Abstract
Background
Severe thrombocytopenia is a major risk factor for haemorrhage, and yet platelet function and bleeding risk at low platelet counts are poorly understood because of limitations of platelet function testing at very low platelet counts.
Objectives
To examine and compare platelet function in severely thrombocytopenic patients with acute myeloid leukaemia (AML) or myelodysplasia (MDS) to patients with immune thrombocytopenia (ITP).
Methods
Whole blood flow cytometric measurement of platelet activation and platelet reactivity to agonists was correlated with the immature platelet fraction (IPF) and bleeding symptoms.
Results
Compared with patients with ITP, patients with AML/MDS had smaller platelets, lower IPF, and substantially lower platelet surface expression of activated GPIIb/IIIa and GPIb both with and without addition of ex vivo ADP or TRAP. In both ITP and AML/MDS, increased platelet surface GPIb on circulating platelets and expression of activated GPIIb/IIIa and GPIb on ex vivo activated platelets correlated with a higher IPF. Whereas platelet reactivity was higher for AML/MDS patients with bleeding than those with no bleeding, platelet reactivity was lower for ITP patients with bleeding than those with no bleeding.
Conclusions
AML/MDS patients have lower in vivo platelet activation and ex vivo platelet reactivity than patients with ITP. The proportion of newly-produced platelets correlates with the expression of platelet surface markers of activation. These differences might contribute to differences in bleeding tendency between AML/MDS and ITP. This study is the first to define differences in platelet function between AML/MDS patients and ITP patients with equivalent degrees of thrombocytopenia.
Keywords: Autoimmunity, bleeding, flow cytometry, haemorrhage, thrombocytopenia, thrombopoiesis
Introduction
The degree of thrombocytopenia is a major determinant of bleeding risk [1]. However, while some patients exhibit little bleeding despite platelet counts of less than 10,000/μl, others suffer significant haemorrhages with platelet counts over 50,000/μl [2-4]. Patients with extreme thrombocytopenia due to lack of platelet production (e.g., acute myeloid leukaemia [AML] or myelodysplastic syndrome [MDS]) or primarily due to accelerated platelet destruction (e.g., immune thrombocytopenia [ITP]) may experience severe bleeding. Platelet function in patients with severe thrombocytopenia has never been adequately studied because of methodological problems related to the assessment of platelet function in thrombocytopenia. The contribution of platelet function to bleeding risk in this setting is therefore poorly understood.
Platelet function in patients with AML or MDS may be impaired due to the underlying malignancy, chemotherapy or other drugs, or concurrent infections. Patients with ITP may also have very low platelet counts, although it has traditionally been assumed that the larger, younger platelets of patients with ITP are better functioning than normal platelets and[5-7], by inference, also better functioning than the platelets of patients with haematological malignancies. However these two types of thrombocytopenic conditions are managed very differently with regard to platelet count thresholds for initiating treatment. It is recommended that adults with ITP are treated at platelet counts of 20-30,000/μl [8, 9], whereas prophylactic platelet transfusions are generally initiated at a platelet count of ≤10,000/μl in patients with thrombocytopenia secondary to haematological malignancy, bone marrow failure or chemotherapy, in part to minimize alloimmunization and platelet refractoriness due to more frequent transfusions [10]. Thus, the typical threshold for treatment of thrombocytopenia is often higher in ITP than in dysplastic or malignant disorders of the bone marrow.
There are very little data describing platelet function in severely thrombocytopenic patients because, with the sole exception of flow cytometry, no platelet function assay can distinguish between the effects of thrombocytopenia and the effects of reduced platelet function. Whole blood flow cytometry can interrogate platelet function independently of platelet count by examining the functional status of individual platelets [11]. Flow cytometry has been widely used and validated in patients with heart disease in whom assessment of platelet function is considered to be important for monitoring of antiplatelet therapy [11-13]. Surprisingly, whole blood flow cytometry has infrequently been tested in the setting of marked thrombocytopenia, where it is uniquely able to assess platelet function [7, 11, 14].
In this study we used whole blood flow cytometry[15] and a standardized bleeding score[16] in patients with platelet counts less than or equal to 30,000/μl to compare platelet function in severely thrombocytopenic patients with AML/MDS to those with ITP. Platelet surface expression of P-selectin, activated glycoprotein (GP) IIb/IIIa (integrin αIIbβ3), and GPIb were measured with and without ex vivo agonist stimulation in order to assess both the activation state of circulating platelets and also platelet reactivity. In addition, we examined whether the proportion of newly synthesized platelets in the circulation contributed to platelet function.
Methods
Twenty-five patients with ITP and 21 patients with AML/MDS were consented and enrolled in this IRB-approved study. Patients were selected on the basis of platelet counts ≤30,000/μl, age > 18 years, not having received platelet transfusions or antiplatelet agents within the previous five days and not having any known disorders of haemostasis or platelet function. None of the patients received cytotoxic chemotherapy on the day of study.
A diagnosis of ITP was based on thrombocytopenia in the absence of another identifiable cause, normal or increased numbers of megakaryocytes (if a bone marrow examination had been performed) and/or response to intravenous immunoglobulin or steroids. Of the 21 patients in the AML/MDS group, 18 had a diagnosis of AML and 3 had myelodysplasia with circulating blasts.
Blood was drawn from patients by antecubital venipuncture into 4.5 mL 3.2% trisodium citrate Vacutainers (Becton Dickinson), a method previously shown not to induce ex vivo platelet activation [17]. Platelet counts were measured in a Bayer-Advia automated CBC counter immediately following the blood draw. Immature platelet fraction (IPF) and the immature platelet count (IPC) were measured for 17/25 ITP patients and 19/21 AML/MDS patients in a Sysmex XE-2100 autoanalyzer within 6 hours of blood draw [18].
Twenty minutes after blood draw, aliquots of whole blood were incubated with fluorescently-labeled monoclonal antibodies and either 0.5 μM adenosine diphosphate (ADP), 20 μM ADP, 1.5 μM thrombin receptor activating peptide (TRAP), 20 μM TRAP, or HEPES-Tyrode’s buffer (10 mM HEPES, 137 mM sodium chloride, 2.8 mM potassium chloride, 1 mM magnesium chloride, 12 mM sodium hydrogen carbonate, 0.4 mM sodium phosphate dibasic, 5.5 mM glucose, 0.35% w/v bovine serum albumin, pH 7.4) for exactly 15 minutes. The reaction was stopped with a 15-fold dilution in 1% formaldehyde in HEPES-saline buffer. Samples were maintained at room temperature and not agitated until fixation to prevent handling activation.
The antibodies used were as follows: phycoerythrin (PE)-conjugated anti-P-selectin monoclonal antibody (CD62P, clone 1E3, Santa Cruz Biotech); fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody PAC1 (Becton Dickinson), which only binds to the activated conformation of GPIIb/IIIa [19]; and PE-Cy5-conjugated anti-CD42b (GPIb) monoclonal antibody (clone HIP1, Becton Dickinson Pharmingen). PE-conjugated MIgG2a isotype (Santa Cruz Biotech), and FITC-PAC-1 together with 2.5 μg/mL of the GPIIb/IIIa antagonist eptifibatide to block specific binding, served as the negative control for P-selectin and PAC-1 respectively.
For flow cytometric analysis of platelet count, anticoagulated blood was labelled with FITC-conjugated anti-GPIIIa (CD61) monoclonal antibody (clone Y2/51, DAKO Cytomation), PE-conjugated anti-GPIIb (CD41) monoclonal antibody (clone 5B12, DAKO Cytomation), and PE-Cy5-conjugated anti-CD42b monoclonal antibody (clone H1P1, Becton Dickinson Pharmingen).
Fixed samples were stored at 4°C and sent by overnight courier to the Center for Platelet Function Studies at the University of Massachusetts Medical School for analysis. A known quantity of RFP-30-5 calibration beads (Spherotech) was added to allow cell counts to be calculated. Analysis was performed in a Becton Dickinson FACSCalibur™ flow cytometer which was calibrated daily to assure proper instrument functioning and consistent fluorescence measurements over time. Platelet surface P-selectin, activated GPIIb/IIIa and GPIb expression were measured relative to the isotype control as mean fluorescence intensity (MFI). For GPIb, the magnitude of change following agonist stimulation was calculated by subtracting the GPIb MFI value with added agonist from the MFI value with no added agonist. Platelets for flow cytometric counting were identified by characteristic forward and side light scatter, and CD61, CD41 and CD42b expression. The platelet count was calculated by determining the volume of blood analyzed by the number of internal standard beads acquired in parallel. Mean forward light scatter (FLS) of platelets was also recorded as an approximation of platelet size. A control sample from a healthy donor was also analyzed in parallel with each study sample to ensure correct sample handling.
At the time of blood draw, bleeding was assessed by history and by physical examination by one of three trained assessors (J.B.B, B.B or C.T.) using a standardized bleeding scale that quantifies bleeding at nine anatomical sites and has been shown to have good inter-user reliability [16]. Bleeding scores were later summarized into three categories: any bleeding vs. no bleeding; skin/oral bleeding vs. no skin/oral bleeding; any non-skin/oral bleeding vs. no non-skin/oral bleeding.
The flow cytometry data was analyzed using a Mixed Model Repeated Measures Analysis of Variance (ANOVA). For all variables, the ANOVA models were checked for Gaussian residuals and equality of variance. Upon finding a significant disease (ITP vs. AML/MDS) by treatment (platelet agonist condition) interaction, five Bonferroni-adjusted pairwise comparisons of AML/MDS vs. ITP for each of the five treatment conditions were performed. A comparison was considered statistically significant if p < 0.01 (Bonferroni-adjustment to maintain an overall 5% significance level). The association between disease and the categorical bleeding score variables was examined using the chi-square test. The Kruskal-Wallis test was carried out to compare the bleeding scores and the continuous variables, IPF and IPC. The Wilcoxon rank sum test compared IPF data for subjects with AML/MDS vs. ITP. Spearman correlations were utilized to examine the association between platelet function flow variables and IPF values. Results were declared significant if P < 0.05. Graphs were drawn using GraphPad Prism 5.0a software (2007).
Results
There was no difference in age between the two patient groups (mean age of the ITP patients was 53 years [range 21 – 80]; mean age of the AML/MDS patients was 59.3 years [range 38-76]; P = 0.06). A higher proportion of the ITP patients were female (68% female for ITP vs. 19% female for AML/MDS, P = 0.001). At the time of study, none of the patients were receiving treatment with thrombopoietin receptor agonists and 60% of the ITP patients had undergone splenectomy.
Mean platelet counts were similar between the ITP and the AML/MDS patient groups (15,850 + 2338 vs. 20,938 + 3729/μl, P = 0.2, Figure 1A). However, the relative and absolute numbers of immature platelets (IPF and IPC) were lower among AML/MDS patients than ITP patients, reflecting a lower rate of platelet production (IPF 11.4 + 1.2% vs. 25.0 + 2.9, P = 0.0002; IPC 2357 + 403 vs. 4000 + 60 /μl, P = 0.03, Figure 1C and 1D). Platelets were much smaller in size in patients with AML/MDS than in those with ITP (69.0 + 4.7 vs. 211.2 + 23.9 arbitrary forward light scatter units, P < 0.0001, Figure 1B).
Figure 1.
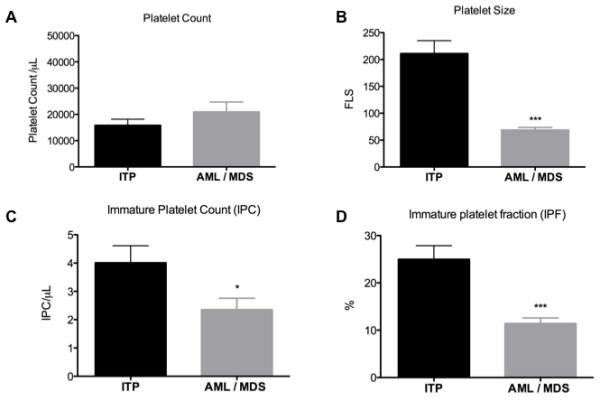
1A. Platelet count, 1B. Platelet size, 1C. Immature platelet count (IPC) and 1D. Immature platelet fraction (IPF). n = 25 for ITP, n = 21 for AML/MDS. Mean values + SEM are shown. * indicates P < 0.05; *** indicates P < 0.001. Abbreviations: AML, acute myeloid leukaemia; FLS, forward light scatter; ITP, immune thrombocytopenic purpura.
When platelets were examined for surface markers of activation with no addition of agonists ex vivo, surface activated GPIIb/IIIa was significantly lower in AML/MDS than in ITP (MFI 6.06 + 0.5 vs. 10.38 + 1.3, P = 0.007, Figure 2A). There was no difference in the platelet surface expression of P-selectin between ITP and AML/MDS (MFI 6.5 + 0.6 vs. 7.3 + 1.2, P = 0.5, Figure 2B).
Figure 2.
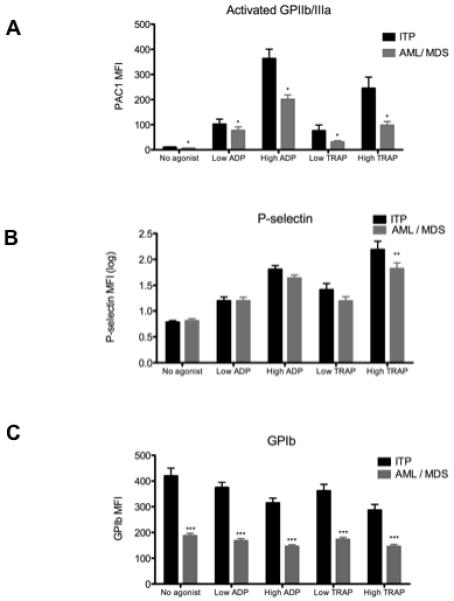
Expression of platelet surface activated GPIIb/IIIa (panel A), P-selectin (panel B) and GPIb (panel C) in ITP and AML/MDS patients with and without addition of platelet agonists ex vivo. n = 25 for ITP, n = 21 for AML/MDS. Mean + SEM is shown. * indicates P <0.05; ** indicates P <0.01; *** indicates P <0.001. Abbreviation: MFI, mean fluorescence intensity.
In response to ex vivo stimulation with ADP and TRAP, each at lower and higher concentrations, an increase in surface activated GPIIb/IIIa and P-selectin and a decrease in surface GPIb expression were observed, as expected, in both AML/MDS and ITP platelets (Figures 2A-C). However, platelet surface activated GPIIb/IIIa remained significantly lower for AML/MDS patients than for patients with ITP (P = 0.025, Figure 2A). Expression of platelet surface P-selectin was also significantly lower in AML/MDS in response to high TRAP stimulation (P = 0.002) although this difference was not significant in the other agonist conditions (Figure 2B). Expression of GPIb was significantly lower in patients with AML/MDS than those with ITP, both with no added agonist (MFI 187.3 ± 9.4 vs. 420.0 ± 30.4, P < 0.0001) and also following ex vivo stimulation, presumably reflecting the relationship between platelet surface expression of GPIb and platelet size (Figure 2C). There was no significant difference between ITP and AML/MDS patient groups with respect to the % decrease in platelet surface GPIb. In ITP patients, the % decrease in platelet surface GPIb compared to no agonist was 7.5% with 0.5 μM ADP, 22.5% with 20 μM ADP, 6.1% with 1.5 μM TRAP and 25.6% with 20 μM TRAP. In AML/MDS patients, the % decrease in platelet surface GPIb compared to no agonist was 10.4% with 0.5 μM ADP, 21.5% with 20 μM ADP, 7.2% with 1.5 μM TRAP and 22.3% with 20 μM TRAP.
There were no significant differences in platelet count, platelet size or IPF between splenectomized and non-splenectomized patients with ITP (Figure 3A), however platelet surface markers of activation with and without agonist stimulation were higher in those ITP patients who had undergone splenectomy than non-splenectomized ITP patients, particularly for activated GPIIb/IIIa (Figure 3B).
Figure 3.
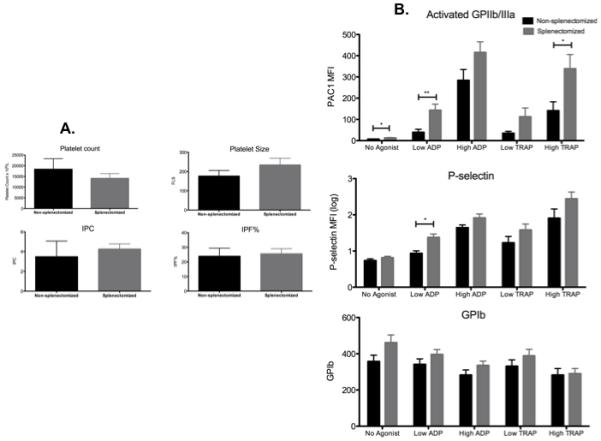
A. Platelet count, platelet size, immature platelet count (IPC) and immature platelet fraction (IPF) in non-splenectomized (black columns) and splenectomized patients (grey columns) with ITP. n=10 for non-splenectomized and n=15 for splenectomized patients.
B. Expression of platelet surface activated GPIIb/IIIa, P-selectin and GPIb in non-splenectomized and splenectomized patients with ITP with and without addition of platelet agonists ex vivo. Mean values + SEM are shown. * indicates P < 0.05; ** indicates P < 0.01.
To investigate whether the lower surface expression of platelet glycoproteins in AML/MDS compared to ITP (Figure 2) was related to the lower proportion of immature platelets in AML/MDS as compared to ITP (Figure 1), we explored the association between IPF and platelet function. Considering both ITP and AML/MDS patients combined, a higher IPF was significantly correlated with increased platelet surface expression on circulating platelets (i.e., no added agonist) of GPIIb/IIIa (P = 0.001), P-selectin (P < 0.01) and GPIb (P < 0.0001) (Figure 4A). The magnitude of change in platelet surface GPIb expression following ex vivo agonist activation was also strongly correlated with IPF, regardless of agonist or agonist concentration (Figure 4B). Similarly, IPF was positively correlated with GPIIb/IIIa expression on high-dose ADP-stimulated platelets (P = 0.0484, data not shown). IPF was not correlated with platelet surface GPIIb/IIIa or P-selectin expression for any of the other agonist conditions (data not shown).
Figure 4.

A. Correlation between markers of activation expressed on the surface of circulating platelets (i.e. with no added agonist) and immature platelet fraction (IPF%). Each dot represents an individual patient (ITP and AML/MDS combined).
B. Immature platelet fraction (IPF%) vs. agonist-induced change in platelet surface GPIb expression for low and high concentrations of ADP and TRAP. The data shown are the magnitude of change in GPIb MFI following agonist stimulation as compared to the level of expression with no added agonist.
There were no major differences in the overall incidence of bleeding between the AML/MDS and ITP patients. However, there was a tendency for non-skin/oral bleeding, i.e. more clinically significant bleeding, to occur more commonly among AML/MDS patients than ITP patients (P=0.118, Table 1). To explore the relationship between platelet function and bleeding, we categorized patients according to types of bleeding symptoms. There were no significant differences in platelet count, IPF or IPC between the 3 bleeding categories: bleeding (any site) vs. no bleeding; any skin/oral bleeding vs. no skin/oral bleeding; any non-skin/oral bleeding vs. no non-skin/oral bleeding. However, for all three bleeding categories, patients with ITP who experienced bleeding had lower platelet reactivity (e.g., lower TRAP-stimulated platelet surface activated GPIIb/IIIa and platelet surface P-selectin) compared to those with ITP who had no bleeding (Figure 5, left column); there was no difference in platelet activation levels with no added agonist. In contrast, the patients with AML/MDS who experienced bleeding had higher platelet activation levels with no added agonist and also higher platelet reactivity (e.g., higher TRAP-stimulated platelet surface activated GPIIb/IIIa and platelet surface P-selectin) than those who had AML/MDS but no bleeding (Figure 5, right column).
Table 1.
| ITP (n=23) | AML/MDS (n=20) | |
|---|---|---|
| Bleeding at any site | 17/23 (74%) | 15/20 (75%) |
| Grade 2 bleeding (any site) | 3/23 (13%) | 5/20 (25%) |
| Skin or oral bleeding | 16/23 (70%) | 14/20 (70%) |
| Non skin/oral bleeding | 2/23 (8%) | 6/20(30%) |
| Description of non skin/oral bleeding events |
Grade 1 GI* (n=1) Grade 1 epistaxis (n=1) |
Grade 2 pulmonary (n=1) Grade 2 GI* (n=1) Grade 1 epistaxis (n=3) Grade 1 urinary (n=1) |
GI - gastrointestinal
Figure 5.

Expression of platelet activation markers with and without ex vivo agonist stimulation in ITP patients (left column) and AML/MDS patients (right column) with vs. without bleeding symptoms. Dark bars: no bleeding (n = 6 for ITP, n = 5 for AML/MDS). Pale bars: bleeding any site (n = 17 for ITP, n = 15 for AML/MDS). * indicates P < 0.05; ** indicates P < 0.01. Abbreviation: PSEL, P-selectin. MFI, mean fluorescence intensity.
DISCUSSION
Platelet count alone does not always predict bleeding in severely thrombocytopenic patients. Although major haemorrhage is more likely at platelet counts below 10,000/μl, significant bleeding may also occur at higher platelet counts and the large majority of patients with even severe thrombocytopenia do not suffer from spontaneous bleeding [3, 4]. Therefore, while severe thrombocytopenia is permissive for bleeding to occur, it is not sufficient.
In a study of patients with haematological malignancy undergoing stem cell transplantation or chemotherapy, bleeding of WHO grade 2 or above was found to occur at a rate of 25% at platelet counts ≤5,000/μl and 17% at platelet counts 20-80,000/μl [4]. In a study of pediatric ITP, while 75% of ICH occurred at platelet counts ≤10,000/μl, 10% of ICH occurred at platelet counts over 20,000/μl [3]. External events, for example trauma or infection, and/or differences in platelet function may contribute to differences in bleeding risk among patients with comparable degrees of thrombocytopenia. Moreover, the types of bleeding may differ as well. Consistent with previous observations [16, 20], in this study bleeding in patients with ITP was characterized by petechiae, ecchymoses, and mouth bleeding whereas patients with AML/MDS more frequently experienced clinically significant GI, GU and pulmonary haemorrhage. A better understanding of platelet function in thrombocytopenia may be one important factor in the evaluation of bleeding risk and, if so, could lead to improved clinical management.
Few studies have examined platelet function in thrombocytopenic individuals. Studies by Karpatkin in 1978 indicated that platelet aggregation was positively correlated with platelet volume in healthy individuals with normal platelet counts, implying that platelet function might be relatively high in ITP patients who have a high proportion of large, young platelets [6]. Other groups have also reported a correlation between platelet size and/or platelet function with bleeding symptoms in thrombocytopenic patients [21, 22]. Examining platelet surface P-selectin expression and platelet aggregation in patients with ITP and healthy controls, Panzer et al. reported that platelet P-selectin expression was higher in patients with ITP than in healthy controls and that this correlated with the size of platelet aggregates formed in vitro although not with bleeding symptoms [14]. Furthermore, baseline platelet surface P-selectin expression ex vivo was found to be inversely correlated with its relative increase after exogenous addition of agonists, suggesting that in vivo activated platelets have reduced capacity for further activation [7]. However, these studies [7, 14] used platelet-rich plasma and standardized the platelet count by dilution, both of which methods introduce the possibility of artifactual in vitro platelet activation. In contrast, in the present study, we examined platelets in whole blood without any separation or dilution of platelets and did not agitate the blood during incubation, thereby minimizing the possibility of artifact.
The main findings of the present study are: 1) compared to patients with ITP with comparable degrees of thrombocytopenia, platelets from patients with AML/MDS were smaller, less immature, and had lower surface expression of GPIb and activated GPIIb/IIIa both with and without addition of the agonists ADP or TRAP; 2) in both ITP and AML/MDS patients, increased platelet surface GPIb on circulating platelets and GPIb and activated GPIIb/IIIa on ex vivo activated platelets were associated with increased IPF; 3) AML/MDS patients with bleeding had higher TRAP-stimulated platelet surface activated GPIIb/IIIa and platelet surface P-selectin than AML/MDS patients with no bleeding, whereas ITP patients with bleeding had lower TRAP-stimulated platelet surface activated GPIIb/IIIa and platelet surface P-selectin than ITP patients with no bleeding.
How may these findings be interpreted? In ITP, platelet production is variable as a result of the effects of autoantibody-mediated inhibition of platelet production [23, 24]. Even when partially inhibited, patients with ITP nevertheless make more platelets than do those with AML/MDS as reflected by a higher IPF and platelet size in ITP than AML/MDS. The activation state of circulating platelets was also higher in ITP than AML/MDS, and ITP platelets also showed higher reactivity in response to the agonists ADP and TRAP. The correlations between IPF and both baseline platelet expression of activation markers and also platelet reactivity in response to agonist stimulation suggest that the younger, larger platelets typically found in greater numbers in ITP have greater function, as was first reported in the 1960’s.[5]
ITP patients with bleeding had lower platelet reactivity in response to agonist than those who had no bleeding, suggesting that platelets from ITP patients with bleeding symptoms have undergone a degree of in vivo activation and therefore have a lower capacity for further ex vivo agonist-stimulated activation. Paradoxically and unexpectedly, patients with AML/MDS who experienced bleeding had significantly higher ex vivo agonist-stimulated platelet reactivity than those who had no bleeding. One interpretation of this finding is that ITP patients with bleeding may have antiplatelet antibodies that interfere with platelet function [25] or else that the platelets are partially activated by the bleeding, while in AML/MDS, although their platelets still have the capacity to respond if stimulated ex vivo, they are not appropriately activated in vivo – suggesting that generation of platelet activators at sites of damaged blood vessels may be impaired or dysregulated. Moreover, other factors in addition to the platelet activation state are necessary to prevent bleeding in severe thrombocytopenia. These findings and hypotheses need to be confirmed and addressed in additional studies.
Platelet surface P-selectin expression is a marker of degranulation and the platelet surface binding of PAC1 (the reporter monoclonal antibody used in the present study) measures the conformational change in platelet surface GPIIb/IIIa that allows fibrinogen to bind and platelet aggregation to occur [11]. These two markers are therefore sensitive indicators of platelet functionality [11]. GPIb is the receptor for von Willebrand factor and platelet surface expression of GPIb decreases upon activation, as the receptor is proteolyzed and/or internalized [26-28]. GPIb expression is strongly related to platelet surface area and therefore to platelet size. As PAC1 binding measures a conformational change in GPIIb-IIIa receptors rather than the absolute number of receptors and P-selectin is not expressed on the surface of the resting platelet [11], the present findings with platelet surface activated GPIIb-IIIa and P-selectin expression in patients with ITP are unlikely to be attributable to differences in platelet size.
Only a moderate difference was observed in bleeding symptoms between the patient groups reflecting the relatively small number of patients with bleeding in this study. In addition to platelet function and count, other factors contribute to bleeding including fever, haemoglobin level, sepsis, uremia, hypoalbuminemia, coagulopathy, leukopenia or leukocytosis, vascular integrity and iatrogenic factors [2]. These factors were not explored in this study
Bleeding in severely thrombocytopenic patients is potentially devastating, and platelets are a commonly transfused blood product that is often in short supply [29]. It has long been postulated that thrombocytopenia due to bone marrow failure carries a higher risk of bleeding than the same degree of thrombocytopenia in ITP, although this hypothesis has never been specifically tested. While flow cytometric assessment of platelet activation is unlikely to be widely available as a routine test in the clinical setting, a better understanding of bleeding risk in thrombocytopenia is likely to have major clinical implications. If bleeding can be anticipated, it may be more feasible to intervene early to ameliorate or prevent it. The present study is the first to examine and define differences in platelet function in patients with equivalent degrees of thrombocytopenia due to ITP or AML/MDS. Further studies need to explore why the sites of bleeding are different in ITP and AML/MDS, how platelet activation/function is regulated in each patient group, for example the role of the endothelial cell, and which type of testing is optimal to study platelet function in each of these patient groups with a view to predicting and preventing clinical bleeding.
Acknowledgements
The authors thank Martin Lesser and Myriam Kline at the Biostatistics Unit, Feinstein Institute, North Shore LUK for assisting with statistical analysis and data interpretation. This study was supported in part by research funding from GSK and Sysmex America Incorporated. B.P. is the recipient of a Kay Kendall Leukaemia Fund travelling fellowship and a Fulbright Scholarship for Cancer Research. JBB receives funding from NIH grant 1U01 HL72196-01-05.
Footnotes
Explanation of Author Contributions
B.P., J.B.B., A.L.F, and A.D.M. participated in study design, sample collection and processing, data analysis and co-wrote the manuscript. B.B. and C.T. recruited patients and participated in data analysis. M.D.L., M.R.B. and Y.L. processed samples and participated in data analysis. E.J.F. participated in study design and recruited patients for the study.
Conflicts of Interest Statement:
JBB currently receives clinical research support from the following companies: Amgen, Cangene, GlaxoSmithKline, Genzyme, IgG of America, Immunomedics, Ligand, Eisai, Inc, Shionogi and Sysmex. JBB’s family owns stock in Amgen and GlaxoSmithKline and he has participated in Advisory Boards and/or consults for Amgen, GlaxoSmithKline, Ligand, Shionogi, Eisai and Portola. Marty Lesser and Myriam Fine from the Biostatistics Unit at Northshore LIJ assisted with the statistical analyses and data interpretation. Their services were funded in part by GlaxoSmithKline. The other authors report no conflicts of interest.
References
- 1.Gaydos LA, Freireich EJ, Mantel N. The quantitative relation between platelet count and hemorrhage in patients with acute leukemia. N Engl J Med. 1962;266:905–9. doi: 10.1056/NEJM196205032661802. [DOI] [PubMed] [Google Scholar]
- 2.Friedmann AM, Sengul H, Lehmann H, Schwartz C, Goodman S. Do basic laboratory tests or clinical observations predict bleeding in thrombocytopenic oncology patients? A reevaluation of prophylactic platelet transfusions. Transfus Med Rev. 2002;16:34–45. doi: 10.1053/tmrv.2002.29403. [DOI] [PubMed] [Google Scholar]
- 3.Psaila B, Petrovic A, Page LK, Menell J, Schonholz M, Bussel JB. Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): study of 40 cases. Blood. 2009;114:4777–83. doi: 10.1182/blood-2009-04-215525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, Gernsheimer TB, Ness PM, Brecher ME, Josephson CD, Konkle BA, Woodson RD, Ortel TL, Hillyer CD, Skerrett DL, McCrae KR, Sloan SR, Uhl L, George JN, Aquino VM, Manno CS, McFarland JG, Hess JR, Leissinger C, Granger S. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362:600–13. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karpatkin S. Heterogeneity of human platelets, II: functional evidence suggestive of young and old platelets. J Clin Invest. 1969;48:1083–6. doi: 10.1172/JCI106064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karpatkin S. Heterogeneity of human platelets. VI. Correlation of platelet function with platelet volume. Blood. 1978;51:307–16. [PubMed] [Google Scholar]
- 7.Panzer S, Hocker L, Rieger M, Vormittag R, Koren D, Dunkler D, Pabinger I. Agonist- inducible platelet activation in chronic idiopathic autoimmune thrombocytopenia. doi: 10.1111/j.1600-0609.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 8.Godeau B, Provan D, Bussel J. Immune thrombocytopenic purpura in adults. Curr Opin Hematol. 2007;14:535–56. doi: 10.1097/MOH.0b013e3282b9748f. [DOI] [PubMed] [Google Scholar]
- 9.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr., Crowther M. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 10.Stanworth SJ, Hyde C, Heddle N, Rebulla P, Brunskill S, Murphy MF. Prophylactic platelet transfusion for haemorrhage after chemotherapy and stem cell transplantation. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD004269.pub2. CD004269. [DOI] [PubMed] [Google Scholar]
- 11.Michelson A, Linden MD, Barnard MR, Furman MI, Frelinger AL. Flow cytometry. In: Michelson A, editor. Platelets. Second Edition edn. Elsevier/Academic Press; San Diego: 2007. pp. 545–63. [Google Scholar]
- 12.Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol. 2007;50:1822–34. doi: 10.1016/j.jacc.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 13.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9:154–69. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 14.Panzer S, Rieger M, Vormittag R, Eichelberger B, Dunkler D, Pabinger I. Platelet function to estimate the bleeding risk in autoimmune thrombocytopenia. Eur J Clin Invest. 2007;37:814–9. doi: 10.1111/j.1365-2362.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- 15.Michelson AD. Evaluation of platelet function by flow cytometry. Pathophysiol Haemost Thromb. 2006;35:67–82. doi: 10.1159/000093547. [DOI] [PubMed] [Google Scholar]
- 16.Page LK, Psaila B, Provan D, Michael Hamilton J, Jenkins JM, Elish AS, Lesser ML, Bussel JB. The immune thrombocytopenic purpura (ITP) bleeding score: assessment of bleeding in patients with ITP. Br J Haematol. 2007;138:245–8. doi: 10.1111/j.1365-2141.2007.06635.x. [DOI] [PubMed] [Google Scholar]
- 17.Kestin AS, Valeri CR, Khuri SF, Loscalzo J, Ellis PA, MacGregor H, Birjiniuk V, Ouimet H, Pasche B, Nelson MJ, et al. The platelet function defect of cardiopulmonary bypass. Blood. 1993;82:107–17. [PubMed] [Google Scholar]
- 18.Ruisi MM, Psaila B, Ward MJ, Villarica G, Bussel JB. Stability of measurement of the immature platelet fraction. Am J Hematol. 2010;85:622–4. doi: 10.1002/ajh.21748. [DOI] [PubMed] [Google Scholar]
- 19.Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–14. [PubMed] [Google Scholar]
- 20.Bolton-Maggs P. Severe bleeding in idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol. 2003;25(Suppl 1):S47–51. doi: 10.1097/00043426-200312001-00011. [DOI] [PubMed] [Google Scholar]
- 21.Eldor A, Avitzour M, Or R, Hanna R, Penchas S. Prediction of haemorrhagic diathesis in thrombocytopenia by mean platelet volume. Br Med J (Clin Res Ed) 1982;285:397–400. doi: 10.1136/bmj.285.6339.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenet G, Lubetsky A, Shenkman B, Tamarin I, Dardik R, Rechavi G, Barzilai A, Martinowitz U, Savion N, Varon D. Cone and platelet analyser (CPA): a new test for the prediction of bleeding among thrombocytopenic patients. Br J Haematol. 1998;101:255–9. doi: 10.1046/j.1365-2141.1998.00690.x. [DOI] [PubMed] [Google Scholar]
- 23.Chang M, Nakagawa PA, Williams SA, Schwartz MR, Imfeld KL, Buzby JS, Nugent DJ. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102:887–95. doi: 10.1182/blood-2002-05-1475. [DOI] [PubMed] [Google Scholar]
- 24.McMillan R, Wang L, Tomer A, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. 2004;103:1364–9. doi: 10.1182/blood-2003-08-2672. [DOI] [PubMed] [Google Scholar]
- 25.Niessner H, Clemetson KJ, Panzer S, Mueller-Eckhardt C, Santoso S, Bettelheim P. Acquired thrombasthenia due to GPIIb/IIIa-specific platelet autoantibodies. Blood. 1986;68:571–6. [PubMed] [Google Scholar]
- 26.Han Y, Nurden A, Combrie R, Pasquet JM. Redistribution of glycoprotein Ib within platelets in response to protease-activated receptors 1 and 4: roles of cytoskeleton and calcium. J Thromb Haemost. 2003;1:2206–15. doi: 10.1046/j.1538-7836.2003.00436.x. [DOI] [PubMed] [Google Scholar]
- 27.Michelson AD, Ellis PA, Barnard MR, Matic GB, Viles AF, Kestin AS. Downregulation of the platelet surface glycoprotein Ib-IX complex in whole blood stimulated by thrombin, adenosine diphosphate, or an in vivo wound. Blood. 1991;77:770–9. [PubMed] [Google Scholar]
- 28.LaRosa CA, Rohrer MJ, Benoit SE, Barnard MR, Michelson AD. Neutrophil cathepsin G modulates the platelet surface expression of the glycoprotein (GP) Ib-IX complex by proteolysis of the von Willebrand factor binding site on GPIb alpha and by a cytoskeletal-mediated redistribution of the remainder of the complex. Blood. 1994;84:158–68. [PubMed] [Google Scholar]
- 29.Stanworth SJ, Dyer C, Choo L, Bakrania L, Copplestone A, Llewelyn C, Norfolk D, Powter G, Littlewood T, Wood EM, Murphy MF. Do all patients with hematologic malignancies and severe thrombocytopenia need prophylactic platelet transfusions? Background, rationale, and design of a clinical trial (trial of platelet prophylaxis) to assess the effectiveness of prophylactic platelet transfusions. Transfus Med Rev. 2010;24:163–71. doi: 10.1016/j.tmrv.2009.11.002. [DOI] [PubMed] [Google Scholar]


