Abstract
This review discusses plant-derived compounds with estrogenic activity. The authors rightly emphasize the need for the intake of foods containing phytoestrogens in view of their positive effects on postmenopausal indications. This is particularly significant in the light of the current wave of enthusiasm for vegetarian food, in general, and phytoestrogens, in particular. Phytoestrogens are plant-derived hormone-like diphenolic compounds of dietary origin. These compounds are weakly estrogenic and could play a role in the prevention of other estrogen-related conditions, namely, cardiovascular diseases, menopausal symptoms, postmenopausal osteoporosis, neuroprotective effects, and hormone-dependent cancers (breast and endometrium cancer).
Keywords: Menopause, menopausal indications, phytoestrogen
INTRODUCTION
The estrogen, androgen, and progesterone receptors, members of the nuclear receptor (NRs) superfamily, act as nuclear transport proteins, cell cycle components, and transcription factors.[1] Most cellular actions of sex steroid hormones are mediated through binding to nuclear receptors that act as ligand-inducible transcription factors.[2] Estrogen plays an important role in the growth, differentiation, and function of many bodily targets, including the female and male reproductive systems. Estrogen also has a variety of pharmacologic functions, Estrogens (especially estradiol) bring out the feminine characteristics, control reproductive cycles and pregnancy, influence skin, immunity, maintenance of bone mass, cardiovascular protection, and brain protection.[3] Estrogen deficiency during menopause can lead to risk for many health problems, such as hot flushes, sleeping disorders, vaginal dryness, joint pain, mood swings, reduced bone density, cardiovascular disease, and so on.[4,5]
ESTROGENS
Estrogens suppress ovulation and with progesterogens form the basis of combined oral contraceptives and hormone replacement therapy (HRT). They are also used to supplement natural estrogen levels where these are insufficient as in some menstrual disorders and to suppress androgen formation, and thus tumor growth of cancers dependent on androgens (prostate cancers). Estrogens appear to offer a number of beneficial effects to women, including protection against osteoporosis and heart attacks. Some cancers (breast and uterine cancers) are dependent on a supply of estrogen for growth, especially during the early stage, so high estrogen levels are detrimental. A success in treating breast cancer has been the introduction of tamoxifen, which contains the stilbene skeleton seen in diethylstilbestrol and related estrogens but acts as an estrogen-receptor antagonist rather than as an agonist in breast tissue, and deprives the cells of estrogen. However, it is an agonist in bone and uterine tissue. Estrogen antagonist can also be used as a fertility drug, occupying estrogen receptors (ERs) and interfering with feedback mechanisms. Clomiphene and to a lesser extent tamoxifen are used in this way, but can lead to multiple pregnancies (Clomiphene leads to ova release by occupying estrogen receptors and interfering with feedback mechanisms and can lead to multiple pregnancies.).[6]
PHYTOESTROGENS
Phytoestrogen is a general definition that has been applied to any plant substance or metabolite that induces biological responses in vertebrates and can mimic or modulate the actions of endogenous estrogens usually by binding to ERs.[7] Sheep grazing on Australian pastures containing a particular type of clover (Trifolium species) rich in formononetin, which is converted to daidzein in the rumen during fermentation, developed a widespread infertility in the 1940s. This problem was traced to these nonsteroidal weak estrogens.[8] Flavonoids, including isoflavones, are natural components in our diet and, with the burgeoning interest in alternative medicine, are increasingly being ingested by the general population. These compounds have a wide range of hormonal and nonhormonal activities in animals or in vitro, and these suggest possible mechanisms for potential physiologic effects of diets rich in isoflavones in humans.[9,10] In addition, experimental and epidemiologic data are available to support the concept that isoflavone-rich diets exert physiologic effects in different parts of the body. The phytoestrogens are synthesized in plants from phenylpropanoids and simple phenols.[11] Molecular modeling studies show the 4-hydroxyl on the B ring of isoflavones to be the binding site for the ER.[12,13]
Why phytoestrogens?
They are nonsteroidal, naturally occurring phenolic compounds that can be divided into two groups: firstly, the flavonoids that are further subdivided into isoflavones, coumestans, and prenyl flavonoids; and secondly, the nonflavonoids, comprising the lignans. All are polyphenols that have a structural similarity to estradiol and possess estrogenic activity due to having a similar ring as that of estradiol and possessing two hydroxyl groups at positions that afford the correct distance between them to facilitate binding to the ER.[14] The isoflavone phytoestrogens share a common structure, with genistein having the important –OH groups at positions 7 and 40. Biochanin A has a methoxy group at position 40 and prunetin has a methoxy group at position 7, resulting in less estrogenic activity as the methoxy groups hinder binding to the ER. In quercetin, the “B” ring is attached to position 2 and there is an –OH group at position 3.[15]
Most of the phytoestrogens are significant contributors of nonsteroidal estrogens of dietary origin and are usually found as glycosides. When consumed by humans, the glycosides are probably hydrolyzed in part by gastric acid and extensively biotransformed in the intestine by the action of bacterial glucosidases, which release aglycones. Hydrolyzed glycosides can be absorbed from the intestinal lumen or further metabolized to many specific metabolites, including equol (the main endproduct of bacterial degradation of daidzein) and 4–ethylphenol (the main endproduct from genistein).[16–19] After consumption of foods containing isoflavones, genistein, daidzein, and their metabolites are found in the urine. Wide individual variations were seen in urinary isoflavonoid phytoestrogen excretion. In women, equol excretion was associated with higher intake of dietary fiber and carbohydrates.[20] Since the binding affinity of equol for the ER and its estrogen potency is greater than that of its precursor daidzein,[21] it might therefore be advantageous to improve the intestinal conversion of daidzein to equol. Thus, the makeup of the diet can alter the metabolism of isoflavones in the intestine. It has been found that under a high carbohydrate environment, fermentation is stimulated and this increases the rate of conversion of daidzein to equol.[22,23] This suggests that the overall composition of the diet may have to be taken into consideration in clinical studies investigating the potential efficacy of isoflavones, and the extent of intestinal bacterial metabolism will therefore determine the bioavailability of dietary estrogens and influence the potential for physiologic effect. Along with steroid hormone metabolism,[24,25] the liver probably plays a key role in the further metabolism of isoflavones by conjugating the aglycone with glucuronic acid.[26–28] The efficiency of conjugation of isoflavones is high and consequently the proportion of circulating free isoflavones is small [Figure 1].
Figure 1.
Chemical structures of some phytoestrogens
MECHANISM OF ACTION OF PHYTOESTROGENS
Phytoestrogens share the basic frame of other steroid hormones, although their potencies are estimated to be approximately 1000-fold weaker than that of 17-α estradiol.[29] To date, more than 300 plants have been found that possess compounds with estrogenic activity.[30,31] Several classes of phytoestrogens have been identified and studied: the hormone-like bis-phenolic phytoestrogens, the isoflavonoids daidzein and genistein, coumestrol, and the lignans and matairesinols are of great interest because of their respective estrogenic, antiestrogenic, anticarcinogenic, and antioxidant activities.[32,33]
Estrogen receptor-mediated mechanisms of action
The estrogenic activity of isoflavones was first described in the 1940s when the infertility of sheep in Western Australia was proposed to be caused by ingestion of clover pastures rich in the isoflavone precursors formononetin and biochanin A.[34] Using an ER-dependent transcriptional response assay, it was reported that, of the isoflavone precursors, genistein had the highest estrogenicity compared with daidzein, biochanin A, and formononetin.[35] It is this estrogenic activity that is thought to be mainly responsible for several of the beneficial effects of isoflavones in hormone-dependent processes, such as reducing bone loss associated with osteoporosis, improving menopausal symptoms, and lowering levels of plasma low-density lipoprotein (LDL) (which accumulates in blood vessel walls leading to arteriosclerosis).[36] There are two types of ER, ER-α and ER-β encoded by distinct genes: ER-α and ER-β appear to perform distinct biological roles, as judged from the different phenotypes of mice each devoid of one of these two genes and the fact that they differ in the C-terminal ligand-binding domain and the N-terminal transactivation domain.[37–39] ER-β is mainly expressed in the nonreproductive tissues, such as the vascular system and bone, and seems to mediate in part the impact of estrogens on the vasculature and the growth-promoting effects of estrogens on nongonadal tissues.[38] By contrast, ER-α is responsible for the classical hormonal effects, such as endometrial proliferation and mammary enlargement. Following ligand binding, the ER dimerizes before binding to target genes and modulating transcription. Activated ERs regulate the transcription of target genes either directly by binding to regulatory DNA elements or indirectly by modulating the expression of other transcription factors, such as AP-1 or NF-B. Thus, ERs can transactivate AP-1- or NF-κB-responsive genes.[37–39] The estrogenic potency of isoflavones is low compared with 17-β estradiol, as soy isoflavones have ∼1/3 and 1/1000 of the affinity of 17-β estradiol for ER-β and ER-α, respectively.[37,38] Since genistein possesses a much higher binding affinity for ER-β than for ER-α, isoflavones can be regarded as a type of natural “selective ER modulator” (SERM). However, recent X-ray crystallographic studies examining the interaction of estrogens, raloxifene and genistein with ER-β suggest that the orientation of raloxifene and genistein with ER-β is different from that of estradiol, in particular in the interaction with helix 12 of the receptor.[36] Isoflavones lack specific lipophilic regions, which undoubtedly affect their ER-β-binding ability and the subsequent initiation of cellular events.
Nonhormonal action of phytoestrogens
Several phytoestrogens have been reported to exert various non-ER-related effects in vitro. These effects include antiproliferative activity, inhibition of tyrosine kinase, protein kinase C, DNA topoisomerase II, antioxidant activity, inhibition of angiogenesis, and inhibition of prostaglandin synthase.[40–44] All of these actions require high concentrations, generally over 10 mM, and it is still questionable whether such concentrations are ever reached in vivo. In theory, these actions could play a role in cancer prevention, but they need to be confirmed in vivo before any conclusions on their biological relevance can be made.
PHYTOESTROGENS AND MENOPAUSAL INDICATIONS
What is menopause?
The term “natural menopause” defines the permanent cessation of menstruation resulting from the loss of ovarian activity. Menopause, the final menstrual period, can only be determined retrospectively, after at least 12 months of amenorrhea (WHO, 1996).[45]
Median age at the menopause is currently around 50 years in Western industrialized societies (in Britain it is 50.78 years, in the United States 49.8 years, and in White South Africans 48.7 years). It occurs earlier in Black women.[46] In addition to race, nutrition and smoking influence the age at menopause. It has been suggested that age at menopause may be a biological marker of aging, later menopausal age being associated with longevity.[47] Although “premature menopause” should be defined as occurring at an age less than two standard deviations below the median estimated for the referent population (WHO, 1996), in practice an age of 40 years is used as an arbitrary cutoff. The term “perimenopause” describes the phase immediately before and the first year after menopause. The term “menopausal transition” is best reserved for the period before the final menstrual period. Its average duration is 3.8 years.[48] The term “postmenopause” defines the phase after the final menstrual period (WHO, 1996). The term “premenopause” refers to the whole of the reproductive period prior to the menopause.
BIOLOGICAL BASIS OF MENOPAUSE
The biological bases of the menopause are changes that occur in the structure and function of the ovary. The number of ovarian follicles present in the ovary, and thus the number of ovarian granulosa cells available for hormone secretion, appears to be a critical determinant of age at menopause. The supply of oocytes is finite: about 7 million germ cells can be found in the ovaries of the human fetus at the fifth month of intrauterine life but these cells do not thereafter divide. The rate of follicle decline is approximately linear on a semi-logarithmic scale until an age of about 35–40 years.[49] It accelerates thereafter until after the menopause, when essentially no follicles remain.[50]
Follicle counts in the women with regular menses were 10-fold greater than in perimenopausal women. Follicles were virtually absent from the ovaries of postmenopausal women (PMW). These observations indicate that the size of the follicular reserve is the major determinant of the transition from regular menses to the perimenopause as well as to the menopause itself.
Richardson in 1993[51] hypothesized that the elevated follicle-stimulating hormone (FSH), seen in the last decade of menstrual life, stimulates a greater proportion of follicles to enter the growing phase and hence to be destined for atresia. However, little is known about the factors affecting the rate of atresia.
As women approach the menopause stage, menstrual cycles become irregular. The progressive shortening of the cycle is caused by shortening of the follicular rather than the luteal phase.[52] About 25% of women aged 40–45 years and 40% in the 45–50 age group have anovulatory cycles.
SIZE AND DISTRIBUTION OF THE POSTMENOPAUSAL POPULATION
In 1990, in the base population, there were approximately 467 million women in the world 50 years and older. This number is projected to increase to 1.2 billion by the year 2030. Figure 2 shows the numbers by region for 1990, 2000, 2010, 2020, and 2030. In 1990, the Established Market Economies (EME) had the largest single number, 128 million, of PMW, 27% of the world total. Sub-Saharan Africa (SSA), Latin America (LAC), and the Middle East (MEC) each had less than 7% of the world total. By 2030, China is expected to have the largest single number of PMW, 281 million, or 23% of the world total; SSA, MEC, and LAC are all expected to have around 100 million; the countries of formerly Socialist Europe (FSE) are expected to have the smallest share, 82 million or about 7%. All the regions of the developing world (SSA, India, China, Other Asia (OAI), LAC, and MEC) show dramatic increases in PMW, typically tripling or more from 1990 to 2030. The increases in the industrialized world (FSE, EME) are much smaller, increasing by about 50% in FSE, and by about 60% in EME.[53]
Figure 2.
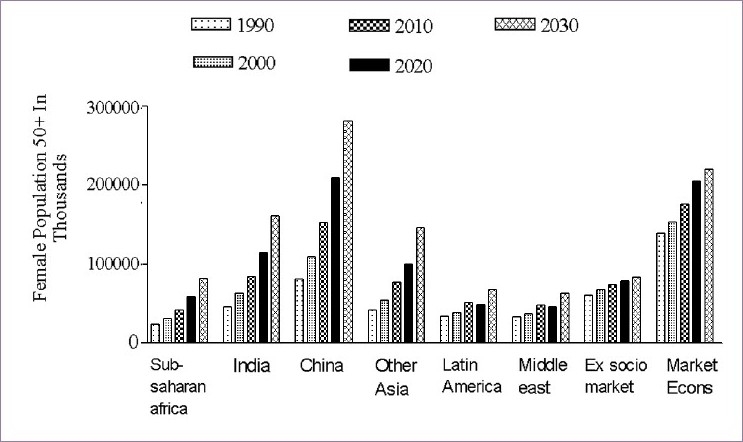
Population of postmenopausal women by region, 1990–2030
MORTALITY RISKS AROUND AND AFTER MENOPAUSE
Menopause is marked by a sharp change in hormonal balance, initial declines in estrogen giving rise to high levels of FSH and lutenizing hormone, and ultimately to reduced progesterone levels and permanent amenorrhea.[54] These sharp changes in hormonal balance over a period of 2 or 3 years may be accompanied by short-run or longer-run changes in mortality associated with menopause. Given the hormonal nature of the changes, the 2 main groups of diseases that might be affected by menopause are diseases of the circulatory system and cancers. Menopause might also affect women, perhaps through the erosion of their societal role in reproduction, or perhaps in some societies through their elevation to the role of mother-in-law. These social changes might also be associated with changes in health risks, for instance, through nutritional effects or through possible changes in exposure to injury, for example, from violence.
In general, that the female reproductive period is associated with reduced mortality, particularly from cardiovascular disease and injury, partially offsets, particularly in high mortality settings, by excess cancer mortality. After menopause, the female mortality advantage gradually erodes, the advantage in cardiovascular mortality eroding particularly rapidly. These patterns are consistent with the hypothesis that estrogen or progesterone reduces mortality risks, and that once the levels of these hormones fall after menopause, mortality risks rise again to levels similar to those of males. There is some corroborative evidence of an overall protective effect of the female reproductive period in clinical studies. Snowdon et al.[55] find that early age at natural menopause in a population of Seventh Day Adventist women with largely healthy lifestyles is associated with substantial excess mortality; the lowest mortality risks were experienced by those who reached menopause in the age range 50–54 years. On the other hand, breast cancer risks appear to be lower in women who reach menopause earlier.[56] This finding also is consistent with the patterns identified above, given the large cardiovascular advantage only partially offset by a smaller neoplasm disadvantage.
POSTMENOPAUSAL INDICATIONS
Complications of the menopause are the immediate symptoms of acute estrogen deficiency and the long-term problems of osteoporosis, coronary heart disease (CHD), and Alzheimer's disease. Acute menopausal symptoms are experienced by about two-thirds of women. Severity and duration are variable but in at least one-third of women who do experience these symptoms, they are severe enough to require medical help. As a rule, symptoms are more pronounced if a woman experiences an abrupt cessation of ovarian function, as after oophorectomy. Typical symptoms, which form the acute climacteric syndrome, can be grouped into vasomotor phenomena and psychosomatic symptoms. While the vasomotor symptoms are more uniform (although the perception of such symptoms by individual women may differ), psychosomatic symptoms and particularly their intensity are strongly determined by psychologic, social, and cultural characteristics of the woman. Genitourinary atrophy, caused by lack of estrogens, leads to symptoms, such as pruritus and dyspareunia. Urethritis, with dysuria, urgency incontinence, and urinary frequency are further results of thinning of the lining of the urethra and bladder. Besides these complications in the PMW, some common and serious diseases, such as breast cancer, endometrium cancer, atherosclerosis, congestive heart diseases, osteoporosis, neurodegeneration, and dementia (Alzhemier's disease).[57]
PHYTOESTROGENS IN POSTMENOPAUSAL INDICATIONS
Soy phytoestrogens are widely used as an alternative to hormone replacement therapy for the treatment of menopausal symptoms. In addition, the conclusion reached in 1999 by the US Food and Drug Administration, that foods containing soy protein included in a diet low in saturated fat and cholesterol may reduce the risk of CHD by lowering blood cholesterol levels, has also contributed to increased dietary soy consumption.
Estrogens prevent heart diseases, atherosclerosis, and vascular diseases, by the following mechanisms.[57] So it is expected that phytoestrogens probably act similarly. However, there is no direct evidence in this regard.
-
A favorable impact on serum lipids and lipoproteins
- Increase high-density lipoprotein cholesterol
- Decrease LDL cholesterol
- Decrease lipoprotein (a) (Lp(a))
Reduce oxidation of LDL cholesterol
-
Direct antiatherosclerotic effect in arteries
- Lower uptake of LDL cholesterol in blood vessels
- Prevent cholesterol deposition in vascular lesions
-
Direct vascular effects
- Reduce vascular tone
- Preserve endothelial function
- Increase production of nitric oxide from blood vessels
- Increase prostacyclin release
- Inhibit endothelial production of endothelin-1
- Inhibit endothelin-1-induced vasoconstriction
Direct inotropic action on the heart
-
Effects on hemostasis
- Decrease plasma fibrinogen
- Reduce plasminogen activator inhibitor
-
Improvement in peripheral glucose metabolism
- Decrease fasting blood glucose
- Decrease serum insulin levels
Antioxidant properties are one of the most important claims for food ingredients, dietary supplements, cosmetics, and anticancer natural products. Phytoestrogen rich plants have established antioxidant activity. Genistein and daidzein isolated from soybean seeds showed stronger antioxidant activity than their glycosides. Red clover extract, a rich source of isoflavones, showed stronger antioxidant activity than soy.[58] Antioxidant activity of phytoestrogens can restore the tissues from stress or oxidative damage.
Both ERs, α and β, have been shown to be involved in neuroprotective effects of estradiol[59–61] and to be expressed in the adult rat hippocampus.[62,63] Therefore, the neuroprotective effects of soy extract and genistein in the hippocampus in vivo may be mediated by ERs. Although soy isoflavones may activate both ER-α- and ER-β-mediated transcription, they are more potent activators of ER-β.[64–66] Therefore, it is possible that the neuroprotective effects of soy extract and genistein may be mainly mediated by the activation of ER-β, which is highly expressed in neurons and glial cells in the adult rat hippocampal formation. In addition, estrogenic compounds may have neuroprotective effects that are independent of the activation of ERs.[67] Indeed, genistein has ER-independent effects, affecting the activity of enzymes, such as protein tyrosine kinases, mammalian DNA topoisomerase I and II, and ribosomal S6 kinase.[68] By its action on tyrosine kinases, genistein may alter phosphorylation events associated with the activation of neurotransmitter receptors. In addition, genistein may directly interact with neurotransmitter receptors and, in consequence, alter neuronal function and neuronal response to injury. Finally, genistein and soy extracts have antioxidant properties,[69] which may contribute to their neuroprotective effects.
Phytoestrogen supplements are routinely advertised as an alternative to traditional hormone replacement therapy and as a way to alleviate both the physical and emotional effects of menopause. To date, there is almost no evidence to support these claims, and data from numerous animal studies suggest that soy may act to suppress sexual function and increase anxiety. The impact of soy-rich diets on human emotional and sexual health deserves to be explored in greater detail, and the numerous clinical trials are now underway to examine the effects of a soy-rich diet on bone density, cancer rates, and cardiovascular health.
CONCLUSIONS
Phytoestrogens are a large group of compounds with different chemical structures and multiple mechanisms of action. Phytoestrogens are ER lignads that act like estrogens in some tissues, such as bone and cardiovascular tissue. Many estrogenic herbs have been used in the treatment of menopausal symptoms for several thousands of years in Asian countries, and phytoestrogens represent a new category of therapeutic agents available for the prevention and treatment of diseases, such as osteoporosis and breast cancer.[70] Based on their chemical structure, phytohormones can be classified as isoflavonoids, flavonoids, anthraquinones, triterpenes, lignans, and saponins. These comprise the major phytoestrogens.
The health care needs of women should be assessed on the basis of a multidimensional evaluation of their mental, physical, and social well-being. Only a multidisciplinary approach allows an assessment of health care needs and the provision of effective care. This review concludes that the intake of phytoestrogens can take out the postmenopausal indications to a large extent.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Fu M, Wang C, Zhang X, Pestell R. Nuclear receptor modifications and endocrine cell proliferation. J Steroid Biochem Mol Biol. 2003;85:133–8. doi: 10.1016/s0960-0760(03)00223-1. [DOI] [PubMed] [Google Scholar]
- 2.Pestell A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 3.Ciocca DR, Roig LM. Estrogen receptors in human nontarget tissues: biological and clinical implications. Endocr Rev. 1995;16:33–62. doi: 10.1210/edrv-16-1-35. [DOI] [PubMed] [Google Scholar]
- 4.Eaker ED, Chesebro JH, Sacks FM, Wenger NK, Whisnant JP, Winston M. Cardiovascular disease in women. Circulation. 1993;88:1999–2009. doi: 10.1161/01.cir.88.4.1999. [DOI] [PubMed] [Google Scholar]
- 5.Rymer J, Wilson R, Ballard K. Making decisions about hormone replacement therapy. Br Med J. 2003;326:322–6. doi: 10.1136/bmj.326.7384.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapiero H, Ba GN, Tew KD. Estrogens and environmental estrogens. Biomed Pharmacother. 2002;56:36–44. doi: 10.1016/s0753-3322(01)00155-x. [DOI] [PubMed] [Google Scholar]
- 7.Food Standards Agency (2002) Report of the COT Working Group on Phytoestrogens. [last accessed on 2010 May 1]. Available from: http://www.food.gov.uk .
- 8.Shutt DA. The effects of plant oestrogens on animal reproduction. Endeavour. 1976;35:110–3. doi: 10.1016/0160-9327(76)90004-1. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy A, Faughnan M. Phytooestrogens through the life cycle. Proc Nutr Soc. 2000;59:489–96. doi: 10.1017/s0029665100000719. [DOI] [PubMed] [Google Scholar]
- 10.Bingham M, Gibson G, Gottstein N, Pascual-Teresa SD, Minihane AM, Rimbach G. Gut metabolism and cardioprotective effects of dietary isoflavones. Current Topics on Nutra Res. 2003;1:31–48. [Google Scholar]
- 11.Hahlbrock K. Flavonoids. In: Conn E, editor. The biochemistry of plants: a comprehensive treatise-secondary plant products. New York: Academic Press; 1981. pp. 425–56. [Google Scholar]
- 12.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–8. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 13.Pike AC, Brzozowski AM, Hubbard R, Bonn T, Thorsell AG, Engstrom O. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999;18:4608–18. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zand RS, Jenkins DJ, Diamandis EP. Steroid hormone activity of flavonoids and related compounds. Breast Cancer Res Treat. 2000;62:35–49. doi: 10.1023/a:1006422302173. [DOI] [PubMed] [Google Scholar]
- 15.Boker LK, Van der Schouw YT, De Kleijn MJ, Jacques PF, Grobbee DE, Peeters PH. Intake of dietary phytoestrogens by Dutch women. J Nutr. 2002;132:1319–28. doi: 10.1093/jn/132.6.1319. [DOI] [PubMed] [Google Scholar]
- 16.Kelly GE, Joannou GE, Nelson C, Reeder AY, Waring MA. The variable metabolic response to dietary isoflavones in humans. Proc Soc Exp Biol Med. 1995;208:40–3. doi: 10.3181/00379727-208-43829. [DOI] [PubMed] [Google Scholar]
- 17.Joannou GE, Kelly GE, Reeder AY, Waring MA, Nelson C. Aurinary profile study of dietary phytoestrogens: the identification and mode of metabolism of new isoflavonoids. J Steroid Biochem Mol Biol. 1995;54:167–84. doi: 10.1016/0960-0760(95)00131-i. [DOI] [PubMed] [Google Scholar]
- 18.Kelly GE, Nelson C, Waring MA, Joannou GE, Reeder AY. Metabolites of dietary (soya) isoflavones in human urine. Clin Chim Acta. 1993;223:9–22. doi: 10.1016/0009-8981(93)90058-c. [DOI] [PubMed] [Google Scholar]
- 19.Axelson M, Kirk DN, Cooley G, Farrant RD, Lawson AM, Setchell KD. The identification of the weak estrogen equol (7-hydroxy-3-4′-hydroxyphenyl chroman) in human urine. Biochem J. 1982;201:353–7. doi: 10.1042/bj2010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slavin JL, Karr SC, Hutchins AM, Lampe JW. Influence of soybean processing, habitual diet and soy dose on urinary isoflavonoid excretion. Am J Clin Nutr. 1998;68:1492S–5S. doi: 10.1093/ajcn/68.6.1492S. [DOI] [PubMed] [Google Scholar]
- 21.Nagel SC, vom Saal FS, Welshons WV. The effective free fraction of estradiol and xenoestrogens in human serum measured by whole cell uptake assays: physiology of delivery modifies estrogenic activity. Proc Soc Exp Biol Med. 1998;217:300–9. doi: 10.3181/00379727-217-44236. [DOI] [PubMed] [Google Scholar]
- 22.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–67S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 23.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med. 1998;217:335–9. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 24.Mackenzie PI, Rodbourne L, Stranks S. Steroid UDP glucuronosyl-transferases. Steroid Biochem Mol Biol. 1992;43:1099–105. doi: 10.1016/0960-0760(92)90338-J. [DOI] [PubMed] [Google Scholar]
- 25.Martucci CP, Fishman J. P450 enzymes of estrogen metabolism. Pharmacol Ther. 1993;57:237–57. doi: 10.1016/0163-7258(93)90057-k. [DOI] [PubMed] [Google Scholar]
- 26.Morton MS, Wilcox G, Wahlqvist ML, Griffiths K. Determination of lignans and isoflavonoids in human female plasma following dietary supplementation. J Endocrinol. 1994;142:251–9. doi: 10.1677/joe.0.1420251. [DOI] [PubMed] [Google Scholar]
- 27.Adlercreutz H, Fotsis T, Lampe J, Wahala K, Makela T, Brunow G. Quantitative determination of lignans and isoflavones in plasma of omnivorous and vegetarian women by isotope-dilution gas chromatography-mass spectrometry. Scand J Clin Lab Investig. 1993;53:5–18. doi: 10.3109/00365519309090693. [DOI] [PubMed] [Google Scholar]
- 28.Coward L, Kirk M, Albin N, Barnes S. Analysis of plasma isoflavones by reversed phase HPLC-multiple reaction ion monitoring mass spectrometry. Clin Chim Acta. 1996;247:121–42. doi: 10.1016/0009-8981(95)06242-4. [DOI] [PubMed] [Google Scholar]
- 29.Lindner HR. Occurrence of anabolic in plants and their importance. Environ Qual Saf Suppl. 1976;5:151–8. [PubMed] [Google Scholar]
- 30.Farnsworth NR, Bingel AS, Cordell GA, Crane FA, Fong HH. Potential value of plants as sources of new antifertility agents II. J Pharm Sci. 1975;64:717–54. doi: 10.1002/jps.2600640504. [DOI] [PubMed] [Google Scholar]
- 31.Price KR, Fenwich GR. Naturally occurring oestrogens in foods-a review. Food Addit Contam. 1985;2:73–106. doi: 10.1080/02652038509373531. [DOI] [PubMed] [Google Scholar]
- 32.Adlercreutz H, Mazur W. Phyto-estrogens and Western disease. Ann Med. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 33.Whitten PL, Naftolin F. Dietary estrogens-a biologically active background for estrogen action. In: Hochberg RB, Naftolin F, editors. New Biology of Steroid Hormones. New York: Ravel Press; 1991. pp. 155–67. [Google Scholar]
- 34.Bennets HW, Underwood EJ, Shier FL. A specific breeding problem of sheep in subterranean clover pastures in western Australia. Aust Vet J. 1946;22:2–12. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- 35.Miksicek RJ. Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J Steroid Biochem Mol Biol. 1994;49:153–60. doi: 10.1016/0960-0760(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 36.Kuiper GG. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 37.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Nat Acad Sci USA. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinol. 1998;139:4252–563. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 39.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758–67. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 40.Akiyama T, Ishida J, Nakagawa S. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 41.Degen GH. Interaction of phytoestrogens and other environmental estrogens with prostaglandin synthase in vitro. J Steroid Biochem. 1990;5:473–9. doi: 10.1016/0022-4731(90)90256-r. [DOI] [PubMed] [Google Scholar]
- 42.Fotsis T, Pepper M, Adlercreutz H. Genistein, a dietary derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci USA. 1993;90:2690–4. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markovits J, Junqua S, Goldwasser F. Genistein resistance in human leukaemic CCRF-CEM cells: Selection of a diploid cell line with reduced DNA topoisomerase II beta isoform A. Biochem Pharmacol. 1995;50:177–86. doi: 10.1016/0006-2952(95)00131-i. [DOI] [PubMed] [Google Scholar]
- 44.Arora A, Nair MG, Strasburg GM. Structure activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radical Biol Med. 1998;24:1355–63. doi: 10.1016/s0891-5849(97)00458-9. [DOI] [PubMed] [Google Scholar]
- 45.Geneva: World Health Organization; 1996. WHO (1996) Research on the Menopause in the 1990s, Technical Report Series 866. [PubMed] [Google Scholar]
- 46.Ginsburg J. What determines the age at the menopause? BMJ. 1991;302:1288–9. doi: 10.1136/bmj.302.6788.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snowden DA. Early natural menopause and the duration of postmenopausal life. J Am Geriatr Soc. 1990;38:402–8. doi: 10.1111/j.1532-5415.1990.tb03537.x. [DOI] [PubMed] [Google Scholar]
- 48.McKinley SM, Brambilla DJ, Posner JG. The normal menopause transition. American J Human Biol. 1992;4:37–46. doi: 10.1002/ajhb.1310040107. [DOI] [PubMed] [Google Scholar]
- 49.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81–7. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- 50.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–7. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 51.Richardson SJ. The biological basis of the menopause. Baillière's Clinical Endocrinology and Metabolism. 1993;7:1–16. doi: 10.1016/s0950-351x(05)80267-8. [DOI] [PubMed] [Google Scholar]
- 52.Lenton EA, Landgren BM, Sexton L, Harper R. Normal variations in the length of the follicular phase of the menstrual cycle: effect of chronological age. Br J Obstet Gynaecol. 1984;91:681–4. doi: 10.1111/j.1471-0528.1984.tb04830.x. [DOI] [PubMed] [Google Scholar]
- 53.Hill K. The demography of menopause. Maturitas. 1996;23:113–27. doi: 10.1016/0378-5122(95)00968-x. [DOI] [PubMed] [Google Scholar]
- 54.Jones GS. Hormonal changes in perimenopause. In: Eskin BA, editor. The Menopause: Comprehensive Management. New York: Masson Publishing USA Inc; 1980. [Google Scholar]
- 55.Snowdon DA, Kane RL, Beeson WL, Burke GL, Sprafka JM, Potter J, et al. Is early menopause a biologic marker of health and aging? Am J Pub Health. 1989;79:709–14. doi: 10.2105/ajph.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kvale G. Reproductive factors in breast cancer epidemiology. Acta Oncol. 1992;31:187–94. doi: 10.3109/02841869209088901. [DOI] [PubMed] [Google Scholar]
- 57.Prelevic GM, Jacobs HS. Menopause and post-menopause. Baillieres Clin Endocrinol Metab. 1997;3:46–54. doi: 10.1016/s0950-351x(97)80317-5. [DOI] [PubMed] [Google Scholar]
- 58.Kroyer GT. Red clover extract as antioxidant active and functional food ingredient. Innov Food Sci Emerg Technol. 2004;5:101–5. [Google Scholar]
- 59.Velísková J, Velísek L, Galanopoulou AS, Sperber EF. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia. 2000;41:S30–5. doi: 10.1111/j.1528-1157.2000.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 60.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, et al. Estrogen receptor α, not β, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;89:1952–7. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fitzpatrick JL, Mize AL, Wade CB, Harris JA, Shapiro RA, Dorsa DM. Estrogen-mediated neuro protection against β amyloid toxicity requires expression of estrogen receptor α or β and activation of the MAPK pathway. J Neurochem. 2002;82:674–82. doi: 10.1046/j.1471-4159.2002.01000.x. [DOI] [PubMed] [Google Scholar]
- 62.Cardona-Gómez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:751–60. doi: 10.1016/s0306-4522(00)00228-1. [DOI] [PubMed] [Google Scholar]
- 63.Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99:605–12. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- 64.Hall JM, Korach KS. Analysis of the molecular mechanisms of human estrogen receptors α and β reveals differential specificity in target promoter regulation by xenoestrogens. J Biol Chem. 2002;277:4455–61. doi: 10.1074/jbc.M200849200. [DOI] [PubMed] [Google Scholar]
- 65.Patisaul HB, Melby M, Whitten PL, Young LJ. Genistein affects ER β- but not ERα-dependent gene expression in the hypothalamus. Endocrinology. 2002;142:2189–97. doi: 10.1210/endo.143.6.8843. [DOI] [PubMed] [Google Scholar]
- 66.Ise R, Han D, Takahashi Y, Terasaka S, Inoue A, Tanji M, et al. Expression profiling of the estrogen responsive genes in response to phytoestrogens using a customized DNA microarray. FEBS Lett. 2005;579:1732–40. doi: 10.1016/j.febslet.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 67.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 68.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 69.Lund TD, Fleming DE, Dayton JR, Lephart ED, Salyer DL. Dietary soy phytoestrogens effects on retinal thickness in rats. Nutr Neurosci. 2003;6:47–51. doi: 10.1080/1028415021000056050. [DOI] [PubMed] [Google Scholar]
- 70.Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5:207–13. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]


