Abstract
Diabetes is a common metabolic disease characterized by abnormally high plasma glucose levels, leading to major complications, such as diabetic neuropathy, retinopathy, and cardiovascular diseases. One of the effective managements of diabetes mellitus, in particular, non–insulin-dependent diabetes mellitus (NIDDM) to decrease postprandial hyperglycemia, is to retard the absorption of glucose by inhibition of carbohydrate hydrolyzing enzymes, such as α-glucosidase and α-amylase, in the digestive organs. α-Glucosidase is the key enzyme catalyzing the final step in the digestive process of carbohydrates. Hence, α-glucosidase inhibitors can retard the liberation of d-glucose from dietary complex carbohydrates and delay glucose absorption, resulting in reduced postprandial plasma glucose levels and suppression of postprandial hyperglycemia. In recent years, many efforts have been made to identify effective α-glucosidase inhibitors from natural sources in order to develop a physiologic functional food or lead compounds for use against diabetes. Many α-glucosidase inhibitors that are phytoconstituents, such as flavonoids, alkaloids, terpenoids,anthocyanins, glycosides, phenolic compounds, and so on, have been isolated from plants. In the present review, we focus on the constituents isolated from different plants having α-glucosidase inhibitory potency along with IC50 values.
Keywords: Alkaloids, anthocyanins, diabetes, flavonoids, α-glucosidase, glycosides, terpenoids
INTRODUCTION
Diabetes mellitus is the most serious, chronic metabolic disorder and is characterized by high blood glucose levels. One therapeutic approach to treat diabetes is to retard the absorption of glucose via inhibition of enzymes, such as α-glucosidase, in the digestive organs.[1,2] α-Glucosidase (α-d-glucoside glucohydrolase) is an exo-type carbohydrase distributed widely in microorganisms, plants, and animal tissues,[3] which catalyzes the liberation of α-glucose from the non reducing end of the substrate. Inhibiting this enzyme slows the elevation of blood sugar following a carbohydrate meal.[4] It is a membrane bound enzyme present in the epithelium of the small intestine, which works to facilitate the absorption of glucose by the small intestine by catalyzing the hydrolytic cleavage of oligosaccharides into absorbable [Figure 1] monosaccharides.[5]
Figure 1.
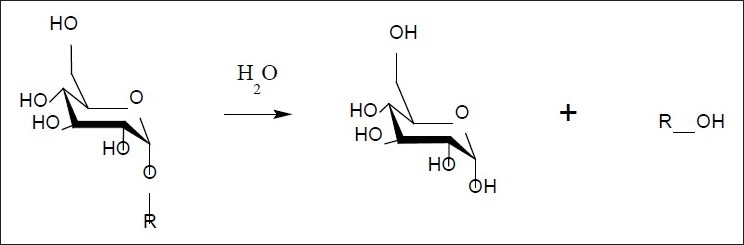
Conversion of oligosaccharide to glucose
By the inhibition of α-glucosidase in the intestine, the rate of hydrolytic cleavage of oligosaccharide is decreased and the process of carbohydrate digestion spreads to the lower part of small intestine. This spreading of digestion process delays the overall absorption rate of glucose into the blood. This has proved to be one of the best strategies to decrease the postprandial rise in blood glucose and in turn help avoiding the onset of late diabetic complications.[5]
There are reports of the presence of α-glucosidase inhibitors, such as acarbose[6,7] andvoglibose,[8] in microorganisms, and nojirimycin[9–11] and 1-deoxynojirimycin[11] in plants, as well as the effects of α-glucosidase inhibitor in wheat kernels on blood glucose levels after food uptake.[12]
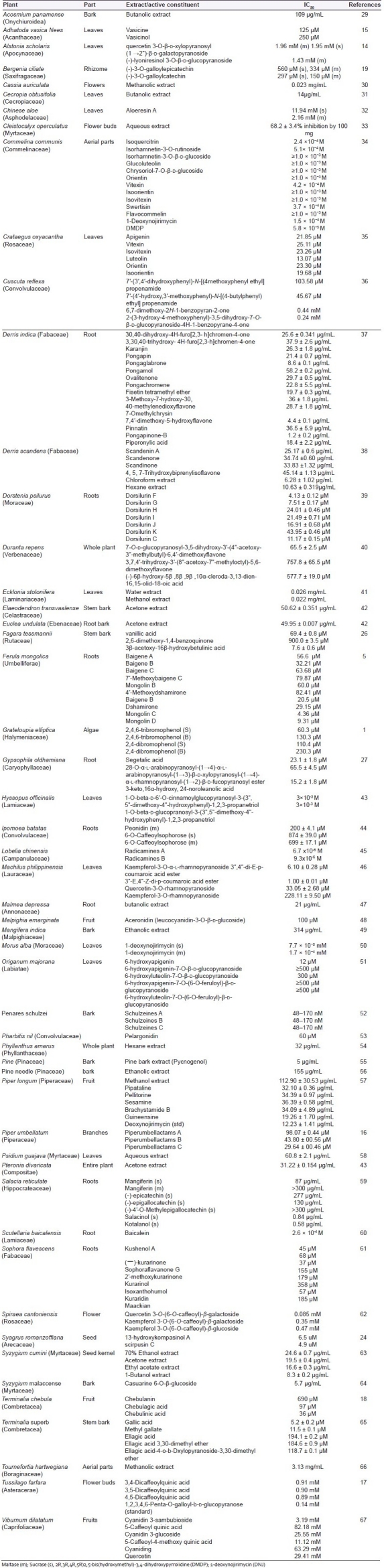
α-Glucosidase inhibitory potency of plant extracts and isolated compounds from different origins are discussed in Table 1.
Table 1.
Extracts/phytoconstituents having α-glucosidase inhibition activity
α-GLUCOSIDASE INHIBITION BY FLAVONOIDS
The inhibitory activity of six groups of flavonoids against α-glucosidase in yeast and rat small intestine was compared, and the chemical structures of flavonoids responsible for the inhibitory activity were evaluated. Yeast α-glucosidase was potently inhibited by the anthocyanidin, isoflavone, and flavonol groups with the IC50 values less than 15 μM. Rat's small intestinal α-glucosidase was weakly inhibited by many flavonoids, and slightly by the anthocyanidin and isoflavone groups.[13]
All the six groups of flavonoids with their chemical structures [Figure 2].
Figure 2.
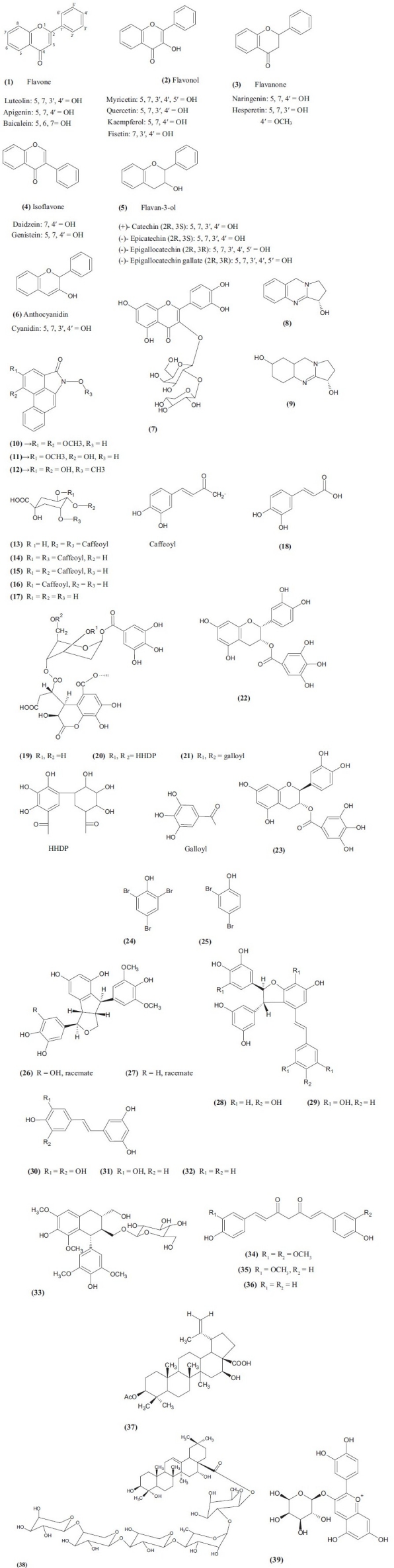
Some of the phytochemicals with their chemical structures
One flavonoid glycoside, quercetin 3-O-β-d-xylopyranosyl (1′′′→2″)-β-d-galactopyranoside(7) from Alstonia scholaris inhibited only maltase with IC50 values of 1.96 mM.[14]
ALKALOIDS
Methanolic extract of Adhatoda vasica Nees was tested in screening experiments for rat intestinal α-glucosidase. Vasicine (8) and Vasicinol (9), which were isolated by assay-guided fractionation of this extract, showed a high sucrase inhibitory activity with IC50 values 125 and 250 μM, respectively. Both of these compounds were shown to be reversible inhibitors of sucrase.[15]
Three alkaloids named piperumbellactam A (10), piperumbellactam B (11) and piperumbellactam C (12) were isolated from branches of Piper umbellatum and these compounds showed moderate α-glucosidase enzyme inhibition with IC50 values 98.07 ± 0.44, 43.80 ± 0.56, and 29.64 ± 0.46, respectively.[16]
The methanolic extract from flower buds of Tussilago farfara showed the highest maltase inhibitory activity, with maltose as a substrate. Enzyme assay-guided fractionation of this extract afforded 3,4-dicaffeoylquinic acid (13), 3,5-dicaffeoylquinic acid (14), and 4,5-dicaffeoylquinic acid (15). Comparison of the activities of these three compounds with others, such as chlorogenic acid (16), quinic acid (17), and caffeic acid (18), suggested that the number of caffeoyl groups attached to a quinic acid core were important for the potency.[17]
Phenolics
The dried Terminalia chebula (Combretaceae) fruits were extracted using 70% methanol at room temperature and its mammalian α-glucosidase inhibitory activity was investigated. It was found to have a potent rat intestinal maltase inhibitory activity. Three active ellagitannins, identified as chebulanin (19), chebulagic acid (20), and chebulinic acid (21) were isolated using bioassay-guided separation. All the three compounds were shown to possess potent intestinal maltase inhibitory activity with IC50 values of 690, 97, and 36 μM, respectively.[18]
The extraction and fractionation of 50% aqueous methanolic extracts of Bergenia cilata led to the isolation of two active compounds, namely, (-)-3-O-galloylepicatechin (22) and (-)-3-O-galloylcatechin (23). These isolated compounds demonstrated significant dose dependent enzyme inhibitory activities against rat intestinal α-glucosidase. The IC50 values of (-)-3-O-galloylepicatechin are 560 and 334 μM for sucrose and maltase, respectively, and that of (-)-3-O-galloylcatechin are 297 and 150 μM for sucrose and maltase, respectively.[19]
Miscellaneous
Two bromophenols, 2,4,6-tribromophenol (24) and 2,4-dibromophenol (25), were purified from Grateloupia elliptica. α-Glucosidase inhibitory activity of these compounds against ?-glucosidasesα-glucosidases was determined compared with acarbose and voglibose. The IC50 values of compounds (24) and (25) against Saccharomyces cerevisiae α-glucosidase were 60.3 and 110.4 μM, respectively, which were lower than the 130.3 and 230.3 μM that was presented against the Bacillus stearothermophilus α-glucosidase.[20] The α-glucosidase inhibitory activities of compound (24) against S. cerevisiae and B. stearothermophilus α-glucosidases were also higher than that for compound (25).[1] It is to be concluded that inhibitory potencies of bromophenol increased with increasing degree of bromo-substitution per benzene ring and with decreasing degree of methyl-substitution.[20] Voglibose and acarbose had high inhibitory effects on mammalian α-glucosidase, but no inhibitory activity against S. cerevisiae α-glucosidase.[21–23]
Bioassay-guided screening indicated that the defatted EtOH extract of the seeds of Syagrus romanzoffiana showed 55% inhibitory activity against α-glucosidase at a concentration of 10 μg/mL. Further fractionation indicated the active ingredients to be concentrated in the BuOH soluble fraction, having 73% inhibition at 10 μg/mL level. This fraction was further separated over Sephadex LH-20 and low pressure RP-18 columns that eventually yielded eight active compounds Of these, seven are stilbenoids, and two of them, 13-hydroxykompasinol A (26) and scirpusin C (27), possess potent inhibitory activity against α- glucosidase type IV from B. stearothermophilus with the IC50 value of 6.5 and 4.9 μM, respectively. The IC50 values of other less potent α-glucosidase inhibitors from this plant are kompasinol A (28) (IC50 = 11.2), scirpusin A (29) (IC50 = 8.3), pentahydroxystilbene (30) (IC50 = 19.2), Piceatannol (31) (IC50 = 23.2), and resveratrol (32) (IC50 = 23.9).[24]
One lignan glucoside, (–)-lyoniresinol 3a-O-b-d-glucopyranoside (33), from Alstonia scholaris exhibited an inhibitory activity against both sucrase and maltase with IC50 values of 1.95 and 1.43 mM, respectively.[14]
Curcuminoids
Natural curcumin (34), demethoxycurcumin (35) and bisdemethoxycurcumin (36) isolated from Curcuma longa (turmeric) were evaluated in vitro for the α-glucosidase inhibitory activity via UV and circular dichroism spectroscopy. The results indicated that natural curcuminoid compound 36 showed a remarkable inhibitory effect with IC50 of 23.0 μM.[25]
Terpinoids
3b-Acetoxy-16b-hydroxybetulinic acid (37) was isolated from Fagara tessmannii, and it was found to be a potent α-glucosidase inhibitor with IC50 value 7.6 ± 0.6.[26]
A new triterpenoid saponin Segetalic acid 28-O-α-l-arabinopyranosyl-(1→4)-α-l-arabinopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→2)-β-d-fucopyranosyl ester (38) has been isolated and elucidated from the roots of Gypsophila oldhamiana and has been evaluated for its α-glucosidase inhibition activity with the IC50 values of about 23.1 ± 1.8 μM.[27]
Anthocyanins
Cyanidin-3-galactoside (39), a natural anthocyanin, was also investigated for its α-glucosidase inhibitory activity. The IC50 value of cyanidin-3-galactoside was 0.50 ± 0.05 mM against intestinal sucrase. A low dose of cyanidin-3-galactoside showed a synergistic inhibition on intestinal α-glucosidase (maltase and sucrase) when combined with acarbose.[28]
Maltase (m); Sucrase (s), 2R,3R,4R,5R)2,5-bis(hydroxymethyl)-3,4-dihydroxypyrrolidine (DMDP); 1-deoxynojirimycin (DNJ)
DISCUSSION
Diabetes is one of the world's greatest health problems, affecting about 171 million people and most of these will be dominated by those suffering from type 2 diabetes.[68] This increasing trend in type 2 diabetes mellitus has become a serious medical concern worldwide, which accounts for 9% of deaths that prompts every effort in exploring for new therapeutic agents to stem its progress. Although the drug treatment for type 2 diabetes mellitus has been improved to some extent during the last decade, drug resistance is still a big concern that needs to be dealt with effective approaches. One of the strategies to monitor blood glucose for type II diabetes mellitus is to either inhibit or reduce the production of glucose from the small intestine. α-Glucosidase inhibitors interfere with the digestion of carbohydrates, achieving better glycemic control. Thus, natural products of great structural diversity are still a good source for searching for such inhibitors, thereby motivating to explore biologically active compounds from the highly diverse plants.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kim KY, Nam KA, Kurihara H, Kim SM. Potent α-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochem. 2008;69:2820–5. doi: 10.1016/j.phytochem.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years. Diabetes Care. 1999;22:960–4. doi: 10.2337/diacare.22.6.960. [DOI] [PubMed] [Google Scholar]
- 3.Kimura K, Lee JH, Lee IS, Lee HS, Park KH, Chiba S. Two potent competitive inhibitors discriminating alpha-glucosidase family I from family II. Carbohydr Res. 2004;339:1035–40. doi: 10.1016/j.carres.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 4.Lebovitz H E. α-Glucosidase inhibitors. Endocrinol Metab Clin North. 1997;26:539–551. doi: 10.1016/s0889-8529(05)70266-8. [DOI] [PubMed] [Google Scholar]
- 5.Irfan Baig. Phytochemical studies on Ferula mongolica and other mongolian medicinal plants. PhD Thesis, International Centre for Chemical Sciences, University of Karachi. 2002 [Google Scholar]
- 6.Truscheit E, Frommer W, Junge B, Muller L, Schmidit DD, Wingender W. Chemistry and biochemistry of microbial alpha-glucosidase inhibitors. Agew Chem Int Ed Engl. 1981;20:744–61. [Google Scholar]
- 7.Wehmeier UF, Piepersberg W. Biotechnology and molecular biology of the alpha-glucosidase inhibitor acarbose. Microbiol Biotechnol. 2004;63:613–25. doi: 10.1007/s00253-003-1477-2. [DOI] [PubMed] [Google Scholar]
- 8.Luo H, Imoto T, Hiji Y. Inhibitory effect of voglibose and gymnemic acid on maltose absorption in vivo. World J Gastroentero. 2001;7:270–4. doi: 10.3748/wjg.v7.i2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inouye S, Tsuruoka T, Ito T, Niida T. Structure and synthesis of nojirimycin. Tetrahedron. 1968;23:2125–44. doi: 10.1016/0040-4020(68)88115-3. [DOI] [PubMed] [Google Scholar]
- 10.Reese ET, Parrish FW. Nojirimycin and d-glucose-1, 5-lactone as inhibitors of carbohydrases. Carbohydr Res. 1971;18:381–8. [Google Scholar]
- 11.Asano N, Tomioka E, Kizu H, Matsui K. Sugars with nitrogen in the ring isolated from the leaves of Morus bombycis. Carbohydr Res. 1994;253:235–45. doi: 10.1016/0008-6215(94)80068-5. [DOI] [PubMed] [Google Scholar]
- 12.Maeda K, Kakabayashi S, Matsubara H. Complete amino acid sequence of an alpha-amylase inhibitor in wheat kernel (0.19-inhibitor) Biochim Biophys Acta. 1985;828:213–2. doi: 10.1016/0167-4838(85)90299-7. [DOI] [PubMed] [Google Scholar]
- 13.Tadera K, Minami Y, Takamastu K, Matsuoka T. Inhibition of α-Glucosidase and α-Amylase by Flavonoids. J Nutr Sci Vitaminol. 2006;52:149–53. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- 14.Anurakkun NJ, Bhandari MR, Kawabata J. α-Glucosidase inhibitors from Devil tree (Alstonia scholaris) Food Chem. 2007;103:1319–23. [Google Scholar]
- 15.Gao H, Huang YN, Gao B, Li P, Inagaki C, Kawabata J. Inhibitory effect on α-glucosidase by Adhatoda vasica Nees. Food Chem. 2008;108:965–72. doi: 10.1016/j.foodchem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Tabopda TK, Ngoupayo J, Liu J, Mitaine-Offer AC, Tanoli SA, Khan SN, et al. Bioactive aristolactams from Piper umbellatum. Phytochem. 2008;69:1726–31. doi: 10.1016/j.phytochem.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Gao H, Huang YN, Gao B, Xu PY, Inagaki C, Kawabata J. α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem. 2008;106:1195–201. [Google Scholar]
- 18.Gao H, Huang YN, Xu PY, Kawabata J. Inhibitory effect on α-glucosidase by the fruits of Terminalia chebula Retz. Food Chem. 2007;105:628–34. [Google Scholar]
- 19.Bhandari MR, Anurakkun NJ, Hong G, Kawabata J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.) Food Chem. 2008;106:247–52. [Google Scholar]
- 20.Kurihara H, Mitani T, Kawabata J, Takahashi K. Inhibitory potencies of bromophenols from Rhodomelaceae algae against α-glucosidase activity. Fish Sci. 1999;65:300–3. [Google Scholar]
- 21.Haslam E. Polyphenol-protein interactions. J Chem Ecol. 1974;139:285–8. doi: 10.1042/bj1390285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cogoli A, Semenza G. 18 A probable oxocarbonium ion in the reaction mechanism of small intestinal sucrase and isomaltase. J Biol Chem. 1975;250:7802–9. [PubMed] [Google Scholar]
- 23.Stern JL, Hagerman AE, Steinberg PD, Mason PK. Phlorotannins-protein interactions. J Chem Ecol. 1996;22:1877–99. doi: 10.1007/BF02028510. [DOI] [PubMed] [Google Scholar]
- 24.Lam SH, Chen JM, Kang CJ, Chen CH, Lee SS. α-Glucosidase inhibitors from the seeds of Syagrus romanzoffiana. Phytochem. 2008;69:1173–8. doi: 10.1016/j.phytochem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Du ZY, Liu RR, Shao WY, Mao XP, Ma L, Gu LQ, et al. α-Glucosidase inhibition of natural curcuminoids and curcumin analogs. Eur E Med Chem. 2006;14:213–8. doi: 10.1016/j.ejmech.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Mbaze LM, Poumale HM, Wansi JD, Lado JA, Khan SN, Iqbal MC, et al. α-Glucosidase inhibitory pentacyclic triterpenes from the stem bark of Fagara tessmannii (Rutaceae) Phytochem. 2007;68:591–5. doi: 10.1016/j.phytochem.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Luo JG, Ma L, Kong LY. New triterpenoid saponins with strong α- glucosidase inhibitory activity from the roots of Gypsophila oldhamiana. Bioorg Med Chem. 2008;16:2912–20. doi: 10.1016/j.bmc.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 28.Adisakwattana S, Charoenlertkul P, Yibchok-Anun S. α-Glucosidase inhibitory activity of cyanidin-3-galactoside and synergistic effect with acarbose. J Enzym Inhib Med Chem. 2009;24:65–9. doi: 10.1080/14756360801906947. [DOI] [PubMed] [Google Scholar]
- 29.Cetto AA, Jim´enez JB, V´azquez RC. Alfa-glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J Ethnopharmacol. 2008;116:27–32. doi: 10.1016/j.jep.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 30.Abesundara KJ, Matsui T, Matsumoto K. Alpha-Glucosidase inhibitory activity of some Sri Lanka plant extracts, one of which, Cassia auriculata, exerts a strong antihyperglycemic effect in rats comparable to the therapeutic drug acarbose. J Agric Food Chem. 2004;52:2541–5. doi: 10.1021/jf035330s. [DOI] [PubMed] [Google Scholar]
- 31.Cetto AA, Jim´enez JB, V´azquez RC. Alfa-glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J Ethnopharmacol. 2008;116:27–32. doi: 10.1016/j.jep.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Anurakkun NJ, Bhandari MR, Hong G, Kawabata J. α-Glucosidase inhibitor from Chinese aloes. Fitoterapia. 2008;79:456–7. doi: 10.1016/j.fitote.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Mai TT, Chuyen NV. Anti-Hyperglycemic Activity of an Aqueous Extract from Flower Buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Biosci Biotechnol Biochem. 2007;71:69–76. doi: 10.1271/bbb.60373. [DOI] [PubMed] [Google Scholar]
- 34.Shibano M, Kakutani K, Taniguchi M, Yasuda M, Baba K. Antioxidant constituents in the dayflower (Commelina communis L.) and their a-glucosidase-inhibitory activity. J Nat Med. 2008;62:349–53. doi: 10.1007/s11418-008-0244-1. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Song F, Xing J, Tsao R, Liu Z, Liu S. Screening and Structural Characterization of α-Glucosidase Inhibitors from Hawthorn Leaf Flavonoids Extract by Ultrafiltration LC-DAD-MSn and SORI-CID FTICR MS. J Am Soc Mass Spectrom. 2009;20:1496–503. doi: 10.1016/j.jasms.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Anis E, Anis I, Ahmed S, Mustafa G, Malik A, Afja N, et al. α-Glucosidase Inhibitory Constituents from Cuscuta reflexa. Chem Pharm Bull. 2002;50:112–4. doi: 10.1248/cpb.50.112. [DOI] [PubMed] [Google Scholar]
- 37.Rao RR, Tiwari AK, Reddy PP, Babu SK, Ali AZ, Madhusudana K, et al. New furanoflavanoids, intestinal a-glucosidase inhibitory and free-radical (DPPH) scavenging, activity from antihyperglycemic root extract of Derris indica (Lam.) Bioorg Med Chem. 2009;17:5170–5. doi: 10.1016/j.bmc.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 38.Rao RV, Dasari KR, Rao MJ. Isolation, characterization and chemobiological quantification of α-glucosidase enzyme inhibitory and free radical scavenging constituents from Derris scandens Benth. J Chromatogr B. 2007;855:166–72. doi: 10.1016/j.jchromb.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 39.Tabopda TK, Ngoupayo J, Awoussong PK, Mitaine-Offer AC, Ali MS, Ngadjui BT, et al. Triprenylated Flavonoids from Dorstenia psilurus and Their α-Glucosidase Inhibition Properties. J Nat Prod. 2008;71:2068–72. doi: 10.1021/np800509u. [DOI] [PubMed] [Google Scholar]
- 40.Iqbal K, Malik A, Mukhtar N, Anis I, Khan SN, Choudhary MI. α-Glucosidase Inhibitory Constituents from Duranta repens. Chem Pharm Bull. 2004;52:785–9. doi: 10.1248/cpb.52.785. [DOI] [PubMed] [Google Scholar]
- 41.Iwai K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in Genetically Diabetic KK-Ay Mice. Plant Foods Hum Nutr. 2008;63:163–9. doi: 10.1007/s11130-008-0098-4. [DOI] [PubMed] [Google Scholar]
- 42.Deutschländera MS, van de Venter M, Roux S, Louw J, Lall N. Hypoglycaemic activity of four plant extracts traditionally used in South Africa for diabetes. J Ethnopharmacol. 2009;124:619–24. doi: 10.1016/j.jep.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 43.Miyazaki H, Matsuura H, Yanagiya C, Mizutani J, Tsuji M, Ishihara C. Inhibitory effects of hyssop (Hyssopus officinalis) extracts on intestinal alpha-glucosidase activity and postprandial hyperglycemia. J Nutr Sci Vitaminol (Tokyo) 2003;49:346–9. doi: 10.3177/jnsv.49.346. [DOI] [PubMed] [Google Scholar]
- 44.Matsui T, Ebuchi S, Kobayashi M, Fukui K, Sugita K, Terahara N, et al. Anti-hyperglycemic Effect of Diacylated Anthocyanin Derived from Ipomoea batatas Cultivar Ayamurasaki Can Be Achieved through the α-Glucosidase Inhibitory Action. J Agric Food Chem. 2002;50:7244–8. doi: 10.1021/jf025913m. [DOI] [PubMed] [Google Scholar]
- 45.Shibano M, Tsukamoto D, Masuda A, Tanaka Y, Kusano G. Two New Pyrrolidine Alkaloids, Radicamines A and B, as Inhibitors of α-Glucosidase from Lobelia chinensis LOUR. Chem Pharm Bull. 2001;49:1362–5. doi: 10.1248/cpb.49.1362. [DOI] [PubMed] [Google Scholar]
- 46.Lee SS, Lin HC, Chen CK. Acylated flavonol monorhamnosides, α-glucosidase inhibitors, from Machilus philippinensis. Phytochem. 2008;69:2347–53. doi: 10.1016/j.phytochem.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Cetto AA, Jim´enez JB, V´azquez RC. Alfa-glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J Ethnopharmacol. 2008;116:27–32. doi: 10.1016/j.jep.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 48.Kawaguchi M, Tanabe H, Nagamine K. Isolation and Characterization of a Novel Flavonoids Possessing a 4,2"-Glycosidic Linkage from Green Mature Acerola (Malpighia emarginata DC.) Fruit. Biosci Biotechnol Biochem. 2007;71:1130–5. doi: 10.1271/bbb.60513. [DOI] [PubMed] [Google Scholar]
- 49.Prashanth D, Amit A, Samiulla DS, Asha MK, Padmaja R. Glucosidase inhibitory activity of Mangifera indica bark. Fitoterapia. 2001;72:686–8. doi: 10.1016/s0367-326x(01)00293-3. [DOI] [PubMed] [Google Scholar]
- 50.Hansawasdi C, Kawabata J. α-Glucosidase inhibitory effect of mulberry (Morus alba) leaves on Caco-2. Fitoterapia. 2006;77:568–73. doi: 10.1016/j.fitote.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Kawabata J, Mizuhata K, Sato E, Nishioka T, Aoyama Y, Kasai T. 6-Hydroxyflavanoids as α-Glucosidase Inhibitors from Marjoram (Origanum majorana) Leaves. Biosci Biotechnol Biochem. 2003;67:445–7. doi: 10.1271/bbb.67.445. [DOI] [PubMed] [Google Scholar]
- 52.Takada K, Uehara T, Nakao Y, Matsunaga S, van Soest RW, Fusetani N. Schulzeines A-C, New α-Glucosidase Inhibitors from the Marine Sponge Penares schulzei. J Am Chem Soc. 2004;126:187–93. doi: 10.1021/ja037368r. [DOI] [PubMed] [Google Scholar]
- 53.Matsui T, Ueda T, Oki T, Sugita K, Terahara N, Matsumoto K. α-Glucosidase Inhibitory Action of Natural Acylated Anthocyanins. 2. α-Glucosidase Inhibition by Isolated Acylated Anthocyanins. J Agric Food Chem. 2001;490:1952–6. doi: 10.1021/jf0012502. [DOI] [PubMed] [Google Scholar]
- 54.Ali H, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006;107:449–55. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Schafer A, Hogger P. Oligomeric procyanidins of French maritime pine bark extract (Pycnogenol1) effectively inhibit a-glucosidase. Diabetes Res Clin Pract. 2007;77:41–6. doi: 10.1016/j.diabres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Kim YM, Jeong YK, Wang MH, Lee WY, Rhee HI. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition. 2005;21:756–61. doi: 10.1016/j.nut.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Pullela SV, Tiwari AK, Vanka UM, Vummenthula A, Tatipaka HB, Dasari KR, et al. HPLC assisted chemobiological standardization of α-glucosidase-I enzyme inhibitory constituents from Piper longum. Linn. An Indian medicinal plant. J Ethnopharmacol. 2006;108:445–9. doi: 10.1016/j.jep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Wang B, Liu HC, Hong JR, Li HG, Huang CY. Effect of Psidium guajava leaf extract on alpha-glucosidase activity in small intestine of diabetic mouse. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38:298–301. [PubMed] [Google Scholar]
- 59.Yoshikawa M, Nishida N, Shimoda H, Takada M, Kawahara Y, Matsuda H. Polyphenol Constituents from Salacia Species: Quantitative Analysis of Mangiferin with α-Glucosidase and Aldose Reductase Inhibitory Activities. Yakugaku Zasshi. 2001;121:371–8. doi: 10.1248/yakushi.121.371. [DOI] [PubMed] [Google Scholar]
- 60.Nishioka T, Kawabata J, Aoyama Y. Baicalein, an α-Glucosidase Inhibitor from Scutellaria baicalensis. J Nat Prod. 1998;61:1413–5. doi: 10.1021/np980163p. [DOI] [PubMed] [Google Scholar]
- 61.Kim JH, Ryu YB, Kang NS, Lee BW, Heo JS, Jeong IY, et al. Glycosidase Inhibitory Flavonoids from Sophora flavescens. Biol Pharm Bull. 2006;29:302–5. doi: 10.1248/bpb.29.302. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida K, Hishida A, Iida O, Hosokawa K, Kawabata J. Flavonol Caffeoylglycosides as α-Glucosidase Inhibitors from Spiraea cantoniensis Flower. J Agric Food Chem. 2008;56:4367–71. doi: 10.1021/jf8007579. [DOI] [PubMed] [Google Scholar]
- 63.Shinde J, Taldone T, Barletta M, Kunaparaju N, Hu B, Kumar S, et al. α-Glucosidase inhibitory activity of Syzygium cumini (Linn.) Skeels seed kernel in vitro and in Goto-Kakizaki (GK) rats. Carbohydr Res. 2008;343:1278–81. doi: 10.1016/j.carres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Jung M, Park M, Lee HC, Kang YH, Kang ES, Kim SK. Antidiabetic agents from medicinal plants. Curr Med Chem. 2006;13:1203–18. doi: 10.2174/092986706776360860. [DOI] [PubMed] [Google Scholar]
- 65.Wansi JD, Lallemand MC, Chiozem DD, Toze FA, Mbaze LM, Naharkhan S. α-Glucosidase inhibitory constituents from stem bark of Terminalia superba (Combretaceae) Phytochem. 2007;68:2096–100. doi: 10.1016/j.phytochem.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 66.Ortiz-Andrade RR, García-Jiménez S, Castillo-España P, Ramírez-Avila G, Villalobos-Molina R, Estrada-Soto S. α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana : an anti-hyperglycmic agent. J Ethnopharmacol. 2007;109:48–53. doi: 10.1016/j.jep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 67.Iwai K, Kim MY, Onodera A, Matsue H. α-Glucosidase Inhibitory and Antihyperglycemic Effects of Polyphenols in the Fruit of Viburnum dilatatum Thunb. J Agric Food Chem. 2006;54:4588. doi: 10.1021/jf0606353. [DOI] [PubMed] [Google Scholar]
- 68.Gershell L. Type 2 diabetes market. Nat Rev Drug Discov. 2005;4:367–68. doi: 10.1038/nrd1723. [DOI] [PubMed] [Google Scholar]


