Abstract
The regeneration of lost periodontal ligament (PDL) and alveolar bone is the purpose of periodontal tissue engineering. The goal of the present study was to assess the suitability of 3 odontogenic progenitor populations from dental pulp, PDL, and dental follicle for periodontal regeneration when exposed to natural and synthetic apatite surface topographies. We demonstrated that PDL progenitors featured higher levels of periostin and scleraxis expression, increased adipogenic and osteogenic differentiation potential, and pronounced elongated cell shapes on barren root chips when compared with dental pulp and dental follicle cells. When evaluating the effect of surface characteristics on PDL progenitors, natural root surfaces resulted in elongated PDL cell shapes, whereas PDL progenitors on synthetic apatite surfaces were rounded or polygonal. In addition, surface coatings affected PDL progenitor gene expression profiles: collagen I coatings enhanced alkaline phosphatase and osteocalcin expression levels and laminin-1 coatings increased epidermal growth factor (EGF), nestin, cadherin 1, and keratin 8 expression. PDL progenitors seeded on natural tooth root surfaces in organ culture formed new periodontal fibers after 3 weeks of culture. Finally, replantation of PDL progenitor-seeded tooth roots into rat alveolar bone sockets resulted in the complete formation of a new PDL and stable reattachment of teeth over a 6-month period. Together, these findings indicate that periodontal progenitor cell type as well as mineral surface topography and molecular environment play crucial roles in the regeneration of true periodontal anchorage.
Introduction
Tissue engineering aims to reconstruct natural tissues through a combination of (i) progenitor and/or stem cells, (ii) biomaterials as scaffold substances, and (iii) growth factors to modulate cell adhesion, proliferation, migration, and differentiation [1]. Although theoretically any type of stem cells, matrices, or factors might be suitable for the regeneration of any type of tissue, recent studies have emphasized the benefits of natural extracellular matrices (ECMs) and tissue-specific progenitors for tissue engineering purposes [2]. Examples for the instructive abilities of natural, tissue-specific ECMs include successful cardiac muscle regeneration using a decellularized heart [3], trachea regeneration using cadaveric trachea and stem cell population [4], and induction of lung-specific lineages by acellular natural lung matrix [5]. Tissue-specific matrices are primed to induce tissue-specific lineage differentiation, whereas tissue-specific progenitor cells are ideally suited for the regeneration of their respective target tissues. For example, it has been shown that only periodontal ligament (PDL), but not gingival connective tissue or bone, contains cells capable of establishing new attachment fibers between cementum and bone [6–8]. Following the concept of the tissue-specific progenitor, periodontal progenitor cells thus emerge as natural first-choice progenitor cells for periodontal tissue engineering and reattachment of teeth.
The idea of following nature's strategies to regenerate periodontal tissues goes back to Bernhard Gottlieb, who almost 6 decades ago suggested that “if these ideas about the biology of the cementum are correct, it is then our task to find out just how nature provides for continuous cementum deposition, and having done so, to imitate the procedure” [9]. Another milestone in the science of periodontal regeneration was the work of Tony Melcher, who proposed that the PDL contains the progenitors for the regeneration of all 3 tissues, PDL, alveolar bone, and root cementum [10]. He believed that although the PDL had lesser regenerative qualities than alveolar bone, the seemless integration of mineralized and soft tissues would be promoted by using ligament cells as progenitors, a strategy that would be further facilitated by the lack of a periostal covering of alveolar bone and the potential for seemless integration [10]. Melcher's work established the biological foundation for the pioneering studies of Sture Nyman, Thorkild Karring, Jan Gottlow, and Jan Lindhe related to guided tissue regeneration [11]. In these studies, a Millipore filter was used to prevent the gingival tissue from contact with the root surface and to allow the PDL cells to reestablish connective tissue attachment [11].
The present study takes advantage of the instructive capacities of the natural tooth root surface topography in combination with lineage-specific periodontal progenitors to engineer a physiological PDL for the reattachment of teeth. As a first step, we have compared key ECM gene expression profiles and multipotency between odontogenic progenitors and tested the effect of ECM proteins on progenitor differentiation. We have then examined the instructive capacity of periodontal mineralized tissue topographies for tissue-specific progenitor cell differentiation and compared 2 different periodontal progenitors from dental follicle (DF) and PDL. Results of this study are a testimony to the instructive capacities of native hydroxyapatite (HAP) surface topographies and the ideal suitability of periodontal progenitors for periodontal regeneration.
Materials and Methods
Isolation and culture of DF, PDL, dental pulp, and bone marrow-derived mesenchymal stem cells
Isolation of DF and PDL cells
DF tissues from developing mouse molar tooth organs of 4-day-old postnatal CD-1 mice (Charles River Laboratories) were dissected using a stereo microscope. The DF forms a loose connective tissue unit around the developing tooth germ that appears translucent compared with the mineralized enamel organ and can be carefully separated. PDL attached to the roots of the erupted first molars of 4-week-old CD-1 mice was scraped off using scalpels. Both DF and PDL tissues were digested in 3 mg/mL of collagenase–dispase (Roche Applied Science) with gentle rotation at 37°C for 1 h. Primary cells released were washed twice with phosphate-buffered saline (PBS) and passed through a 70-μm strainer to obtain single cells. Cells were then plated at a density of 1×105 cells/100-mm dish containing Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic–antimycotic. Cells with colony-forming ability from both populations were used in subsequent experiments.
Isolation of dental pulp cells
For isolation of dental pulp (DP) cells, erupted third molars from 2-month-old CD-1 mice were collected. Cell isolation and culture were carried out as previously described [12] with some modifications. The pulp chamber was exposed using dental fissure burs to cut around the cementum–enamel junction. The pulp tissue was isolated from the crown and the roots and then digested in DMEM containing 3 mg/mL of collagenase/dispase for 1 h at 37°C. Released cells were washed twice with DMEM and single cells were obtained by passing the cells through a 70-μm strainer. Cells were plated at a density of 1×105 cells per 100-mm dish and cultured in DMEM supplemented with 10% FBS, 2 mM l-glutamine, and 1% antibiotic–antimycotic in a 95% air, 5% CO2, 37°C humidified incubator. Colony-forming cells from these DP cultures were used in subsequent experiments.
Isolation of bone marrow-derived mesenchymal stem cells
Mouse bone marrow-derived mesenchymal stem cells (BM-MSCs) were harvested from 2-month-old male CD-1 mice (Jackson Laboratory) as previously described [13,14]. After gaining access to the marrow cavities of tibia and femur, the BM plugs were flushed out using a 20-gauge needle on a 10-mL syringe with DMEM (low glucose) supplemented with 10% FBS and 1% antibiotic–antimycotic. Marrow samples were collected and mechanically disrupted by suction and release through a 25-gauge needle and syringe. The subsequent cell suspension was centrifuged for 10 min at 1,000 rpm and pelleted cells were resuspended in media. To count cells, a small volume of the cell suspension was mixed with 4% acetic acid to lyse red blood cells and nucleated cells were counted using hemocytometer. Cells were then plated at a density of 5×107 cells per 100-mm dish and incubated in 95% air and 5% CO2 at 37°C, with fresh media changes every 3 days. After 2 weeks in culture, large colonies formed and resulted in 80% confluence. The cells were washed with PBS, trypsinized, and subcultured at a density of 5–7×105 cells per 100-mm dish. This plastic-adherent colony-forming subpopulation of cells derived from murine BM is multipotential in nature as shown in subsequent experiments. The cell plating density was based on previous studies [15,16], resulting in efficient cell proliferation at 5×107 cells per 100-mm dish for BM-derived stromal cells and 5×105 cells per 100-mm dish for DF, PDL, and DP. All primary cells were maintained in culture conditions as described by previous studies and gene expression profiles were characterized from third-passage cells as previously described [17–19]. All animal experiments received approval from the Institutional Animal Care Committee at University of Illinois, Chicago.
In vitro culture of PDL cells on collagen type I– and laminin-1–coated plates
PDL cells were plated at a density of 3,500 cells/cm2 on collagen type I-coated plates (Beckton Dickinson) or laminin-1–coated plates (5 μg/cm2) and cultured in vitro for 1 week. Both collagen type I and laminin-1 were also added as a supplement in respective cultures at a concentration of 50 μg/mL. Cell cultures on noncoated tissue culture plates served as controls.
Semiquantitative reverse transcriptase–polymerase chain reaction
Total RNA was extracted from DF, PDL, DP, and MSCs that were cultured for 3 passages. Alternatively, total RNA was also extracted from third-passage PDL cells cultured on collagen type I-coated plates and on laminin-1–coated plates for 1 week using TRIZOL reagent (Invitrogen). Quality and quantity of the RNA was tested using a NanoDrop spectrophotometer and agarose gel electrophoresis. Two micrograms of the total RNA was reverse transcribed using the sprint reverse transcriptase (RT) complete-double preprimed kit (Clontech) and the cDNA was amplified using selected primers (Table 1) and polymerase chain reaction (PCR). RT-PCR products were then confirmed for their product size by running them through an agarose gel electrophoresis alongside a DNA ladder and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. Semiquantitative RT-PCR experiments were done in triplicates for each cell type and each culture condition.
Table 1.
Primer Sequences for Semi-Quantitative RT-PCR
| Gene | Orientation | Sequence | Product size (bp) |
|---|---|---|---|
| KRT 8 | S | GCGCTGTCCCACCGTCTAGAAGC | 196 |
| AS | GCTGGAAGAGCTGATGCGGGC | ||
| CDH1 | S | TCACGCGCGCTGAGATGGAC | 279 |
| AS | CGCTGGCCCCATGGGTTAGC | ||
| EGF | S | CCTGCAGCTCGGGTCAGTGC | 164 |
| AS | TCTCCGGCCCCACAGGTCTG | ||
| NES | S | GGAAGAGGGCCCGGAGAGGG | 309 |
| AS | TCGAGGGGCTCAGCAGGGAC | ||
| OPN | S | ATGTCCCCAACGGCCGAGGT | 176 |
| AS | GCTCAGAAGCTGGGCAACAGGG | ||
| GAPDH | S | GACGGCCGCATCTTCTTGTGC | 317 |
| AS | AGCACCGGCCTCACCCCATT | ||
| POSTN | S | AAAGGCTGCCCCGCAGTGAT | 157 |
| AS | AAGCCTCGTTACTCGGCGCG | ||
| SCX | S | ACGGGCAACCATGCCACTCG | 173 |
| AS | ACGGTCTTTGCTCAACTTTCTCTGG | ||
| COL1A1 | S | GGTGAACGTGGTGCTCCCGG | 304 |
| AS | CAGGACCAACAGCGCCAGGG | ||
| ALPL | S | CCACTCGGGTGAACCACGCC | 169 |
| AS | CCGCCACCCATGATCACGTCG | ||
| DSPP | S | CTGGGCCATTCCGGTTCCCCA | 429 |
| AS | CCAGCCTGTCCGTGGACACTG | ||
| OCN | S | CCTCACAGATGCCAAGCCCAGC | 101 |
| AS | ACCCAAGGTAGCGCCGGAGT | ||
| RUNX2 | S | GGCCACTTCGCTAACTTGTGGC | 440 |
| AS | GGCTACAACCTTGAAGGCCACGG |
S, sense; AS, anti sense.
Osteogenic and adipogenic differentiation of DF, PDL, DP, and MSCs
To differentiate DF, PDL, DP, and MSCs toward an osteogenic lineage, cells were seeded at a density of 3,500 cells/cm2 and cultured in differentiation basal medium–osteogenic medium (Lonza) and maintained for 21 days. Alizarin Red-S staining was used to assess the osteogenic mineralization nodules after 21 days of treatment. To differentiate DF, PDL, DP, and MSCs toward an adipogenic lineage, respective cells, approximately 2.5×105, were plated into each well of a 6-well plate. Starting at confluence, cells were subjected to 3 cycles of adipogenic induction/maintenance. Each cycle involved treating the cells with adipogenic induction medium for 3 days followed by adipogenic maintenance medium for the next 3 days. At the end of 21 days of culture, mature adipocytes were detected by Oil Red-O staining. Both osteogenic and adipogenic differentiation media were purchased from Lonza.
In vitro culture of DF, PDL, DP, and MC3T3 cells on root cementum chips
To observe the attachment behavior of odontogenic cells to microstructured root cementum, about 1×106 DF, PDL, DP, and nonodontogenic preosteoblast MC3T3 cells along with root cementum chips (1 mm thick) were suspended in 1 mL of DMEM, respectively, and subjected to end-to-end rotation in an eppendorf tube that was rotated at 60 rotations/min for 2 h at 37°C. Root cementum chips were prepared by chipping off the cementum from the surface of lyophilized bovine teeth from 6-month-old steers. Chips were cut into 2×2×1 mm, sterilized in 70% ethanol for 30 min, washed 3 times with ultrapure water, air dried in a fume hood, and stored in a sterile container until used. After washing out unattached cells on cementum chips with PBS, cementum chip–cell constructs were cultured for 3 days and cell attachment pattern on and around the chips was observed by staining the cells with 50% paragon epoxy. Images were then captured using phase-contrast microscopy.
In vitro culture of DF and PDL cells on HAP blocks and root cementum surface
DF and PDL cells (1×106 cells in 200 μL DMEM) were seeded on EDTA-etched rat first molars or HAP blocks (3 mm3) that were a generous gift from Biomatlante SARL. After 2 h of incubation at 37°C, nonattached cells were washed off with 1×PBS and the cell–material constructs were cultured in vitro for 2 weeks. At the end of 2 weeks, the constructs were fixed, decalcified, and processed for paraffin embedding. Subsequently, 5-μm-thick sections were cut and stained with hematoxylin and eosin.
In vitro organ culture of PDL cells on root cementum surface
PDL, MSCs, DF, and DP cells (1×106 cells in 200 μL DMEM) were seeded on EDTA-etched mouse first molars and incubated for 2 h. Briefly, denuded mouse first molars from 4-week-old CD-1 mice were treated with 5% EDTA solution (pH 7.4) for 5 min (helps in surface demineralization and exposure of organic matrix), washed extensively with distilled water, and fixed in 70% ethanol. Teeth were then washed in DNAse/RNAse-free water for 4 h with 3 changes to fresh water and air dried in a sterile hood prior to being subjected to cell seeding. After washing out nonattached cells, the cell-seeded molars were cultured in vitro in an organ culture system in DMEM supplemented with 10% FBS and 1% antibiotic–antimycotic for 1 or 3 weeks in 95% air and 5% CO2 at 37°C. At the end of 1 week, PDL cells attached on the tooth surfaces were labeled with fluorescent dye DiI (Molecular Probes) according to the manufacturer's protocol. At the end of 3 weeks, the cell–tooth root constructs were fixed, decalcified, and processed for paraffin embedding. Subsequently, 5-μm-thick sections were cut and stained with Masson's trichrome stain (Sigma-Aldrich) according to manufacturer's protocol.
First maxillary molar extraction and subsequent replantation for 6 months
Four athymic nude rats (∼275 g, body weight) were fed powdered rat chow containing 0.4% beta aminopropionitrile (Sigma-Aldrich) for 2 days to reduce the tensile strength of collagen and to promote gentle tooth extraction with minimum impairment of the surrounding periodontal tissues [20]. Under anesthesia with ketamine (100 mg/kg)/xylazine (5 mg/kg), first maxillary molars were extracted using forceps and subjected to collagenase/dispase treatment to digest the attached PDL fibers and cells. The denuded teeth were then treated with 5% EDTA solution (pH 7.4) for 10 min (aids surface demineralization and exposure of organic matrix), washed extensively with distilled water, and fixed in 70% ethanol overnight. Tooth samples were then washed in DNAse/RNAse-free water for 4 h with 3 changes to fresh water and air dried in a sterile hood to prepare for cell seeding. Immediately after extraction, the sites were cleaned with surgical dental burs, plugged with a collagen sponge, and allowed to heal until replantation. After 4 days of healing, the extraction sites were reopened and cleaned with a dental bur under constant irrigation to promote easy reentry of the extracted maxillary molars. Molars used for replantation were either seeded with PDL cells and cultured for 3 days or left untreated. Once the tooth was replanted back in its socket, a thin coat of glass ionomer dental restorative was used to stabilize it with the adjacent second molar to maintain physiologic occlusion with the corresponding mandibular molar. Of the 8 molars that were replanted in their respective tooth socket, 4 were seeded with PDL cells prior to replantation and the rest were cell-free replants. Rat maxillae were harvested after 6 months. All animal procedures were approved by the Institutional and Animal Care and Use Committee (IACUC) at University of Illinois, Chicago, and all of the committee's guidelines were complied with.
Results
Odontogenic progenitors are distinguished by unique gene expression profiles and differentiation potential
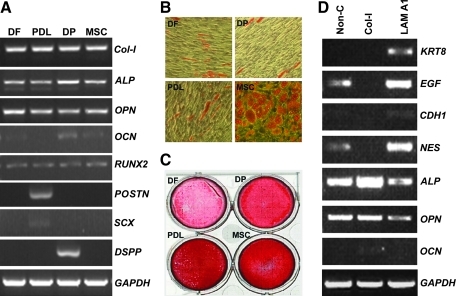
We hypothesized that odontogenic progenitors were characterized by unique gene expression profiles and various degrees of multipotency. To test our hypothesis, major ECM gene expression patterns were analyzed using RT-PCR and adipogenic and osteogenic differentiation potentials were compared (Fig. 1A–C). Our analysis indicated that collagen I, alkaline phosphatase, osteopontin, and runt-related transcription factor 2 (runx2) were expressed at similar levels in DF cells, PDL progenitors, DP progenitors, and MSCs. In contrast, osteocalcin (OCN) was preferentially expressed in DP and MSCs, periostin (POSTN) and scleraxis (SCX) only in PDL, and dentin sialophosphoprotein (DSPP) only in DP. Although all 3 odontogenic progenitors demonstrated adipogenic differentiation potential, the overall level of Oil Red stain was highest in MSCs, followed by PDL, and the adipogenic differentiation potential was less pronounced in DF and DP. A similar trend was observed when analyzing osteogenic differentiation potential using Alizarin Red stain, with highest Alizarin Red levels in MSCs and PDL, followed by DP, and DF cells only displaying weak Alizarin Red levels.
FIG. 1.
Differences in gene expression and adipogenic and osteogenic differentiation between 3 odontogenic progenitors, DF, DP, and PDL, compared with MSCs. (A) Differences in gene expression between DF, PDL, DP, and MSCs as revealed by reverse transcriptase–polymerase chain reaction (RT-PCR). Note the elevation of POSTN and SCX in periodontal progenitors and high levels of DSPP and OCN in DP progenitors. DP, dental pulp; PDL, periodontal ligament; DF, dental follicle; MSCs, mesenchymal stem cells; OCN, osteocalcin; Col-I, collagen I; ALP, alkaline phosphatase; OPN, osteopontin; RUNX2, runt-related transcription factor 2; POSTN, periostin; SCX, scleraxis; DSPP, dentin sialophosphoprotein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (B) Adipogenic differentiation of odontogenic progenitors compared with MSCs as revealed by Oil Red-O staining for detection of lipids. Note the presence of numerous high-density lipid vacuoles in MSCs and some lipid vacuoles in PDL progenitors. (C) Osteogenic differentiation of odontogenic progenitors compared with MSCs as revealed by Alizarin Red-S staining for detection of mineral nodules. PDL and MSCs demonstrated the highest levels of mineralized nodules, followed by DP progenitors. (D) Effect of extracellular matrix surface coating on PDL progenitor gene expression.
ECM proteins affect gene expression of periodontal progenitors
In a follow-up experiment, we asked the question how various ECM surface coatings affected gene expression in PDL progenitors. For this purpose, culture dishes were coated with either laminin-1 or collagen I or not coated (Fig. 1D). Our study indicated that collagen I coating of culture dishes substantially reduced epidermal growth factor (EGF) and nestin expression levels in PDL progenitors, whereas alkaline phosphatase and OCN levels were increased. In contrast, surface coating with laminin-1 resulted in an increase of EGF, nestin, cadherin 1, and keratin 8.
Apatite surface structure affects odontogenic progenitor cell shape and orientation
Here, we asked the question whether odontogenic progenitors reacted differently to structured tooth root surfaces. To determine progenitor cell behavior in relationship to surfaces, DP, DF, PDL, and MC3T3 cells were cultured with barren root cementum chips, and cell shape and orientation were determined, either adjacent to chips (Fig. 2A–D) or on the surface of chips (Fig. 2E–H). Our study demonstrated that DP, DF, and MC3T3 cells were either rounded or polygonal (Fig. 2A, B, D, E, G, H), whereas PDL progenitors were stretched and elongated (Fig. 2D, H), both adjacent to (Fig. 2D) and on the surface of cementum chips (Fig. 2H). We then decided to compare the behavior of PDL progenitors on synthetic apatite blocks (Fig. 3B) with that on natural root surfaces (Fig. 3C). PDL progenitors tightly attached to both synthetic apatite (Fig. 3B) and natural root surfaces (Fig. 3C). Elongated and perpendicular fibers resembling PDL were only detected on natural root surfaces (Fig. 3C), whereas PDL progenitors formed a layer of rounded and polygonal cells surrounding synthetic apatite blocks (Fig. 3B). In this set of experiments, DF cells were used as a control. DF cells attached to synthetic apatite blocks with their secreted ECM fibers aligned parallel to the apatite block surface (Fig. 3A).
FIG. 2.
Effect of surface topography on odontogenic progenitor cell shape. (A–D) The effect of EDTA-etched root cementum particle surface structure on progenitor cell polarization in close proximity. (E–H) The behavior of odontogenic progenitors on EDTA-etched root cementum chips. Note elongated cell shapes of PDL progenitors on apatite surfaces compared with DP, mouse calvaria preosteoblast (MC3T3), and DF cells. Scale bars=250 μm (A–D), 500 μm (E–H).
FIG. 3.
Effect of apatite surface structure on periodontal progenitor cell shape in vitro. The arrangement of DF cells (A) and PDL progenitors (B) on apatite blocks. (C) PDL progenitor shape and fiber orientation (fib) on the surface of rat molar root chips. Scale bar=100 μm (A–C). df, dental follicle; HAP, hydroxyapatite; pdl, PDL progenitors.
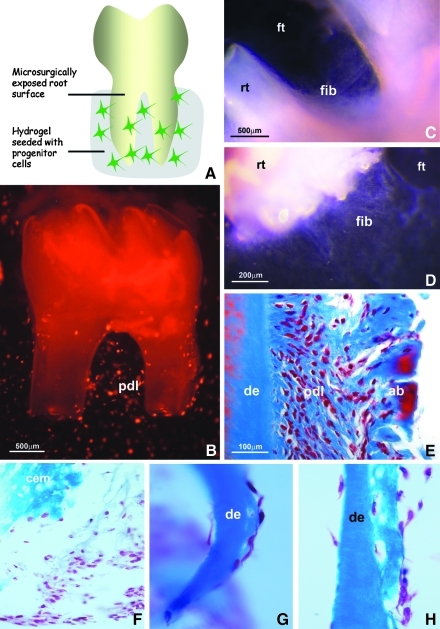
Only PDL progenitors but not DF, DP, and MSCs formed new PDL-like fibrous tissues with parallel fibers aligned perpendicular to the root surface in vitro
To determine whether PDL progenitors had the capacity to regenerate periodontal tissues, mouse molar teeth were extracted from 4-week-old mice, and attachment tissues were enzymatically removed from root surfaces. Barren root surfaces were incubated with collagen gels seeded with DiI-labeled PDL progenitors for 3 weeks (Fig. 4A). After 1 week, PDL progenitor cells surrounded the root surface (Fig. 4B) and fibrous tissues had formed between root surface and the filter disc on which the teeth were cultured (Fig. 4C, D). Histological analysis after 3 weeks of culture documented formation of new PDL-like fibrous tissues with fibers aligned parallel to each other and perpendicular to the root surface (Fig. 4E). However, DF, DP, and MSCs in collagen gels cultured on barren root surfaces for 3 weeks under identical conditions did not form fibers perpendicular to the root surface. Instead, extended stretches of root surface cultured together with DF, DP, and MSCs were devoid of cells after 3 weeks, and if cells did attach, they formed only a thin monolayer aligned parallel to the root surface, and the dense ECM typical for PDL regenerates did not form at all (Fig. 4F–H).
FIG. 4.
PDL progenitor cell growth on tooth root surfaces in organ culture. (A) Periodontal root organ culture model. Mouse molars were extracted, covered with PDL progenitors suspended in collagen gel, and cultured for 1 or 3 weeks in vitro. (B) DiI-labeled PDL progenitors (pdl) after 1 week of culture. (C, D) Newly formed fibers (fib) on barren root surfaces (rt) after 1 week of culture on nitrocellulose filter disks (ft). (E) Histology of newly formed periodontal tissues in proximity to cultured barren root surfaces after 3 weeks in vitro. (F–H) Histology of cells in proximity to the root surface after 3 weeks of culture. Progenitor cells were either from DF (F), DP (G), or MSCs (H). Adjacent root surface (cem/de) is marked for orientation. de, root dentin; cem, cementum; pdl, newly formed periodontal ligament; ab, alveolar bone; rt, root; ft, nitrocellulose filter surface. Magnification is indicated on individual micrographs.
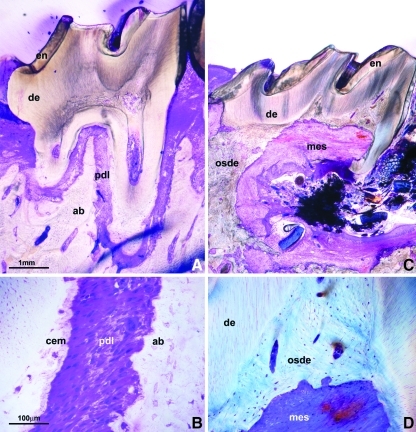
Complete formation of a new PDL following implantation of progenitor-seeded first rat molars after 6 months
To verify whether periodontal progenitors regenerate periodontal tissues on surface-exposed root surfaces in vivo, surface cleaned, EDTA-etched, PDL progenitor-preseeded rat molars were replanted into rat molar tooth sockets, where they were kept without further treatment for 6 months. Control teeth were replanted into alveolar bone sockets without PDL progenitor seeding. Histological analysis after 6 weeks revealed formation of a complete new PDL in progenitor-treated molars (Fig. 5A, B), whereas control molars exhibited extensive resorption and osteodentin formation (Fig. 5C, D). Periodontal progenitor-preseeded and -replanted rat molars were attached to the surrounding alveolar bone by a physiological PDL featuring polarized and parallel-oriented PDL fibers (Fig. 5B). Overall, all 4 teeth replanted with preseeded PDL cells maintained their physiological occlusions and did not show any signs of resorption. Of the 4 cell-free implants, 2 were lost and the remaining 2 were resorbed in some areas and ankylosed in the others.
FIG. 5.
Comparison between PDL progenitor-preseeded and -replanted rat first molars and nontreated controls after 6 months of implantation in nude rats. (A) A sagittal cross-section through the implanted molar and the adjacent jaw bone. The newly formed PDL is highlighted in red color by the Paragon-Epoxy stain on ground sections. (B) High-magnification view of the engineered PDL, revealing parallel oriented Sharpey's fibers between root surface (cem) and alveolar bone (ab). (C) Replanted rat molar without prior pretreatment after 6 months of implantation in nude rats. Note massive resorption, obliteration, and osteodentin formation in the area of the anatomical tooth root. (D) A high-magnification micrograph showing the osteodentin (osde) and invasive connective tissue (mes) formed in the DP region. en, enamel; de, dentin; pdl, periodontal ligament; ab, alveolar bone; cem, cementum; osde, osteodentin; mes, invasive connective tissue. Scale bars=1 mm (A, C), 100 μm (B, D).
Discussion
During periodontal disease, the entire periodontal attachment apparatus that surrounds the tooth root is destroyed by inflammation. Thus, periodontal regeneration encompasses the restoration of the entire periodontium consisting of alveolar bone, PDL, and root surface cementum, and not just of a singular tissue. In the present study, we have focused on the interface between periodontal progenitors and mineralized tissues as a unique environment to study reciprocal interactions between periodontal tissue components and take advantage of the instructive capacities of highly specialized mineralized tissue topographies for PDL regeneration. Consequently, we have used the periodontal mineralized tissue/soft tissue interface to study the cellular and topographic aspects necessary for complete periodontal regeneration by varying surface topography and by testing the suitability of 3 different odontogenic progenitor populations from DF, PDL, and DP.
The somatic progenitors and stem cells of the odontogenic region are typical mesenchymal cells and appear fairly similar from a morphological point of view. Thus, in light of adult stem cell plasticity, any of them might be a suitable candidate for periodontal tissue engineering. However, in earlier studies, we have demonstrated that even the seemingly homogeneous DF contains several vastly heterogeneous progenitor populations [16]. A thorough system biological analysis of all 4 periodontal progenitors, including alveolar bone osteoblasts, cementoblasts, PDL cells, and DF cells, revealed substantial differences between all 4, with greatest differences between DF progenitors and cementoblasts [21]. Our present findings indicate that PDL progenitors are significantly different from DP and DF cells in terms of gene expression, differentiation potential, and especially in their ability to assume elongated cell shapes on structured surfaces, supporting previous findings from our group that indicate substantial differences between periodontal progenitor populations [16,21–23]. Together, these studies indicate that the progenitor lineage derived from the target tissue may be the most suitable progenitor population for many tissue engineering applications.
In the present study, we have demonstrated that apatite surface structure dramatically affects odontogenic progenitor cell shape and orientation, with PDL progenitors elongating in a perpendicular fashion on natural root surfaces, whereas they were rounded on synthetic apatites. Substantial effects of substrate surface topography on cell shape, adhesion, and cell behavior have been reported in earlier studies [24–26]. The present findings indicate that the natural root surface topography appears to present a unique trigger for perpendicular cell elongation, ideally suited for periodontal fiber regeneration. As a substrate for tooth attachment regeneration, the instructive properties of the root surface are also suitable for therapeutic application, as natural tooth surfaces from extracted teeth are readily available for replantation.
Our data indicate that not only surface topography but also ECM composition affects periodontal progenitor gene expression. In our study, collagen I coatings enhanced alkaline phosphatase and OCN expression levels, triggering increased osteogenic behavior, whereas laminin-1 coatings increased EGF, nestin, cadherin 1, and keratin 8 expression, directing cells toward neurogenic cell behavior. These findings confirm previous data on the effect of surface coatings on cell function [27–29] and suggest that surface coatings may be used to further enhance the regenerative potential of periodontal progenitors.
Among the genes profiled for expression in PDL, DF, DP, and MSCs, POSTN and SCX were uniquely expressed in PDL progenitors. POSTN is a critical ECM protein important for periodontal homeostasis and PDL space maintenance [30]. Its expression changes dynamically in response to tension and compression in the PDL, and loss of POSTN resulted in traumatic dental-alveolar stimuli and a phenotype resembling an early onset of periodontal disease [30,31]. POSTN may play an important role in the characteristic response of PDL cells to mechanical and surface stimuli. SCX is a transcription factor expressed in the tendon progenitor population and is continuously expressed during their differentiation into mature tendons [32]. Tendons are similar to the PDLs, in that both tissues form an unmineralized, load bearing attachment. In case of tendons the attachment is between a muscle and a mineralized tissue (bone) while PDLS form attachment between two mineralized tissues (bone and cementum). Similar to POSTN, SCX expression changes dynamically in the PDL and was shown to be enhanced on the tension side following application of orthodontic stress [33]. Both of these molecules might contribute to the unique cellular response of periodontal progenitors to surfaces.
Reattachment of tooth roots with complete new PDL formation is one of the highlights of the present study. Previously, we have demonstrated using GFP labeling that the PDL engineered using our preseeding approach is derived from cultured periodontal progenitors and not from surrounding tissues [34]. In that study, we also documented that replanted teeth were mechanically stable after 4 months of implantation [34]. Although a number of groups have attempted to promote reattachment of teeth [35,36], regeneration of a fibrous PDL has been rarely accomplished. The ability of periodontal progenitors in conjunction with natural root surface topographies to form true periodontal tissues is likely a result of a stepwise predifferentiation of periodontal progenitors beyond the less-differentiated state of intermediary pluripotent progenitors such as the DF [22] in concert with the instructive capacities of the natural tooth root surface [34].
One of the intriguing findings of the organ culture study was the formation of a significant amount of alveolar bone directly on the culture membrane. Alveolar bone was formed only by PDL progenitors and not by DF, DP, and MSCs. In the present study, alveolar bone was formed as part of an interface between root surface, new cementum, PDL, alveolar bone, and, surprisingly, nitrocellulose filter membrane. This finding suggests that PDL progenitors might be programmed to form mineralized attachment tissues on both ends of the ligament: the root surface and the alveolar bone surface, here replaced by the culture membrane. In addition, this study suggests that the culture environment provides sufficient mechanical clues for the PDL progenitors to thrive and to secrete minerals at attachment sites.
Our data also suggest that of the 4 types of progenitors/stem cells tested, only periodontal progenitors have the capability to form elongated shapes on root cementum surfaces. We suggest that these elongated cell shapes may be a prerequisite for the formation of relatively long and extremely strong PDL fibers, such as Sharpey's fibers, explaining the success of our replantation studies. Future studies will uncover the signaling factors and differentiation events responsible for periodontal progenitors to form elongated and parallel periodontal attachment fibers. Such information would facilitate the use of nonperiodontal progenitors and stem cells for periodontal regeneration.
Acknowledgments
This study was generously supported by the National Institute for Dental Research Grants DE15425 (to T.G.H.D.) and DE019463 (to X.L.). T.G.H.D. expresses his gratitude for the mentorship of Dr. Lavin Flores-de-Jacoby, Philipps-University of Marburg, Germany, who first introduced him to the concept of periodontal progenitors and periodontal regeneration.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Langer R. Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Traphagen S. Yelick PC. Reclaiming a natural beauty: whole-organ engineering with natural extracellular materials. Regen Med. 2009;4:747–758. doi: 10.2217/rme.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott HC. Matthiesen TS. Goh SK. Black LD. Kren SM. Netoff TI. Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 4.Macchiarini P. Jungebluth P. Go T. Asnaghi MA. Rees LE. Cogan TA. Dodson A. Martorell J. Bellini S. Parnigotto PP. Dickinson SC. Hollander AP. Mantero S. Conconi MT. Birchall MA. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 5.Cortiella J. Niles J. Cantu A. Brettler A. Pham A. Vargas G. Winston S. Wang J. Walls S. Nichols JE. Influence of a cellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–2580. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 6.Karring T. Nyman S. Lindhe J. Healing following implantation of periodontitis affected roots into bone tissue. J Clin Periodontol. 1980;7:96–105. doi: 10.1111/j.1600-051x.1980.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 7.Nyman S. Karring T. Lindhe J. Planten S. Healing following implantation of periodontitis-affected roots into gingival connective tissue. J Clin Periodontol. 1980;7:394–401. doi: 10.1111/j.1600-051x.1980.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 8.Ivanovski S. Gronthos S. Shi S. Bartold PM. Stem cells in the periodontal ligament. Oral Dis. 2006;12:358–363. doi: 10.1111/j.1601-0825.2006.01253.x. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb B. Biology of the cementum. J Periodontol. 1942;13:13–19. [Google Scholar]
- 10.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–260. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 11.Nyman S. Gottlow J. Karring T. Lindhe J. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J Clin Periodontol. 1982;9:257–265. doi: 10.1111/j.1600-051x.1982.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 12.Gronthos S. Brahim J. Li W. Fisher LW. Cherman N. Boyde A. DenBesten P. Robey PG. Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 13.Friedenstein AJ. Chailakhjan RK. Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 14.Alhadlaq A. Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 15.Alhadlaq A. Elisseeff JH. Hong L. Williams CG. Caplan AI. Sharma B. Kopher RA. Tomkoria S. Lennon DP. Lopez A. Mao JJ. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911–923. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- 16.Luan X. Ito Y. Dangaria S. Diekwisch TG. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15:595–608. doi: 10.1089/scd.2006.15.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gronthos S. Mankani M. Brahim J. Robey PG. Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura M. Gronthos S. Zhao M. Lu B. Fisher LW. Robey PG. Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo BM. Miura M. Gronthos S. Bartold PM. Batouli S. Brahim J. Young M. Robey PG. Wang CY. Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 20.Wikesjo UM. Claffey N. Christersson LA. Franzetti LC. Genco RJ. Terranova VP. Egelberg J. Repair of periodontal furcation defects in beagle dogs following reconstructive surgery including root surface demineralization with tetracycline hydrochloride and topical fibronectin application. J Clin Periodontol. 1988;15:73–80. doi: 10.1111/j.1600-051x.1988.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 21.Dangaria SJ. Ito Y. Luan X. Diekwisch TG. Differentiation of neural-crest-derived intermediate pluripotent progenitors into committed periodontal populations involves unique molecular signature changes, cohort shifts, and epigenetic modifications. Stem Cells Dev. 2011;20:39–52. doi: 10.1089/scd.2010.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luan X. Dangaria S. Ito Y. Walker CG. Jin T. Schmidt MK. Galang MT. Druzinsky R. Neural crest lineage segregation: a blueprint for periodontal regeneration. J Dent Res. 2009;88:781–791. doi: 10.1177/0022034509340641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dangaria SJ. Ito Y. Walker C. Druzinsky R. Luan X. Diekwisch TG. Extracellular matrix-mediated differentiation of periodontal progenitor cells. Differentiation. 2009;78:79–90. doi: 10.1016/j.diff.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitton JH. Dalton BA. Beumer G. Johnson G. Griesser HJ. Steele JG. Surface topography can interfere with epithelial tissue migration. J Biomed Mater Res. 1998;42:245–257. doi: 10.1002/(sici)1097-4636(199811)42:2<245::aid-jbm9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Glass-Brudzinski J. Perizzolo D. Brunette DM. Effects of substratum surface topography on the organization of cells and collagen fibers in collagen gel cultures. J Biomed Mater Res. 2002;61:608–618. doi: 10.1002/jbm.10243. [DOI] [PubMed] [Google Scholar]
- 26.Linder S. Pinkowski W. Aepfelbacher M. Adhesion, cytoskeletal architecture and activation status of primary human macrophages on a diamond-like carbon coated surface. Biomaterials. 2002;23:767–773. doi: 10.1016/s0142-9612(01)00182-x. [DOI] [PubMed] [Google Scholar]
- 27.Yun JK. DeFife K. Colton E. Stack S. Azeez A. Cahalan L. Verhoeven M. Cahalan P. Anderson JM. Human monocyte/macrophage adhesion and cytokine production on surface-modified poly(tetrafluoroethylene/hexafluoropropylene) polymers with and without protein preadsorption. J Biomed Mater Res. 1995;29:257–268. doi: 10.1002/jbm.820290217. [DOI] [PubMed] [Google Scholar]
- 28.Webster TJ. Ergun C. Doremus RH. Siegel RW. Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J Biomed Mater Res. 2000;51:475–483. doi: 10.1002/1097-4636(20000905)51:3<475::aid-jbm23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Verrier S. Pallu S. Bareille R. Jonczyk A. Meyer J. Dard M. Amedee J. Function of linear and cyclic RGD-containing peptides in osteoprogenitor cells adhesion process. Biomaterials. 2002;23:585–596. doi: 10.1016/s0142-9612(01)00145-4. [DOI] [PubMed] [Google Scholar]
- 30.Rios H. Koushik SV. Wang H. Wang J. Zhou HM. Lindsley A. Rogers R. Chen Z. Maeda M. Kruzynska-Frejtag A. Feng JQ. Conway SJ. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;24:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilde J. Yokozeki M. Terai K. Kudo A. Moriyama K. The divergent expression of periostin mRNA in the periodontal ligament during experimental tooth movement. Cell Tissue Res. 2003;3:345–351. doi: 10.1007/s00441-002-0664-2. [DOI] [PubMed] [Google Scholar]
- 32.Brent AE. Schweitzer R. Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 33.Atlas B. Scleraxis expression in the murine tooth germ and periodontal ligament. British Society for Dental Research. J Dent Res; Annual Scientific Meeting; Dundee, England. 2005. B. [Google Scholar]
- 34.Dangaria SJ. Ito Y. Yin L. Valdre G. Luan X. Diekwisch TG. Apatite microtopographies instruct signaling tapestries for progenitor-driven new attachment of teeth. Tissue Eng Part A. 2011;17:279–290. doi: 10.1089/ten.tea.2010.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonoyama W. Liu Y. Fang D. Yamaza T. Seo BM. Zhang C. Liu H. Gronthos S. Wang CY. Wang S. Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim K. Lee CH. Kim BK. Mao JJ. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010;89:842–847. doi: 10.1177/0022034510370803. [DOI] [PMC free article] [PubMed] [Google Scholar]