorders. Indian Pat Appl Publ. 2009:28. [Google Scholar]
- 23.Ikeda Y, Motoune S, Ono M, Mohri Y. Stable liquid preparation/Stable liquid preparation coated with water-soluble cellulose derivative. US Pat App. 2006 [Google Scholar]
- 24.Beneke CE, Viljoen AM, Hamman JH. Polymeric plant-derived excipients in drug delivery. Molecules. 2009;14:2602–20. doi: 10.3390/molecules14072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkes, Richard S. Food compositions incorporating stearidonic acid. US Pat App Pub. 2009:48. [Google Scholar]
- 26.Wolf A, Pouny Y, Marton I, Dgany O, Altman A, Shoseyov O. Use of robust plant chaperonin-like Sp1 proteins to stabilize therapeutic proteins in pharmaceutical use. PCT Int App. 2007:155. [Google Scholar]
- 27.Diederichs J. Plant extract stabilized by polyvinylpyrrolidone and its therapeutic use. PCT Int App. 2006:13. [Google Scholar]
- 28.Jean D. Preparing stabilized suspensions of fresh plants for use as pharmaceuticals, cosmetics or food. Br UK Pat Appl. 1989 [Google Scholar]
- 29.Ludwikowska K, Radecki A, Piekos R. Characteristics of ethoxypolysiloxane oil emulsions.Akad Med Gdansk. Pol Przemysl Chem. 1969;48:216–20. [Google Scholar]
- 30.Victor CG, Miguel CG, Barbara GS, Martha M. Continuous multi-microencapsulation process for improving the stability and storage life of biologically active ingredients in foods, cosmetics and drugs. J Microencaps. 2005;22:913–9. [Google Scholar]
- 31.Stahl, Chris R, Fernandez, Aldo O, Woodin, Frederick W. Stabilized compositions containing an oxygen-labile active agent. US Pat Appl Publ. 2002:6. [Google Scholar]
- 32.Iwu M, Okunji C, Tchimene M, Anele N, Chah K, Osonwa U, et al. Stability of cough linctus (Streptol) formulated from named medicinal plant extracts. Chem Pharm Bull. 2009;57:229–32. doi: 10.1248/cpb.57.229. [DOI] [PubMed] [Google Scholar]
- 33.Miller TJ, Fatnon MJ, Webb SR. Transgenic plants producing soluble, stable immunoprophylactic and therapeutic compositions which released by disrupting plant cell wall. PCT Int App. 2004:102. [Google Scholar]
- 34.Shin HJ, Lee SC, Hahn YH, Ryou HW. Stable tablets containing volatile liquid drugs. Repub Korea. 1999 [Google Scholar]
- 35.Boyong L, Cheng XX. Stable pharmaceutical compositions without a stabilizer. US Pat App Pub. 2004:5. [Google Scholar]
- 36.Nagy KM, Balazs R, Marcisz J, Nagy KW, Tajthy J, Judit E, et al. Plant pigments as light stabilizers for photosensitive drugs. Ger Offen. 1992:10. [Google Scholar]
- 37.Sakurada S, Yoshino S. Storage stable powders containing oil compositions containing aqueous active substances. 2000:13. [Google Scholar]
- 38.Gamay A. Stabilized vitamin solutions: Use thereof; process for their production; and formulations comprising the same U.S. Pat. Appl. Publ. 2009:11. [Google Scholar]
- 39.Cho HJ, Won YH, Kim EJ, Choi MH, Jeon IS, Choi WS. Herbal liquid composition capable of effectively preventing precipitation with improved storage stability by comprising β-cyclodextrin, and method for preparing thereof. Repub. Korean Kongkae Taeho Kongbo. 2007 [Google Scholar]
- 40.Shen Z, Zhang L, Yang A. Hair dyes containing Chinese herb Impatiens with good safety and stability. Faming Zhuanli Shenqing Gongkai Shuomingshu. 2007:17. [Google Scholar]
- 41.Ishida H, Makino T, Hisaka Y. Stable pharmaceutical compositions with high contents of herb medicine extracts formulated with porous calcium silicate. dJpn. Kokai Tokkyo Koho. 1997:4. [Google Scholar]
- 42.Souza CR, Donida MW, Oliveira WP. The role of colloidal silicon dioxide in the enhancement of the drying of herbal preparations in suspended state. Chem Engg Commun. 2008;196:391–405. [Google Scholar]


