Abstract
Ficus hispida (FH) Linn. is a moderate sized tree found throughout the year and is grown wild or cultivated for its edible fruits and folklore value. Traditionally, different parts of the plant have been used in the treatment of ulcers, psoriasis, anemia, piles jaundice, vitiligo, hemorrhage, diabetes, convulsion, hepatitis, dysentery, biliousness, and as lactagogue and purgative. FH contains wide varieties of bioactives from different phytochemical groups like alkaloids, carbohydrates, proteins and amino acids, sterols, phenols, flavonoids, gums and mucilage, glycosides, saponins, and terpenes. Various scientific works have been published to establish the scientific basis of traditional medicinal values attributed to FH. Furthermore, newer pharmacological activities like antineoplastic, cardioprotective, neuroprotective and anti-inflammatory effects were also reported recently. Till now, no work has been published to elaborate the pharmacognostic features of FH Linn. The present review is, therefore, an effort to give a detailed account on its pharmacognosy and phytochemistry, and an extensive survey on its pharmacological activities. Moreover, we are trying to establish the mechanism of action behind its earlier reported pharmacology. The review also looks at the future formulation based delivery approaches of its lipophilic bioactives, which is done to enhance its dissolution so as to increase its bioavailability, and thus the associated pharmacological action.
Keywords: Bioavailability, ethnomedicinal properties, extraction method, Ficus hispida, pharmacognosy
INTRODUCTION
The history of herbal medicine is as old as human civilization. India has an ancient heritage of traditional system of medicine including Ayurveda, Siddha, Unani and Homoeopathy. The Ayurvedic medicine system and Indian Materia Medica provide a great deal of information on folklore practices and traditional aspects of therapeutically important herbs. With the emerging worldwide interest in adopting and studying traditional system of medicine and exploiting their potential based on different health care systems, the evaluation of rich heritage of therapeutically active herbs is primarily carried out on the basis of morphological, phytochemical, pharmacological and various instrumental techniques such as chromatography, etc. The use of foods in curing various ailments and improving health is nearly as old as humanity. Among such foods, none may be older than the fig, recent investigations of which have indicated that it has been cultivated for over 11,000 years.[1] The genus Ficus represents an important group of trees, not only for their immense value but also for their growth habits and religious value. The genus Ficus is an exceptionally large pantropical genus with over 700 species and belongs to the family moraceae. Among the species of genus Ficus, Ficus hispida Linn. (FH) is a valuable plant due to its various pharmacological activities. It is a rough leaved fig commonly known as peyatti (Tamil), dumoor (Bengali) and gobla (Hindi). It is a shrub or moderate sized tree, found throughout the year, growing in evergreen forest, moist localities, banks of stream, deciduous forests, to an elevation of 1800 m above sea level, often cultivated in villages for shade and its edible fruits in India, Sri Lanka, Myanmar, southern region of the Republic of China, New Guinea, Australia and Andaman island.[2] Almost all parts of this plant are used as a folklore remedy for the treatment of various ailments by the Indian traditional healers, but the leaves are of particular interest from a medicinal point of view[3] as an antidiarrheal,[4] hepatoprotective,[5] anti-inflammatory,[6] antitussive, antipyretic, astringent, vulnerary, hemostatic and anti-ulcer drug, among other parts.[3,7] In spite of its significant traditional medicinal value, this plant has not been explored extensively till now with respect to pharmacognostical, traditional use, phytochemical and therapeutic parameters. The present attempt is to review and compile updated information on the above mentioned aspects of FH, with mechanism-based pharmacological activity of the plant and possible strategy to increase the bioavailability of its lipophilic bioactives like alkaloids, sterols, flavonoid, coumarin, etc. This article will enhance the existing knowledge of FH, and also create awareness of the possible new therapeutic uses for the development of pharmaceutical entities for better health care in the near future.
PHARMACOGNOSY
Taxonomical classification[8]
Domain: Eukaryote Kingdom: Plantae
Division: Magnoliopsida Order: Rosales
Family: Moraceae Genus: Ficus
Species: hispida
Common names
FH, derived from the Latin word FIK-us “for Fig” and HISS-pih- duh “with bristly hairs”, is commonly known as devil fig, hairy fig, opposite-leaved fig-tree, and rough-leaved fig. In India, it is recognized by different names in different languages like in Gujarati as umbar; Hindi as gobla, kagsha, kala umbar, katgularia, phalgu; Kannada as adavi atti, kada atti; Konkani as kharvoti; Malayalam as erumanaakk, kaattaththi, paarakam; Marathi as bokeda, bokhada, bokheda, dhed umbar, karavati; Sanskrit as kakodumbarika, malayuhu, phalgu, phanika; Tamil as peyatti; and Telugu as bomma-medi. Apart from India, it is widely known as hpauwu, kadut, kha-aung, mai-nawt-hpu in Burmese, dui ye rong in Chinese, kota dimbula in Sinhalese, and ma duea plong in Thai. Other synonyms which are commonly used are Covellia hispida (Linnaeus f.) Mique; Ficus compressa S. S. Chang; F. heterostyla Merrill; F. letaqui H. Lιveillι and Vaniot; F. sambucixylon H. Lιveillι.[9]
Morphology
It is a coarsely hairy shrub or medium sized tree, up to 10 m tall, grows in secondary forests, open lands and river banks, up to 1200 m in altitude.[3] Bark is generally brownish or blaze pink with lantecellate . Leaves are simple, decussate, ovate, oblong, or obovate-oblong, thickly papery, covered with coarse hairs and oppositely arranged on 1–4 cm long petiole; lamina 7–35 × 3–16 cm (40 × 18 cm in saplings); is narrow elliptic-oblong, ovate or obovate with rounded subcordate or truncate-subcordate base; margin is entire or dentate, sometimes irregularly toothed, scabrid on both surfaces and hispid beneath having acute to mucronate apex; midrib is three-nerved at base; secondary nerves are four to nine pairs, often branched, ascending; tertiary nerves broadly reticulo-percurrent. Ovate-lance shaped stipules are usually four in number and are visible on leafless fruiting branchlets. Inflorescence is of syconia type, clustered on tubercles of main trunk, older branches and sometimes on pendulous leafless branches.[10] Male flowers are many and found near apical pore; calyx is three lobed, thinly membranous; stamen 1. Gall flowers: calyx is absent; style subapical, short, and thick. Female flowers: calyx lobes absent; style lateral with hairs appearing during the months of June and July.[11] Figs appear in leaf axil on normal leafy shoots, sometimes on leafless branchlets, solitary or paired, yellow or red when mature, top-shaped and 1.2–3 cm in diameter.
Microscopy
The fruit shows a single layered epidermis covered with thick cuticle having a few unicellular trichomes. Epidermis is followed by four to six layers of hexagonal to polygonal collenchymatous cells. A few cells contain rosette crystals of calcium oxalate; mesocarp is composed of large, oval to polygonal, thick-walled parenchymatous cells; a few vascular vessels show spiral thickening. Root shows 5–10 layers of cork consisting of thin-walled, compressed cells, with outer layers exfoliating; secondary cortex is a wide zone consisting of irregularly arranged, tangentially elongated, thin-walled, parenchymatous cells, some of which contain rosette crystals of calcium oxalate and dark red colored contents; secondary phloem consists of usual elements comprising thin-walled cells; cellulosic phloem fibers are found scattered throughout the secondary phloem in singles and in groups of two to three; a few phloem parenchyma and phloem ray cells contain rosette crystals of calcium oxalate; secondary xylem is situated centrally, consisting of usual elements, all being lignified; xylem vessels are numerous and equally distributed throughout the secondary xylem region, in singles as well as in groups of two to six; xylem rays are numerous, straight and one to five cells wide.[12]
Extraction methods
Since ages, extracts of leaves, bark and root have been used by the tribals of Assam and Manipur in the treatment of jaundice[13] and diabetes.[14] The extraction methods for these plant parts are well documented in the literature and supported by various scientists. Dhanasekaram et al, prepared the methanolic extract of powdered dried leaves by extracting it with methanol on a water bath (50°c). The solvent was removed by filtration and fresh solvent was added to the plant material. This process was repeated twice. The obtained extract was stored at 0–4°C for further use.[15] Some workers independently adopted a slightly different approach of first defatting the dried leaves using petroleum ether (60–80mL) and then extracting with methanol.[16–18] Ghosh et al, ground the dried bark and extracted it with double distilled ethanol and isolated the water soluble portion.[19] The extraction from roots was carried out by a method similar to that used by Dhanasekaram et al.[20] with leaves.
Phytochemistry
Preliminary phytochemical investigations of FH have shown the presence of alkaloids, carbohydrates, proteins and amino acids, sterols, phenols, flavonoids, gums and mucilage, glycosides, saponins, and terpenes.[15] Previous reports by Acharya et al, show that FH bark contains lupeol acetate, β-amyrine acetate, β-sitosterol.[21] In another study, purification of acetates of n-triacontanol, β-amyrin and gluanol was carried out from the petroleum ether extracts of the dried bark powder.[21] S. Wang and D. A. Coviello obtained a new and unusual compound, 10-ketotetracosyl arachidate, when the bark was extracted with light petroleum.[22,23] Venkatachalam et al, isolated two substantial phenanthroindolizidine alkaloids, 6-O-methyltylophorinidine and 2-demethoxytylophorine, and a novel biphenylhexahydroindolizine hispidine from stem and leaves of FH.[24] Recently, hispidin has been reported to have anticancer activity.[25] Peraza-Sanchez et al, revealed the occurrence of known phenanthroindolizidine alkaloid, n-alkanes, coumarins, and triterpenoid and identified a new norisoprenoid ficustriol from the methanolic extract of leaves and twigs of this plant.[26] Recently, Huong et al, demonstrated that leaves contain hispidin, oleanolic acid, bergaptine, β-amyrine, and β-sitosterol.[25] Quishi song et al, implicated the presence of a number of volatiles from the fruit, that include linalool, linalool oxide, terpeneol, and 2,6-dimethyl-1,7-octadiene-3,6-diol, which may act as the attractants of the wasps responsible for pollination of FH.[27] The structures of some of the important constituents are shown in Figure 1.
Figure 1.
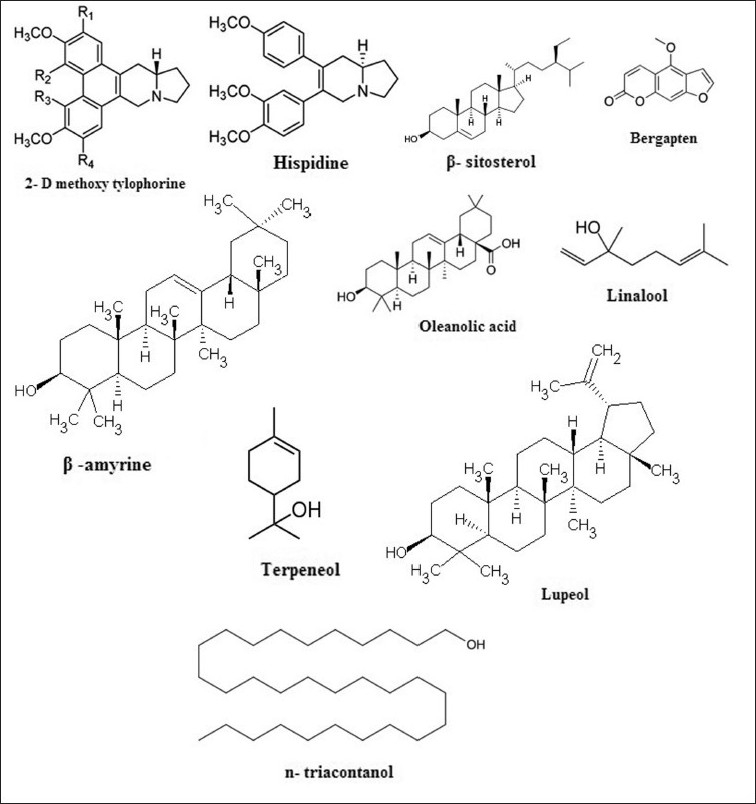
Some important bioactive constituents with their structure, obtained from Ficus hispida Linn.
ETHNOMEDICINAL PROPERTIES
History of the use of FH dates back to the time of Charaka when he advised the juice obtained from the fig to be taken with jaggery as a sramsana (mild purgative) in the treatment of switra (vitiligo).[28] Tribals of Meghalaya use the unripe fruit for preparation of curry.[29] The fruit juices along with honey act as a good antihemorrhagic. All parts of this plant are found to be acrid, astringent, bitter, coolant and have activity against dysentery, ulcers, biliousness, psoriasis, anemia, piles and jaundice. Additionally, the fruit is known to be active as aphrodisiac, tonic, lactagogue and emetic.[3,30–32] Rural people residing in the villages of Khatra, Bankura, West Bengal in India chew the leaves to cure diarrhea.
Literature survey revealed that various important pharmacological activities like antioxidant, cardioprotective, hepatoprotective, anticancer, anti-inflammatory, etc. are associated with different parts of FH. Furthermore, it is needed to establish the mechanism of action behind the activities, which in turn will help in the establishment of bioactives in clinical application. Keeping these aspects in mind, we are trying to elucidate the mechanism of the established pharmacological activity in FH along with its therapeutic activity.
Hypoglycemic activity
Ghosh et al, have successfully demonstrated the hypoglycemic activity of FH bark in diabetic albino rats. They reported that water soluble portion of ethanolic extract of the bark showed significant reduction of blood glucose level, increase in the uptake of glucose and increase in the glycogen content of liver, skeletal muscle and cardiac muscle. They also revealed the interaction of the constituent of the extract with insulin on concomitant administration, but the compound involved is not yet established.[19]
Mechanism of action
The possible mechanism behind this activity is thought to be multidirectional as the antioxidant constituents of FH bark, such as lupeol acetate, b-amyrine acetate, and β-sitosterol, that may reduce oxidative stress, may prevent the damage of β cells of islets of Langerhans.[21] Furthermore, the enhanced expression of glucose receptors on the cell which increases the glucose uptake may also be involved.
Cardioprotective effect
Shanmugarajan et al, evaluated the cardioprotective effect of FH leaf extract on cyclophosphamide mediated myocardial injury due to oxidative stress in rat heart. This study showed that the extract exhibited a significant inhibition of lipid peroxidation and increased the level of superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, glutathione-S-transferase and reduced the glutathione activity in heart tissue provoked by cyclophosphamide.[17]
Mechanism of action
The antioxidant potential of the phytoconstituents is responsible for its cardioprotective activity. It has been accepted that many constituents of leaves, such as hispidin, β-sitosterol, β-amyrin, and bergaptin, act as antioxidants.[24] Furthermore, du et al, reported that oleanolic acid has its antioxidant property mediated by glutathione and a-tocopherol that provide protection against myocardial ischemia-reperfusion injury.[33–35] This antioxidant property is responsible for the restoration of antioxidant level and prevention of lipid peroxidation. Another constituent that also contributes to the augmentation effect is β-amyrin which has GSH restoration effect that has been recently investigated by Oliveira.[36]
Antidiarrheal activity
Mandal et al, prepared the extracts from the leaves of FH and assessed the antidiarrheal activity in rats against castor-oil induced diarrhea and PEG2-induced interpooling in rats. The methanolic extract of leaves showed a significant and dose-dependent antidiarrheal activity and also reduced the propulsion of charcoal meal through the gastrointestinal tract when administered orally. They also established the dose of the extract and found that at the level of 600 mg/kg, the response was equivalent to that produced by 5 mg/kg of diphenoxylate.[16]
Mechanism of action
It has been considered that tannins which are accountable for denaturation of proteins and form protein tannate may be responsible for the antidiarrheal activity as this protein tannate minimizes the intestinal mucosa permeability.
Antiulcerogenic effect
Sivaraman et al, conducted a study on the methanolic extract of FH root in aspirin ulcerated rats and showed that a dose of 200 and 400 mg/kg substantially decreased the incidence of ulcers, enhanced the healing of ulcers and significantly reduced free and total acidity.[20]
Mechanism of action
The antiulcer activity of the methanolic extract of FH root might be due to its cytoprotective action owing to the presence of its antioxidant constituent. It prevents degradation of mucosa, causes restoration of carbohydrates as well as carbohydrate/protein ratio and enhances the synthesis of cytoprotective prostaglandins like PGI.[37]
Sedative and anticonvulsant effects
Dhanasekaran et al, administered the methanolic extract of leaves at a dose of 200 and 400 mg/kg and investigated phenobarbitone induced sleeping time and hole-board exploratory behavior for sedation test, and strychnine, picrotoxin, and phenylenetetrazole-induced convulsion in swiss albino mice. The result proved that there was a significant and dose-dependent reduction in the onset and prolongation of sleep duration elicited by phenobarbitone. In addition to this, suppression of exploratory action was reported. Moreover, complete inhibition of seizures induced due to picrotoxin and strychnine was seen at a dose of 400 mg/kg, along with significant prolongation of both clonic and tonic seizures.[15]
Mechanism of action
Its sedative and anticonvulsant activity might involve both GABAergic and glycinergic inhibitory mechanisms.[38] They have not revealed the constituent responsible for these activities. The occurrence of flavonoids, phenols, and terpenes might lead to these actions. GABAergic effect of extract of leaves may involve a) opening of chloride channel associated with the GABA-A receptor, b) facilitating the binding of GABA to the receptor site, c) protecting the GABAergic neurons against oxidative stress elicited damage due to its antioxidant activity.
Neuroprotective effects
Sivaraman et al, established the neuroprotective effect of methanolic extract of leaves of FH on b-amyloid induced cognitive deficits and oxidative stress in mice. Study showed that the extract impairs the cognitive behavior and memory deficit and suppresses the increased level of thiobarbituric acid reactive species in brain. Additionally, increased activities of antioxidant enzymes like glutathione peroxidase, glutathione reductase and superoxide dismutase were also seen in the study. These activities may be helpful in the holistic treatment of Alzheimer′s disease and other age-related memory impairments.[39]
Mechanism of action
Neurodegenerative disorders of brain like Alzheimer′s disease and dementia are due to the oxidative stress induced death of the neurons. Furthermore, they may be caused by a decrease in the level of acetylcholine which may be due to the overexpression of acetylcholinesterase.[40] So, the oxidation protective bioactives may inhibit the neuron cellular damage along with reduction in amyloid plug content. Lately, Sivaraman et al, confirmed the acetylcholine enhancing activity which was attributed to the depression of enzymatic activity on acetylcholine .[41]
Hepatoprotective effect
Shanmugarajan et al, prepared the methanolic extract of leaves of FH and successfully confirmed the hepatoprotective effect on cyclophosphamide mediated oxidative liver injury in Wistar rat. In another study (performed by the same group), methanolic leaf extract was explored against azathioprin elicited liver injury in Wistar rat liver.[42] Mandal et al, extracted leaves of FH and investigated for hepatoprotection in rats by inducing acute liver damage by paracetamol. The results were found to be comparable to that of a standard Liv-52 formulation, a known hepatoprotective formulation .[43]
Mechanism of action
The hepatoprotective property of F. hispida extract may be due to its constituents, oleanolic acid and β-sitosterol, which have membrane stabilizing property.[44–45] The preliminary phytochemical studies indicated the presence of triterpenoids and flavonoid in the methanolic extract of leaves of FH.15 Since these constituents have antioxidant activity,[46–49] it may be speculated that they may also be responsible for the observed protective effects. Liu et al, demonstrated the role of triterpenoid in glutathione restoration, an enzyme responsible for protection of cell membrane and regulation of cell function.[34–36] In accordance with the previous finding reported by Balanehru et al, oleanolic acid, hispidin and β-sitosterol act as free radical scavengers and thus might be associated with hepatoprotective effect.[45–46] Moreover, recently, it has been investigated that oleanolic acid,[47–48] β-amyrin[36] and phenanthroindolizidine[49] have potent hepatoprotective effects attributed to the various activities.
Antineoplastic activity
Pratumvinit et al, depicted the antineoplastic effect of FH stem successively extracted with crude ethanol; water, methanol; water, methanol and ethyl acetate fractions against SKBR3, MDA-MB435, MCF7 and T47D human breast cancer cell lines in vitro. Only the ethanolic fraction was found to have anti-neoplastic activity against T47D cells.[50]
Mechanism of action
The mechanism behind this activity was ascribed to the presence of constituent O-methyltylophorinidine which has been reported to exhibit cytotoxicity for lung, colon, nasopharynx and prostrate cancer cell lines.[50] This cytotoxic effect may be attributed to cell growth inhibition and induction of apoptosis. Encouragingly, recent studies have demonstrated that tylophorine analogues have a unique mode of action different from those of the current known anticancer agent. One of these actions is an inhibitory effect on NF-KB binding mediated transcription, leading to apoptosis. A recent fascinating report by Huong et al, assessed hispidin as a strong anticancer agent found in the bark of FH.[25]
Anti-inflammatory and antipyretic
Vishnoi et al, showed significant activity in the carraggenan induced paw edema in albino rat model, reducing inflammation by approximately 64.07% compared to only 45.13% with the standard, diclofenac sodium.[51]
Mechanism of action
Its antioxidant activity and production of protective prostaglandins may be suggested for anti-inflammatory and antipyretic effects. Shielding of oxidative damage reduces the generation of free radical or inflammatory mediators.
BIOAVAILABILITY ENHANCEMENT APPROACHES FOR LIPOPHILIC CONTENTS OF FH
The problem of oral bioavailability may arise with the use of alcoholic extract of FH root, bark, leaves, etc. because of its lipophilic constituents which are responsible for various activities. It is a well-known fact that deliveries of lipophilic molecule through oral route have poor bioavailability due to poor dissolution. This necessitates the incorporation of FH into a dosage form that can increase its delivery and hence its bioavailability. Earlier reported methods for dissolution enhancement, such as solid dispersion, microemulsion, lipid carriers like liposome, noisome, lipid emulsion, solid lipid nanoparticle (SLN), micellar formation technique, nanosuspension, complexation with cyclodextrin or phospholipids, etc. can be applied with the active therapeutic constituent. Furthermore, appearance of activity at lower dose level of the drug is the main advantage with the use of these advanced novel formulation approach.[52–56]
CONCLUSIONS
In a country like India, providing modern health care facility with expensive medicines is still in infancy, especially in rural sections. So, it is prudent to look for options in herbal medicines. Hence, in the recent years, ethnomedical studies have received much attention as they bring to light the numerous known and unknown medical virtues, especially of plant origin, which need studies on modern scientific lines like phytochemical investigation, pharmacological screening and human studies. F. hispida possesses various important pharmacological activities as discussed in the present review. Additionally, it is imperative that more pre-clinical and clinical studies along with the establishment of better quality control methods should be conducted to elucidate the unexplored potential of this plant.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kislev ME, Hartmann A, Bar-Yosef O. Early domesticated fig in the Jordan valley. Science. 2006;312:1372–4. doi: 10.1126/science.1125910. [DOI] [PubMed] [Google Scholar]
- 2.Ripu M, Kunwar I, Rainer WB. Ficus (Fig) species in Nepal a review of diversity and indigenous uses. J Ecol Appl. 2006;11:85–7. [Google Scholar]
- 3.Nadkarn AK. Mumbai: Popular Prakashan; 1976. Indian Materia Medica; pp. 1031–5. [Google Scholar]
- 4.Mandal SC, Kumar CK. Studies on antidiarrhoeal activity of ficus hispida. leaf extract in rats. Fitoterapia. 2002;73:663–7. doi: 10.1016/s0367-326x(02)00225-3. [DOI] [PubMed] [Google Scholar]
- 5.Mandal SC, Saraswathi B, Kumar CK, Lakshmi SM, Maiti BC. Protective effect of leaf extract of Ficus hispida Linn.Against paaracetamol-induced hepatotoxicity in rats. Phytother Res. 2000;14:457–9. doi: 10.1002/1099-1573(200009)14:6<457::aid-ptr610>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Vishnoi SP, Jha T. Evaluation of anti-inflammatory activity of leaf extracts of Ficus hispida. Indian J Nat Prod. 2004;20:27–9. [Google Scholar]
- 7.Rastogi, Mehrotra BN. Lucknow, New Delhi: Publication and Information Directorate, II; 1993. Compendium of Indian Medicinal Plants, Central Drug Research Institute; pp. 27–30. [Google Scholar]
- 8. [cited in 2010]. Available from: http://www.biotik.org/india/species/f/ficuhisp/ficuhisp_en.html .
- 9. [cited in 2010]. Available from: http://www.flowersofindia.net/catalog/slides/Hairy%20Fig.html .
- 10.Ayurvedic pharmacopoeia of India. Part 1. :149–51. [Google Scholar]
- 11.Sivaraman D, Muralidaran P. Sedative and anticonvulsant activities of the methanol leaf extract of ficus hispida linn. Drug Invent Today. 2009;1:23–7. [Google Scholar]
- 12.Mandal SC, Kumar CK. Studies on antidiarrhoeal activity of ficus hispida leaf axtract in rats. Fitoterapia. 2002;73:663–7. doi: 10.1016/s0367-326x(02)00225-3. [DOI] [PubMed] [Google Scholar]
- 13.Shanmugarajan TS, Arunsundar M, Somasundaram I, Krishnakumar E, Silverman D, Ravichandiran V. Cardioprotective effect of ficus hispida linn: On cyclophosphamide provoked oxidative myocardial injury in a rat model. Int J Pharmacol. 2008;1:1–10. [Google Scholar]
- 14.Arunsunder M, Shanmugarajan TS. Ficus hispida modulates oxidative-inflammatory damage in a murine model of diabetic encephalopathy. Ann Biol Res. 2010;1:90–7. [Google Scholar]
- 15.Ghosh R, Sharatchandra KH, Rita S, Thokchom IS. Hypoglycemic activity of ficus hispida (bark) in normal and diabetic albino rats. Indian J Pharmacol. 2004;36:222–5. [Google Scholar]
- 16.Sivaraman D, Muralidharan P. anti-ulcerogenic evaluation of root extract of ficus hispida linn: In aspirin ulcerated rats. Afr J Pharma Pharmacol. 2010;4:72–82. [Google Scholar]
- 17.Acharya BM, Kumar KA. Chemical examination of the bark of ficus hispida Linn. Curr Sci. 1984;53:1034–5. [Google Scholar]
- 18.Wang S, Coviello DA. The isolation, characterization and synthesis of 10-ketotetracosyl arachidate from Ficus hispida. Tetrahedron. 1975;31:929–32. [Google Scholar]
- 19.Venkatachalam SR, Mulchandani NB. Isolation of phenanthroindolizidinealkaloids and a novel biphenylhexahydroindolizine alkaloid from Ficus hispida. Naturwissenschaften. 1982;69:287–8. [Google Scholar]
- 20.Huong VN, Trang VM. Hispidin: A strong anticancer agent isolated from the leaves of Ficus hispida L. Tap Chi Hoa Hoc. 2006;44:345–9. [Google Scholar]
- 21.Peraza-Sánchez SR Chai H, Shin Y, Santisuk T, Reutrakul V, Farnsworth NR, et al. Constituents of the leaves and twigs of Ficus hispida. Planta Med. 2002;68:186–8. doi: 10.1055/s-2002-20257. [DOI] [PubMed] [Google Scholar]
- 22.Song Q, Yang D, Zhang G, Yang C. Volatiles from Ficus hispida and their attractiveness to fig wasps. J Chem Ecol. 2001;27:516–23. doi: 10.1023/a:1012226400586. [DOI] [PubMed] [Google Scholar]
- 23.Kayang H. Tribal knowledge on wild edible plants of Meghalaya, northeast India. Indian J Trad Know. 2007;6:177–81. [Google Scholar]
- 24.Kirtikar KR, Basu BD. Allahabad, India: Lalit Mohan Basu; 1956. Indian medicinal plants; p. 2322. [Google Scholar]
- 25.Rastogi R, Mehrotra BN. II. New Delhi, Lucknow: CDRI, Publication and Information Directorate; 1993. Compendium of Indian medicinal plants; p. 27. [Google Scholar]
- 26.The wealth of India, raw materials. Vol. 4. New Delhi: CSIR; 1956. Anonymous. [Google Scholar]
- 27.The useful plants of India. New Delhi: Publication and Information Directorate; CSIR; 1986. Anonymous; p. 221. [Google Scholar]
- 28.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Liu YP, Klaassen CD. Effect of oleanolic acid on hepatic toxicant-activating and detoxifying systems in mice. J Pharmacol Exp Ther. 1995;275:768–74. [PubMed] [Google Scholar]
- 30.Liu J, Liu YP, Klaassen CD. Protective effects of oleanolic acid on acetaminophen hepatotoxicity in mice. J Phamacol Exp Ther. 1993;266:1607–13. [PubMed] [Google Scholar]
- 31.Oliveira FA, Chaves MH, Almeida FR, Lima RC, Maia JL, Rao VS. Protective effect of α and β-amyrin, a triterpene mixture from protium heptaphyllum (Aubl.) March. Trunk wood resin, against acetaminophen-induced liver injury in mice. J Ethanopharmacol. 2005;98:103–8. doi: 10.1016/j.jep.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 32.Tseng CF, Iwakami S, Mikajiri A, Shibuya M, Hanaoka F, Ebizuka YK, et al. Inhibition of in vitro prostaglandin and leukotriene biosyntheses by cinnamoyl-beta-phenethylamine and N-acyldopamine derivatives. Chem Pharm Bull. 1992;40:396–400. doi: 10.1248/cpb.40.396. [DOI] [PubMed] [Google Scholar]
- 33.Biggio G, Cibin M, Diana M, Fadda F, Ferrara SD, Gallimberti L, et al. Suppression of voluntary alcohol intake in rats and alcoholics by gamma hydrobutyric acid: A non-GABAergic mechanism. Biochem Psychopharmacol. 1992;47:281–8. [PubMed] [Google Scholar]
- 34.Sivaraman D, Muralidharan P. Neuroprotective effect of ficus hispida linn on â-amyloid induced cognitive dysfunction. Indian J Pharmacol. 2008;40:S119. [Google Scholar]
- 35.Geula C, Mesulam M. Cholinergic systems and related neuropathological predilection patterns in Alzheimer disease. In: Katzman T, Bick R, editors. Alzheimer Disease. New York: Raven Press; 1994. pp. 263–91. [Google Scholar]
- 36.Sivaraman D, Muralidaran P. Acetylcholine enhancing activity of methanol leaf extract of ficus hispida linn in rat hippocampus. J Herbal Med Toxicol. 2009;3:147–50. [Google Scholar]
- 37.Shanmugarajan TS, Arunsundar M, Somasundaram I, Sivaraman D, Ravichandaran V. Ameliorative effect of ficus hispida linn leaf extract on cyclophosphamide-induced oxidative hepatic injury in rats. J Pharmacol Toxicol. 2008;3:363–72. [Google Scholar]
- 38.Mandal SC, Saraswathi B, Kumar CK, Mohana Lakshmi S, Maiti BC. Protective effect of leaf extract of ficus hispida linn. against paracetamol-induced hepatotoxicity in rats. Phytother Res. 2000;14:457–9. doi: 10.1002/1099-1573(200009)14:6<457::aid-ptr610>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Yokota JD, Takuma A, Hamada M, Onogawa S, Yoshioka, Nishioka Y. Scavanging of reactive oxygen species by eriobotrya Japonica seed extract. Biol Pharm Bull. 2006;29:467–71. doi: 10.1248/bpb.29.467. [DOI] [PubMed] [Google Scholar]
- 40.Tang XH, Gao J, Fang F, Chen J, Xu LZ, Zhao XN, et al. Hepatoprotection of oleanolic acid is related to its inhibition on mitochondrial permeability transition. Am J Chir Med. 2005;33:627–37. doi: 10.1142/S0192415X05003223. [DOI] [PubMed] [Google Scholar]
- 41.Augusti KT, Anuradha SP, Prabha KB, Smitha M, Joseph MC. Neutraceutical effects of garlic oil its non polar fraction and a ficus flavonoid as compared to vitamine E in carbon tetrachloride induced liver damage in rats. Indian J Exp Biol. 2005;43:437–44. [PubMed] [Google Scholar]
- 42.Bai X, Qiu A, Guan J, Shi Z. Antioxidant and protective effect of an oleanolic acid enriched extract of A.deliciosa root on carbon tetrachloride induced rat liver injury. Asia Pac J Clin Nutr. 2007;16:169–73. [PubMed] [Google Scholar]
- 43.Buniatian ND, Chikitkina VV, Lakavleva LV. The hepatoprotective action of ellagotannins. Eksp Klin Formakol. 1998;61:53–5. [PubMed] [Google Scholar]
- 44.Daniel RS, Devi KT, Nair CR. Mechanism of action of antiatherogenic and related effects of Ficus bengalensis Linn. Flavonoids in experimental animals. Indian J Exp Biol. 2003;41:296–303. [PubMed] [Google Scholar]
- 45.Balanehru S, Nagaranjan B. Protective effect of oleanolic acid and Ursolic acid against lipid peroxidation. Biochem Int. 1991;24:981–90. [PubMed] [Google Scholar]
- 46.Park IH, Chung SK, Lee KB, Yoo YC, Kim GS, Song KS. An antioxidant hispidin from the mycelial cultures of phellinus linteus. Arch Pharm Res. 2004;27:615–8. doi: 10.1007/BF02980159. [DOI] [PubMed] [Google Scholar]
- 47.Abdel-Zaher AO, Abdel-Rahman MM, Hafez MM, Omran FM. Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2007;234:124–34. doi: 10.1016/j.tox.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Kim NY, Lee MK, Park MJ, Kim SJ, Park HJ, Choi JW, et al. Momordin Ic and oleanolic acid from Kochiae fructus reduces carbon tetrachloride-induced hepatotoxicity in rats. J Med Food. 2005;8:177–83. doi: 10.1089/jmf.2005.8.177. [DOI] [PubMed] [Google Scholar]
- 49.Malathi R, Gomez MP. Hepatoprotective effect of methanolic leaf extracts of Tylophora asthamatica against paracetamol induced liver damage in rats. J Pharmacol Toxicol. 2007;2:737–42. [Google Scholar]
- 50.Pratumvinit B, Srisapoomi T, Worawattananon P, Opartkiattikul N, Jiratchariyakul W, Kummalue T. In vitro antineoplastic effect of ficus hispida l: Plant against breast cancer cell lines. J Med Plants Res. 2009;3:255–61. [Google Scholar]
- 51.Vishnoi SP, Jha T. Evaluation of anti-inflammatory activity of leaf extracts of Ficus hispida. Indian J Nat Prod. 2009;35:57–63. [Google Scholar]
- 52.Oppenheim RC, Stewart NF, Gordon L, Patel HM. The production and evaluation of orally administered insulin nanoparticles. Drug Develop Ind Pharm. 1982;8:531–46. [Google Scholar]
- 53.Touitou E, Rubinstein AA. Targeted enteral delivery of insulin to rats. Int J Pharm. 1986;30:95–9. [Google Scholar]
- 54.Arunkumar N, Deecaraman M, Rani C. Nanosuspension technology and its applications in drug delivery. Asian J Pharm. 2009;3:168–73. [Google Scholar]
- 55.Shah P, Bhalodia D, Shelat P. Nanoemulsion: A pharmaceutical review. Syst Rev Pharm. 2010;1:24–32. [Google Scholar]
- 56.Bhole PG, Patil VR. Enhancement of water solubility of felodipine by preparing solid dispersion using poly-ethylene glycol 6000 and poly-vinyl alcohol. Asian J Pharm. 2009;3:240–4. [Google Scholar]


