Abstract
The relationship between brain aging and Alzheimer’s disease (AD) is contentious. One view holds AD results when brain aging surpasses a threshold. The other view postulates AD is not a consequence of brain aging. This review discusses this conundrum from the perspective of different investigative lines that have tried to address it, as well as from the perspective of the mitochondrion, an organelle that appears to play a role in both AD and brain aging. Specific issues addressed include the question of whether AD and brain aging should be conceptually lumped or split, the extent to which AD and brain aging potentially share common molecular mechanisms, whether beta amyloid should be primarily considered a marker of AD or simply brain aging, and the definition of AD itself.
Keywords: aging, amyloid, brain, dementia, Alzheimer’s disease, mitochondria
1. Introduction
Alzheimer’s disease (AD), the most common neurodegenerative disease, affects 5.4 million Americans and is believed to cost the economy $385 billion per year [1, 2]. Currently available treatments minimally impact the disease and more effective interventions are needed. Accomplishing this will require a sophisticated understanding of its cause or causes.
The definition of AD has varied over the years [3]. Initially, and a number of decades thereafter, AD was considered a form of presenile dementia [4, 5]. Elderly demented individuals were not generally diagnosed with AD, even though their brains frequently contained neuritic plaques and neurofibrillary tangles. Later, the definition was changed to include all patients, regardless of age, who were felt to have a plaque-and-tangle related dementia.
Large numbers of elderly individuals develop a dementia syndrome and have enough plaques and tangles to qualify for an AD diagnosis [3]. One in every eight persons over 65 is estimated to have AD, 40–50% past the age of 85 may have it, and in some very elderly subgroups the figure appears to surpass 50% [2, 6–8]. If AD diagnostic criteria are relaxed so that individuals with lesser degrees of cognitive decline and lower brain plaque and tangle burdens are included, the number of those affected becomes even larger [7, 9–11].
For epidemiologic reasons alone AD is therefore a somewhat unique disease. According to some studies its incidence and prevalence continuously increases with advancing age, and appears to reach a point at which a majority of those beyond a certain age likely have it [3, 8, 12]. Because of the profound relationship between advancing age and AD, it is worth considering the extent to which AD and brain aging constitute discrete phenomena, and to also consider the extent to which they overlap. This review will consider this topic from a clinical perspective, a brain pathology perspective, and ultimately from the perspective of the mitochondrion, an organelle implicated in both aging and AD paradigms. In doing so, it will attempt to address the following questions: what is brain aging, and what is AD?
2. Alzheimer’s Disease Versus Brain Aging: Clinical Perspective
Dementia is AD’s salient clinical feature. While cognitive decline represents the dementia syndrome’s central characteristic, additional criteria are specified and must be satisfied [13, 14]. Most importantly, a patient’s cognitive decline should be so extensive it degrades independence, social abilities, or job performance. This part of the definition helps distinguish benign, minor degrees of cognitive change from more serious, major degrees of cognitive change.
Applying a syndromic diagnosis of dementia, though, is often difficult. For the clinician, the decision over whether a patient has experienced functional degradation is subjective and not always clear-cut. Also, some individuals may decline substantially yet still be able to compensate, while small declines may significantly impair other individuals. To help address this latter limitation, in addition to determining how a patient performs relative to age-matched “normals” on cognitive tests, whether performance occurred at or below the patient’s own “expected” level is also assessed. This approach can identify cognitive deficits in persons whose exam performance surpasses that of their peer group.
Another problem arises from the fact that when it comes to cognitive decline, “benign” is an arbitrary term. Cognitive decline only remains benign for as long as it remains compensated. For much of the latter part of the 20th century it was assumed that although cognitive changes occur during aging, “age-related” decline rarely affects functional abilities and, from an etiology perspective, is unrelated to cognitive changes that occur in AD [3].
This view was supported by studies of “healthy” brain aging, which concluded advancing age adversely affects some cognitive skills but typically spares others [15–18]. Memory encoding, working memory (a component of executive function), and processing speed all decline with age. Although this decline probably proceeds throughout adulthood it truly becomes evident after the age of 60. Semantic knowledge and autobiographical memory, on the other hand, tend to remain intact.
Clinical studies of age-related cognitive change have been further interpreted within the context of neuroimaging studies of non-demented adults [15]. These neuroimaging studies show advancing age and prefrontal cortical atrophy positively correlate, while hippocampal volumes change to a lesser extent. Therefore, cognitive skills that decline with age localize to the prefrontal lobes, a part of the brain that steadily atrophies with age in non-demented individuals. Cognitive skills that are relatively stable in non-demented, aged individuals localize to the hippocampi, a part of the brain that shows some but relatively limited age-related atrophy in non-demented individuals. To many, this suggests an age-related decline in prefrontal lobe-associated cognitive performance is normal and expected, while an age-related decline in medial temporal lobe-associated cognitive performance is abnormal and not expected in healthy individuals.
In the 1980s and 1990s operational criteria designed to define this benign, age-related cognitive change phenomenon were developed. “Age-associated memory impairment” (AAMI) applied to elderly individuals with cognitive complaints, no dementia, and cognitive test scores below those of young adults [19]. “Age-associated cognitive decline” (AACD) could be diagnosed in elderly individuals with cognitive complaints, no dementia, and cognitive test scores more than one standard deviation below the mean of age-matched, complaint-free cohorts [20, 21].
In practice, this approach to age-related cognitive change now appears excessively optimistic. Over time, cognitive decline in those with AAMI and AACD tends to progress, the line from no dementia to dementia is frequently crossed, and AD is often diagnosed [3, 22–31]. Among Memory Disorders specialists there is an increasing realization that objective and possibly even subjective cognitive decline in elderly individuals could represent incipient AD. To acknowledge this concern, a “mild cognitive impairment” (MCI) syndrome was defined and formal criteria developed [32]. These criteria have undergone several minor modifications over the past decade, but in general MCI is diagnosed in persons with changing cognitive abilities, objective evidence of cognitive decline, and functional independence (i.e. no dementia) [32–35].
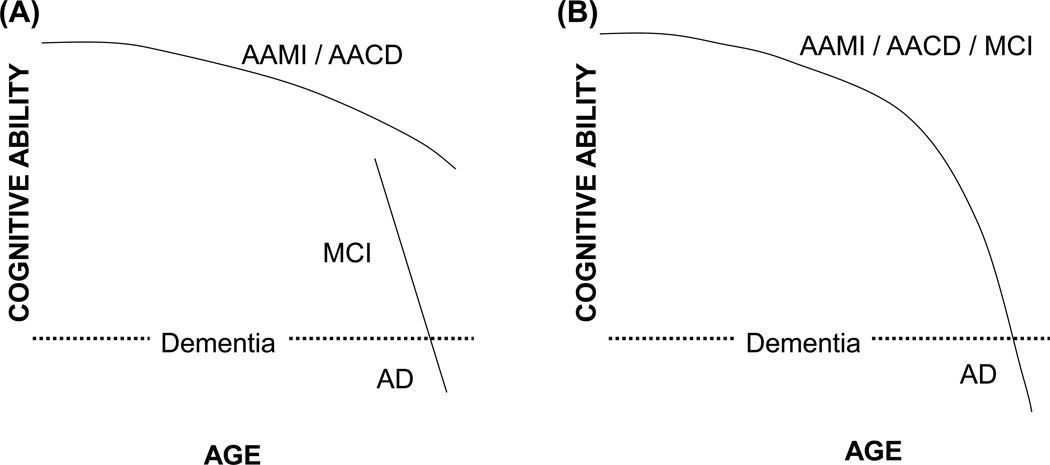
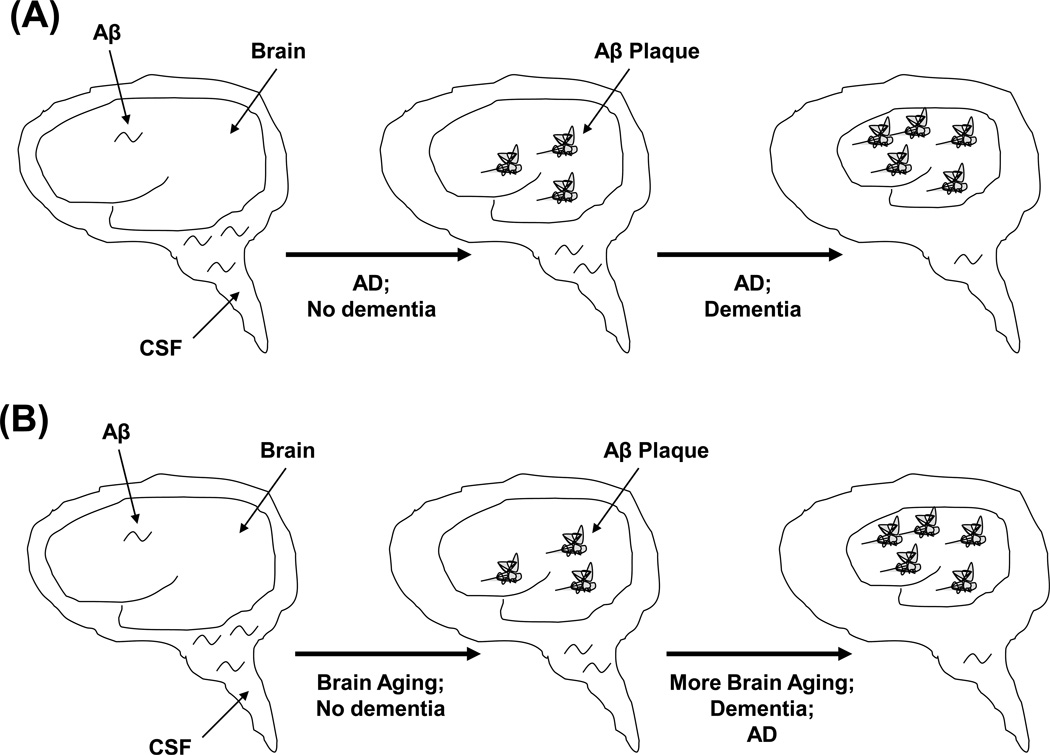
MCI and AACD criteria overlap. The biggest difference between these two syndromes is that AACD implies changes are due to brain aging and of minimal concern, while MCI implies changes are not due to brain aging and are of substantial concern (Figure 1). This point brings us back to the questions posed in the Introduction section: what is brain aging, and what is AD?
Figure 1. Two different cognitive decline schemes.
(A) Cognitive decline that accompanies brain aging does not cause dementia and is mechanistically distinct from the cognitive decline that occurs during MCI and AD. This model is inferred from neuropsychology studies. (B) Cognitive decline is part of a single continuum that, given enough time, has the ability to progress to dementia in most people. This model suggests cognitive decline in aging individuals does not necessarily represent a mechanistically or clinically benign process and implies the presence of a common underlying mechanism. This view is consistent with the experience of Memory Disorders Clinics that longitudinally evaluate patients with memory complaints.
3. Alzheimer’s Disease Versus Brain Aging: Pathology Perspective
Several phenomena commonly observed in aging brains are considered consequences of aging. Atrophy and small degrees of white matter change, especially in the vicinity of the lateral ventricles, are not felt to necessarily represent harbingers of disease [36–40]. This does not infer changes seen in the “healthy” aging brain do not also occur in AD, although patterns may differ. Hippocampal atrophy is more noticeable in demented subjects with AD than it is in non-demented subjects [41]. AD subjects show more pervasive white matter change [42–46].
Differences between hippocampi from AD and age-matched, cognitively intact individuals are reported. In brains from cognitively intact subjects, hippocampal CA1 neuron counts do not correlate with age [47–49]. Brains from AD subjects have fewer CA1 neurons than control subjects [47]. The CA1 neuron count data have been used to argue brain aging and AD are discrete events. Deducing mechanistic inferences from descriptive, observational studies, though, is often dangerous. A more conservative conclusion is that reduced CA1 neuron counts associate with dementia, and as long as an aging individual is not demented CA1 neuron counts should remain relatively constant.
From a pathology perspective, neuritic plaques and neurofibrillary tangles serve as a dividing line between AD and other dementias. Neuritic plaques are extracellular cortical structures that contain amyloid protein. The main plaque amyloid protein constituent is beta amyloid (Aβ), which itself derives from processing of a larger protein called the amyloid precursor protein (APP). Neurofibrillary tangles are intraneuronal cortical structures that consist of tau protein aggregates. Demented individuals who do not have plaques and tangles do not qualify for a diagnosis of AD.
In demented patients without plaques and tangles, alternative pathology that suggests a non-AD diagnosis is often detected. Frequently, however, alternative pathologies are also seen in demented patients with plaques and tangles [50–52]. In these cases, a judgment must be made as to which pathology is most likely responsible for the clinical dementia syndrome. The histological dividing line between AD and other dementias, therefore, is not always clear.
In addition, the simple presence of plaques and tangles do not distinguish demented from non-demented individuals because brains of non-demented individuals frequently contain plaques and tangles. Tangles are commonly observed in aged individuals irrespective of cognitive status [53–56]. Tau phosphorylation, an event believed to precede tangle formation, is actually found in the temporal lobe transentorhinal region of young adults and with advancing age tau phosphorylation anatomically spreads to include additional regions [57]. Decades of clinical-pathological correlative studies, now corroborated by a decade of neuroimaging studies, also demonstrate a sizable percentage of the non-demented elderly have plaques [40, 51, 54, 55, 58–68]. To date, Pittsburgh Compound B positron emission tomography (PIB PET) constitutes the most extensively used plaque imaging approach. PIB binds fibrillar Aβ. Brain retention may reflect plaque or non-plaque fibrillar Aβ binding, and possibly also Aβ-independent phenomena, but PIB retention within a brain generally indicates the presence of Aβ plaques [69, 70]. Studies of non-demented elderly cohorts overall report approximately 20–40% are PIB-positive [67, 68, 71–78]. When analyzed according to age, though, the percentage of non-demented subjects who are PIB-positive increases with advancing age and the majority of those past 80 years of age (65%) show PIB retention [67]. Brain histology surveys suggest that brains from individuals surviving to age 85 and beyond have at least some degree of plaque and tangle pathology [9, 54, 79]. Plaque burdens in brains of very elderly, non-demented APOE2 carriers are reported to reach very high levels [80].
For those who have plaques but not dementia, what does the presence of brain Aβ pathology imply? One possibility is that relationships between Aβ and brain aging exist independent of AD. If so, then Aβ could constitute either a marker of brain aging or else an actual cause of brain aging. The other possibility is that relationships between Aβ and brain aging do not exist, and that changes in Aβ always indicate the presence of AD. Much of the AD research field now seems headed in the latter direction. This is reflected by the recent adoption of AD diagnostic criteria that classify cognitively normal individuals with brain plaques or low cerebrospinal fluid Aβ levels as having “preclinical AD” [77].
4) Brain Aging and the New Alzheimer’s Disease Diagnostic Criteria
According to the new AD diagnostic criteria cognitive decline and an absence of alternative dementia-associated pathology are no longer required features. At least for research purposes, to qualify for a diagnosis of AD altered brain or CSF Aβ is now the sole requirement [77]. Because most very elderly individuals have Aβ plaques, by extension most very elderly people have AD. Also, since most young individuals will develop Aβ deposits if they live long enough [9, 54, 79], almost the entire population that doesn’t already have AD is on their way to developing AD [3]. According to the preclinical AD criteria, therefore, it is difficult to see how brain amyloidois and brain aging could be considered separate entities arising through independent mechanisms.
It is important to note some persons may not develop brain amyloid pathology no matter how long they live [81]. Exemplifying this is a report of a 115 year-old supercentenarian (a term applied to individuals beyond 110 years of age) who did not clinically dement during life and whose brain, on autopsy, showed very limited tau pathology and almost no fibrillar Aβ deposition [82]. It is difficult, though, to view this as an example of normal brain aging. Supercentenarians are so exceedingly rare this report may more correctly be considered an exception to, as opposed to example of, normal brain aging.
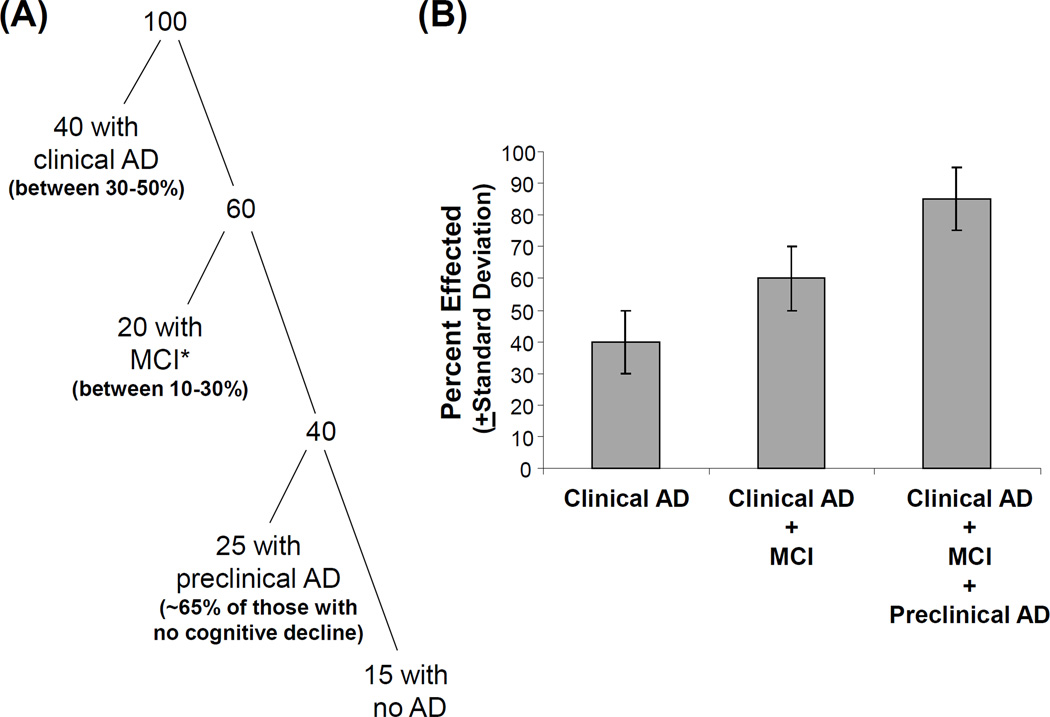
The 2011 AD criteria reflect the impression, now held by many AD clinicians, that cognitive decline in AD begins long before dementia syndrome criteria are met [3]. Various studies suggest this pre-dementia period of cognitive decline lasts at least a decade and perhaps even many decades [83–86]. The new AD criteria are therefore designed to include rather than exclude persons who may be in the earliest stages or at a high risk of developing AD. This is accomplished by stipulating everyone with either fibrillar Aβ deposits or low CSF Aβ has AD, or at least a modified form of AD [77]. Those who meet MCI clinical criteria and have Aβ changes qualify for a diagnosis of “MCI due to AD” [35] or “prodromal AD” [87]. As was discussed earlier, cognitively intact individuals with Aβ changes qualify for a diagnosis of preclinical AD [77]. Based on studies that show clinical AD [6, 8], MCI [88], and preclinical AD [67] are common in the elderly, by this scheme that vast majority of those past the age of 85 have AD (Figure 2).
Figure 2. New AD criteria imply most of the elderly population has some form of AD.
An attempt was made to estimate the percentage of the population over the age of 85 that has at least some form of AD. The studies of Evans et al. and Corrada et al. suggest probably between 30–50% of this group has AD; the study of Lopez et al. suggests up to 30% of this group has MCI, although that study did not incorporate biomarker endpoints; and the PIB-PET study of Rowe et al. suggests approximately 65% of cognitively intact individuals in this age group are PIB positive. This breakdown is shown in (A) as a classification of 100 individuals, while (B) shows the estimated data in bar graph form with estimated error bars. *The figure assumes not all of those with clinical MCI would qualify for a diagnosis of MCI due to AD, and therefore estimates that with biomarker criteria applied a more conservative 20% would qualify for MCI due to AD.
According to the new criteria, the primary dividing line between AD and brain aging is the presence or absence of brain or CSF Aβ changes. Those with Aβ changes have AD, regardless of their clinical status. Those with cognitive decline but no Aβ changes may be manifesting signs of brain aging, AD that for unclear reasons does not yet show but will eventually show Aβ changes, or another cause of cognitive decline.
The new AD diagnostic criteria will no doubt affect clinical study design. In the future, what will constitute a “control” subject? This question is important to address, as study design influences results and by extension interpretation. After all, age-dependent increases in AD incidence and prevalence suggest many of today’s control subjects are, in fact, tomorrow’s AD subjects. Looking back, the new criteria further imply that over the past several decades a substantial percentage of subjects included in control groups were not pure controls. This has implications for a variety of investigations, such as association studies. In the future, instead of assuming cohort purity, we will increasingly need to distinguish cohorts by age of symptom, sign, or biomarker onset.
In addition to using Aβ phenomena to distinguish AD from “normal” brain aging, the new criteria make one critical mechanistic assumption. Aβ is identified as an “upstream” AD biomarker [77]. Other biomarkers, including increased CSF tau, CSF phospho-tau, and decreased glucose utilization (as demonstrated by fluorodeoxyglucose PET; FDG PET), are identified as “downstream” biomarkers. This is based on studies that suggest fibrillar Aβ deposition or low CSF Aβ either can or does precede the other changes [76, 89]. Chronological precedence for Aβ over tau is mostly deduced from studies that find CSF Aβ levels are already low during the earliest stages of cognitive change and do not drop more over time; CSF tau concentrations are more dynamic over the course of MCI and AD and changes correlate to some extent with cognitive decline; and tau changes correlate better with acknowledged late-appearing biomarkers such as hippocampal atrophy [90–94]. Whether Aβ changes precede either age-related or AD-related brain bioenergetic changes is less clear-cut, especially since abundant data from human and cell-based studies either suggest or demonstrate bioenergetic factors regulate both APP processing and Aβ production [95–100].
In any event, as far as “upstream-downstream” relationships are concerned biomarker correlation or chronology does not address whether one biomarker change drives another. Basing mechanistic inferences on descriptive observation is notoriously unreliable; such inferences are frequently inaccurate and occasionally misleading. Finally, using Aβ phenomena to distinguish between AD and brain-aging does not address what causes these phenomena in the first place. To address the question of whether brain aging and AD constitute discrete or continuous entities will probably require a critical understanding of underlying mechanisms.
5) Mitochondria and the Aging Brain
Brain aging associates with or results from particular molecular phenomena. As aging is itself AD’s strongest risk factor, it is worth considering whether such phenomena also play a role in AD. Aging is an incompletely understood process that different hypotheses attempt to explain. Most predict time-dependent, cumulative change plays a role, specify a potentially responsible change, and postulate possible underlying mechanisms. The proposed change variably implicates a toxic accumulation, functional decline, or DNA alteration. Such changes, however, may not occur in isolation and increasingly appear to be inter-related [101].
Some aging paradigms feature mitochondria [102–105]. Numerous studies certainly find mitochondrial changes occur with advancing age [106, 107]. These changes involve both gain and loss of function. Electron transport chain function declines, while free radical production increases. These functional alterations may be initiated by events outside the mitochondria, or primarily by internal phenomena. Several studies report mitochondrial DNA (mtDNA) mutation can initiate aging, or at least accelerate the progression of age-associated phenotypes [108, 109].
Descriptive studies of mitochondria and brain aging indicate relationships do exist, although these studies cannot assign causality [107, 110, 111]. Such investigations are complicated by the fact that from a bioenergetics perspective the brain is not a homogeneous organ. Neurons, which have highly aerobic terminals, and astrocytes, which depend more on glycolysis to generate energy, increasingly appear to constitute a reciprocal bioenergetic network. Neuron neurotransmitter release seems to activate astrocyte glycolysis and lactate production; astrocyte-generated lactate in turn fuels neuron oxidative phosphorylation [112–114]. In some respects this bioenergetic network is reminiscent of the one formed between type I and type II muscle fibers.
Within the brain, age-related mitochondrial changes include a decline in complex I (NADH:ubiquinone oxidoreductase) and complex IV (cytochrome oxidase; COX) activity, with preserved complex II (succinate dehydrogenase) activity [107]. Complex I and IV are partly mtDNA-encoded, while complex II is entirely encoded by nuclear genes. This pattern of electron transport chain (ETC) decline suggests mtDNA changes may mediate the observed complex I and IV functional changes.
Evidence indicates age-related mtDNA changes do in fact occur within the human brain. Levels of a 5-kilobase deletion that particularly affects mtDNA COX genes increase with age [115]. Another study catalogued low abundance heteroplasmic mutations in COX1, a COX subunit gene located on the mtDNA [110]. Multiple mutations were detected and the mutation frequency increased with age. For the most part each individual mutation was present in very low amounts, but the number of mutations seemed to have a cumulative impact as the quantity of COX1 mutations within a given brain correlated inversely with its COX activity.
Mitochondrial mass, or at least parameters related to it, also appear to change during brain aging. Studies are limited, but overall suggest that with advancing age mitochondrial mass increases or attempts to increase. One polymerase chain reaction (PCR)-based study reported brain mtDNA content increases with age [116]. An immunochemistry-based study that compared hippocampi from old and young human subjects also found that compared to young subjects, elderly subjects had higher neuron mtDNA levels [111].
Oxidative stress markers also increase in the aging brain. This manifests in the form of protein carbonylation, lipid oxidation, and mtDNA oxidation [117]. MtDNA oxidation, in turn, renders mtDNA more susceptible to mutation because oxidized bases are misread during replication and this leads to nucleotide substitutions [118–120]. Oxidative stress may partly account for mtDNA’s relatively high mutation rate; mtDNA acquires mutations at approximately 10 times the rate of nuclear DNA [121].
The overall context and meaning of these age-related mitochondrial changes is unclear but speculation is possible. According to some versions of mitochondrial aging theory, for various reasons mtDNA mutation accumulates over time. These mutations eventually induce ETC dysfunction, most likely in the form of perturbed complex I and IV activity. This functional decline may activate a compensatory upregulation of mitochondrial mass.
6) Mitochondria and Alzheimer’s Disease
Most investigators agree mitochondria from AD subjects differ from those of age-matched, non-demented subjects. These differences are reviewed in detail elsewhere, and only those changes that are most relevant to the AD versus brain-aging debate are now considered [122, 123].
Relative to control brains, in AD brains several mitochondrial enzymes have reduced Vmax activities. Examples include two dehydrogenase enzymes, pyruvate dehydrogenase and ketoglutarate dehydrogenase [124]. These are nuclear-encoded enzymes that are translated in the cytosol and imported into mitochondria. The basis of these changes is unknown, although oxidative stress has been postulated to play a role [125].
COX activity is also reduced in AD brain mitochondria [122, 126]. The anatomic distribution of the AD COX defect was initially a subject of debate. Semi-quantitative histochemical techniques used in some early studies reported it existed only in certain brain regions, and mRNA expression analyses found low mtDNA gene expression in AD brains [122]. Based on these findings some proposed reduced COX activity was a secondary response to neurodegeneration. Toxic inhibition by Aβ, inability to produce adequate levels of the enzyme, and physiologic downregulation were all considered potential causes.
Numerous studies, though, indicate the AD COX defect is not limited to specific brain regions and is in fact anatomically widespread [122, 123]. In addition to the brain, reduced COX activity is also seen in AD subject platelets and fibroblasts [127, 128]. Spectrophotometric analysis of the holoenzyme reveals the absence of its high affinity cytochrome c binding site and an altered Km [129]. These data argue the AD COX defect is not simply a consequence of neurodegeneration or reduced COX protein.
COX activity is lower in MCI subject platelet mitochondria than it is in platelet mtiochondria from control subjects [130]. Cognitively normal, middle-aged individuals with AD mothers, who have a higher risk of developing AD than middle aged individuals with AD fathers or no AD-affected parents [131], have relatively low platelet COX activities [132]. Young adult APOE4 carriers, who also have an increased AD risk, have lower posterior cingulate COX activities than young adult non-APOE4 carriers [133]. In most of the young adult APOE4 carriers evaluated by this study COX activity was low even in the absence of fibrillar Aβ. Findings such as these suggest reduced AD COX activity develops either very early in the course of AD, or that low COX activity represents an inherited AD risk factor.
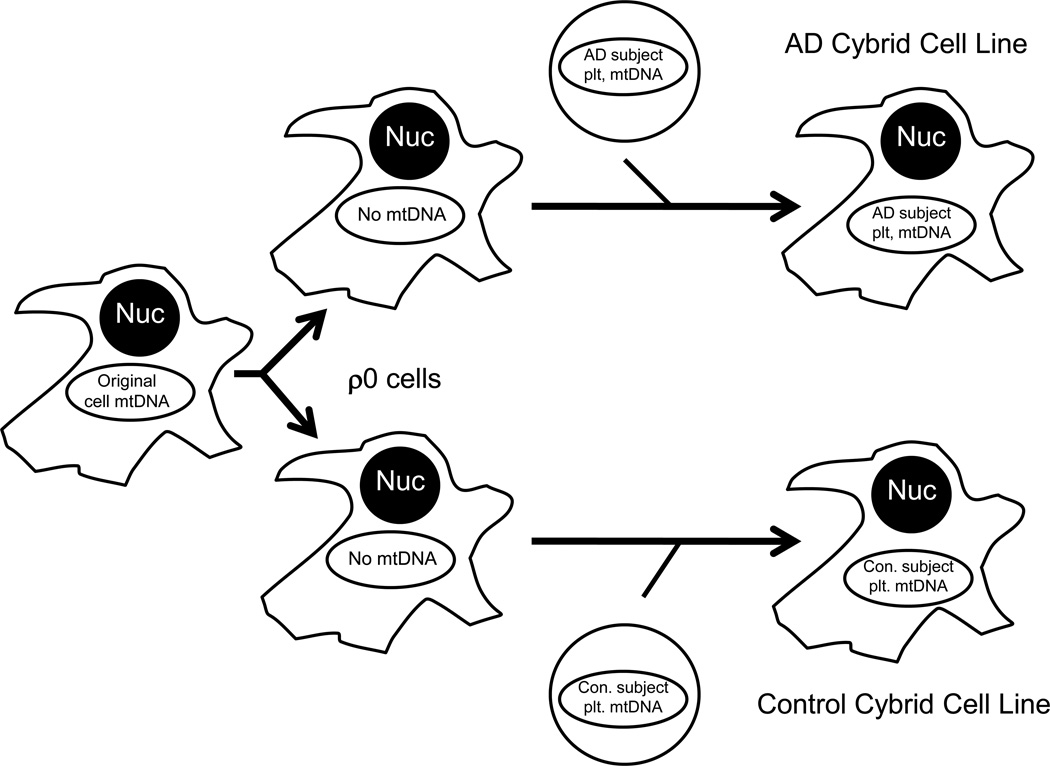
Reduced COX activity in non-degenerating tissues, in conjunction with its apparent maternal transmission, suggests a genetic etiology. COX has 13 protein subunits. Ten of these are encoded on nuclear genes and the other three are mtDNA-encoded. Studies were previously conducted to investigate a possible mtDNA contribution. These studies used a cytoplasmic hybrid (cybrid) approach in which neuronal cell lines were first depleted of endogenous mtDNA (Figure 3) [123, 134]. These mtDNA-depleted cells, called ρ0 cells, were mixed with platelets isolated from different individual AD and control subjects. ρ0 cells that incorporated platelet mitochondria were isolated and expanded to create unique cybrid cell lines. All cybrid cell lines created from a common ρ0 cell strain had the same nuclear background and differed only in their mtDNA. COX activity in cybrid lines containing AD subject mtDNA was lower than it was in cybrid lines containing control subject mtDNA. Other biochemical and molecular parameters were altered in AD cybrids, and most likely represent downstream consequences of their mtDNA-dependent COX defect. Many of these AD cybrid alterations recapitulate phenomena observed in AD brains and include increased oxidative stress, apoptosis markers, and processing of APP to Aβ [123].
Figure 3. The cybrid technique.
The cybrid technique can be used to evaluate the functional consequences of different mtDNA molecules. To perform this type of cybrid study, a cell line is depleted of its endogenous mtDNA; these mtDNA-depleted cells are called ρ0 cells. ρ0 cells are then mixed with non-nucleated cytoplasts or cells (such as platelets) in the presence of a detergent, which disrupts membranes. This facilitates incorporation of non-nucleated cell mitochondria, which contain mtDNA, within the cytosol of the nucleated cells. The transferred mtDNA repopulates the nucleated cell to create a “cytoplasmic hybrid”, or cybrid. Cybrid cell lines containing mtDNA from different sources vary only in the origination of their mtDNA, since the nuclear DNA is equivalent between the cell lines. Each line that is generated can be expanded for use in biochemical or molecular assays. Functional differences between cell lines are believed to reflect and arise through differences in their mtDNA. Nuc=nucleus; Con=control; Plt=platelet; AD=Alzheimer’s disease.
A relationship between APP processing and cell bioenergetics certainly appears to exist [123]. This was directly demonstrated in cell culture experiments in which COX inhibition and ATP depletion both reduced non-amyloidogenic, α secretase-mediated APP processing [95, 96, 98]. Preventing the α-secretase APP cleavage preserves Aβ integrity and makes it possible for the β-secretase (BACE) to produce Aβ derivatives. Free radicals, a byproduct of ETC electron transfer, further appear to activate BACE [135].
Other evidence of an APP-bioenergetics relationship is indirectly inferred from in vivo studies. Brain plaque deposition anatomically corresponds to the functional magnetic resonance imaging (fMRI)-defined default mode network (DMN), a brain region characterized by relatively high glycolysis rates [69, 99, 136]. Soluble Aβ levels also vary throughout the day, rising during wakefulness and falling during sleep [100]. Soluble interstitial Aβ levels fall in the setting of trauma-induced coma, and rising levels signal clinical recovery [137]. These data imply APP processing is a highly regulated process, and suggests cell bioenergetics at least partly regulate APP processing.
Similar to what is observed in brains of non-demented individuals, mitochondrial mass changes occur in the AD brain. Most studies report the number of intact, normal appearing mitochondria declines [111, 138]. AD subject hippocampal neurons, however, contain more degrading or degraded mitochondria than control subject hippocampal neurons [111]. In general, PCR-amplifiable mtDNA declines but when hippocampal neuron mtDNA is quantified by a non-PCR method that can detect mtDNA in degraded mitochondria total hippocampal neuron mtDNA levels may actually increase [111, 139]. In at least some hippocampal neurons COX subunit protein levels also increase [140]. This increase, though, appears to relate to a neuron’s structural integrity. In one study, COX protein was increased in tangle-free neurons, and reduced in tangle-containing neurons [140].
A case can be made that similar to what is observed in non-demented aged subjects, the brains of AD subjects show perturbed mitochondrial function and a subsequent compensatory response. Perhaps the main differences are that in AD brains defects are more profound, compensation is often inadequate, and compensatory responses are more likely to simply break down.
7) Reconciling Brain Aging and Alzheimer’s Disease: The Mitochondrial Cascade Hypothesis
The amyloid cascade hypothesis has enormously impacted the AD field. It was formulated in the early 1990s after an APP mutation was discovered in an autosomal dominant AD kindred [141, 142]. In its current version, the amyloid cascade hypothesis speculates either increased Aβ production or decreased Aβ disposal increases brain Aβ [143]. The extra Aβ eventually generates fibrils and deposits as plaques, but before this can happen toxic Aβ oligomers form and cause neurodysfunction, neurodegeneration, neurofibrillary tangles, and clinical dementia [144].
Mutations that potentially increase Aβ, or at least its 42 amino acid species (Aβ42), are found in very rare autosomal dominant AD kindreds that mostly develop AD before the age of 60 [145]. This form of AD is mutation-dependent, not age-related. Why Aβ42 accumulates in the brains of demented and non-demented elderly individuals is not known. The amyloid cascade hypothesis assumes in the common, late-onset AD variants mutations or polymorphisms in genes that regulate Aβ production or removal directly control Aβ levels, oligomer formation, and AD itself. If as stipulated Aβ levels are the primary cause of late-onset AD then other, more upstream events cannot determine Aβ levels.
APP processing, though, is a highly regulated event. Bioenergetic metabolism certainly affects APP processing and Aβ production. With advancing age, mitochondrial function and dynamics change. These changes alter bioenergetic metabolism and would predictably increase Aβ production.
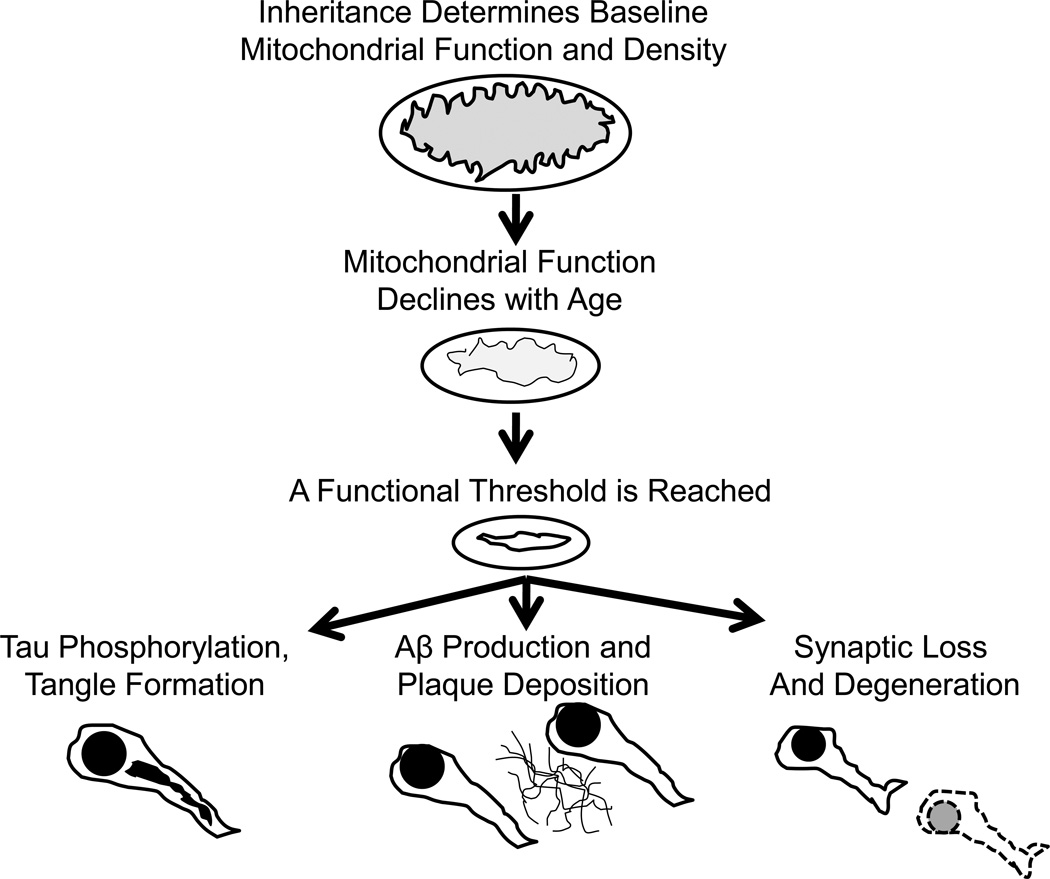
The bioenergetics-centric “sporadic AD mitochondrial cascade hypothesis” reflects this relationship (Figure 4) [123, 146–148]. Rather than assuming brain aging and AD are mechanistically divergent phenomena, the mitochondrial cascade hypothesis assumes brain aging and AD are mechanistically related. According to the mitochondrial cascade hypothesis, mitochondrial function constitutes the crucial link between brain aging and AD. It deviates from the amyloid cascade hypothesis by predicting age-related changes in mitochondrial function, rather than mutations or polymorphisms in APP or Aβ processing genes, primarily account for age-associated brain Aβ changes. In other words, toxic cascades in AD are not initiated by Aβ, but by mitochondria.
Figure 4. The mitochondrial cascade hypothesis.
The mitochondrial cascade hypothesis believes common mechanisms drive brain aging and AD pathology. The hypothesis specifically postulates mitochondrial function and cell bioenergetics constitute the shared upstream mechanism. The hypothesis states an individual’s nuclear and mitochondrial genes determine baseline mitochondrial function and durability. Mitochondrial function declines with advancing age, which influences brain aging and initiates compensatory responses. At some point these compensatory responses are no longer functionally adequate. Aβ production, tau phosphorylation, and synaptic degeneration are all downstream consequences of perturbed mitochondrial function.
The mitochondrial cascade hypothesis does not address whether Aβ functions as a toxic intermediate in late-onset, non-autosomal dominant AD. According to the hypothesis Aβ is not an “upstream” biomarker of AD, but is instead a “downstream” marker of evolving mitochondrial dysfunction. Accordingly, Aβ deposition within an aging brain simply indicates critical age-related changes are occurring. Initially, adaptive changes likely engage to help preserve physiologic and clinical integrity. Eventually, a point is reached where adaptive changes are no longer adequate or attempted. At this point neurodegeneration and clinical dementia result, and brain aging segues into AD.
If correct, the mitochondrial cascade hypothesis has several inconvenient implications. The main implication is that early-onset, autosomal dominant and late-onset, non-autosomal dominant AD cases do not share a common etiology. Models based on early-onset, autosomal dominant AD may therefore provide limited insight into late-onset, non-autosomal dominant AD. Also, even if anti-Aβ treatments are ultimately found to meaningfully benefit early-onset, autosomal dominant AD patients, this approach may turn out not to benefit or only minimally benefit late-onset, non-autosomal dominant AD patients.
8) Conclusions
The AD field sits at a crossroads. According to one paradigm, AD is caused by the accumulation of Aβ, accumulating Aβ necessarily signifies the presence of AD, and reducing Aβ should effectively treat AD. The other paradigm alternatively postulates in late-onset, non-autosomal dominant AD Aβ accumulates because of a more upstream physiologic process, Aβ accumulation marks this upstream process, and reducing Aβ will only benefit patients if Aβ itself also induces neurodysfunction.
A better understanding of the processes that mediate AD and brain aging should inform the brain aging-AD splitting versus lumping debate (Figure 5). One view, which seems particularly pertinent to cases of autosomal dominant AD, holds divergent mechanisms drive brain aging and AD. The other view is that shared mechanisms drive both brain aging and AD. This latter possibility may apply mostly to late-onset AD and could perhaps explain noted associations between advancing age, AD prevalence, and AD incidence [8, 12].
Figure 5. Are AD and brain aging distinct or overlapping entities?
As shown in (A), changes to brain and CSF Aβ are due to and representative of AD. Whether or not an individual with Aβ changes has a dementia syndrome is a function of how long the patient has had AD. This view tends to split brain aging and AD. As shown in (B), Aβ changes constitute a biomarker of brain aging. If the molecular and biochemical pathways that regulate APP and Aβ metabolism surpass a functional threshold beyond which compensation is no longer possible, dementia and neurodegeneration occur. This view tends to lump together brain aging and AD.
An alternative to the amyloid cascade hypothesis [141–143], the mitochondrial cascade hypothesis [123, 146–148], sees brain aging and AD as part of a continuum. This continuum is influenced by common underlying mechanisms. The mitochondrial cascade hypothesis proposes mitochondrial function declines with age, and this decline promotes some features of brain aging including Aβ accumulation. When mitochondrial decline surpasses a threshold compensatory mechanisms fail and late-onset AD ensues. In contrast to the amyloid cascade hypothesis, which views Aβ as the primary upstream AD biomarker, the mitochondrial cascade hypothesis in general sees Aβ more as a downstream biomarker of brain aging. If the mitochondrial cascade hypothesis is correct, treatments designed to improve mitochondrial function should benefit late-onset AD patients more than treatments designed to directly reduce Aβ.
Highlights.
Whether Alzheimer’s disease and brain aging are mechanistically linked is contentious.
New definitions of Alzheimer’s disease infer most very elderly individuals have some form of it.
Beta amyloid homeostasis is regulated by bioenergetic stress.
Mitochondria may constitute a mechanistic link between Alzheimer’s disease and brain aging.
Acknowledgements
The author is supported by P30AG035982 and the Morgan Family Foundation.
Abbreviations
- AACD
age-associated cognitive decline
- AAMI
age-associated memory impairment
- Aβ
beta amyloid
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- BACE
beta secretase
- COX
cytochrome oxidase
- CSF
cerebrospinal fluid
- cybrid
cytoplasmic hybrid
- DMN
default mode network
- ETC
electron transport chain
- FDG
fluorodeoxyglucose
- fMRI
functional magnetic resonance imaging
- PET
positron emission tomography
- PIB
Pittsburgh compound B
- MCI
mild cognitive impairment
- mtDNA
mitochondrial DNA
- PCR
polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer's Association. 2011 Alzheimer’s disease facts and figures. Alzheimer's and Dementia. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow RH. Is aging part of Alzheimer's disease, or is Alzheimer's disease part of aging? Neurobiol Aging. 2007;28:1465–1480. doi: 10.1016/j.neurobiolaging.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Amaducci LA, Rocca WA, Schoenberg BS. Origin of the distinction between Alzheimer's disease and senile dementia: how history can clarify nosology. Neurology. 1986;36:1497–1499. doi: 10.1212/wnl.36.11.1497. [DOI] [PubMed] [Google Scholar]
- 5.Boller F, Forbes MM. History of dementia and dementia in history: an overview. J Neurol Sci. 1998;158:125–133. doi: 10.1016/s0022-510x(98)00128-2. [DOI] [PubMed] [Google Scholar]
- 6.Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported, JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 7.Yaffe K, Middleton LE, Lui LY, Spira AP, Stone K, Racine C, Ensrud KE, Kramer JH. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011;68:631–636. doi: 10.1001/archneurol.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71:337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 9.Polvikoski T, Sulkava R, Rastas S, Sutela A, Niinisto L, Notkola IL, Verkkoniemi A, Viramo P, Juva K, Haltia M. Incidence of dementia in very elderly individuals: a clinical, neuropathological and molecular genetic study. Neuroepidemiology. 2006;26:76–82. doi: 10.1159/000090252. [DOI] [PubMed] [Google Scholar]
- 10.Morishima-Kawashima M, Oshima N, Ogata H, Yamaguchi H, Yoshimura M, Sugihara S, Ihara Y. Effect of apolipoprotein E allele epsilon4 on the initial phase of amyloid beta-protein accumulation in the human brain. Am J Pathol. 2000;157:2093–2099. doi: 10.1016/s0002-9440(10)64847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O'Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 12.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010;67:114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 16.Borson S. Cognition, aging, and disabilities: conceptual issues. Phys Med Rehabil Clin N Am. 2010;21:375–382. doi: 10.1016/j.pmr.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folstein M, Folstein S. Functional expressions of the aging brain. Nutr Rev. 2010;68 Suppl 2:S70–S73. doi: 10.1111/j.1753-4887.2010.00351.x. [DOI] [PubMed] [Google Scholar]
- 18.Salthouse TA. Memory aging from 18 to 80. Alzheimer Dis Assoc Disord. 2003;17:162–167. doi: 10.1097/00002093-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Crook T, Bahar H, Sudilovsky A. Age-associated memory impairment: diagnostic criteria and treatment strategies. Int J Neurol. 1987;21–22:73–82. [PubMed] [Google Scholar]
- 20.Levy R. Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr. 1994;6:63–68. [PubMed] [Google Scholar]
- 21.Richards M, Touchon J, Ledesert B, Richie K. Cognitive decline in ageing: are AAMI and AACD distinct entities? Int J Geriatr Psychiatry. 1999;14:534–540. doi: 10.1002/(sici)1099-1166(199907)14:7<534::aid-gps963>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Goldman WP, Morris JC. Evidence that age-associated memory impairment is not a normal variant of aging. Alzheimer Dis Assoc Disord. 2001;15:72–79. doi: 10.1097/00002093-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer's disease: a meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 24.Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs DM, Sano M, Dooneief G, Marder K, Bell KL, Stern Y. Neuropsychological detection and characterization of preclinical Alzheimer's disease. Neurology. 1995;45:957–962. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- 26.Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44:1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 28.Sacuiu S, Sjogren M, Johansson B, Gustafson D, Skoog I. Prodromal cognitive signs of dementia in 85-year-olds using four sources of information. Neurology. 2005;65:1894–1900. doi: 10.1212/01.wnl.0000188873.13444.85. [DOI] [PubMed] [Google Scholar]
- 29.Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, Newman AB, Kuller L. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- 30.Small BJ, Fratiglioni L, Viitanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population-based sample. Arch Neurol. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 31.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 32.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 33.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 34.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 35.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enzinger C, Fazekas F, Ropele S, Schmidt R. Progression of cerebral white matter lesions -- clinical and radiological considerations. J Neurol Sci. 2007;257:5–10. doi: 10.1016/j.jns.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haug H, Eggers R. Morphometry of the human cortex cerebri and corpus striatum during aging. Neurobiol Aging. 1991;12:336–338. doi: 10.1016/0197-4580(91)90013-a. discussion 352-335. [DOI] [PubMed] [Google Scholar]
- 39.Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O'Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study, Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 40.Anderton BH. Ageing of the brain. Mech Ageing Dev. 2002;123:811–817. doi: 10.1016/s0047-6374(01)00426-2. [DOI] [PubMed] [Google Scholar]
- 41.Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rezek DL, Morris JC, Fulling KH, Gado MH. Periventricular white matter lucencies in senile dementia of the Alzheimer type and in normal aging. Neurology. 1987;37:1365–1368. doi: 10.1212/wnl.37.8.1365. [DOI] [PubMed] [Google Scholar]
- 43.Burns JM, Church JA, Johnson DK, Xiong C, Marcus D, Fotenos AF, Snyder AZ, Morris JC, Buckner RL. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch Neurol. 2005;62:1870–1876. doi: 10.1001/archneur.62.12.1870. [DOI] [PubMed] [Google Scholar]
- 44.Gouw AA, Seewann A, Vrenken H, van der Flier WM, Rozemuller JM, Barkhof F, Scheltens P, Geurts JJ. Heterogeneity of white matter hyperintensities in Alzheimer's disease: post-mortem quantitative MRI and neuropathology. Brain. 2008;131:3286–3298. doi: 10.1093/brain/awn265. [DOI] [PubMed] [Google Scholar]
- 45.Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W. Histopathologic correlates of white matter changes on MRI in Alzheimer's disease and normal aging. Neurology. 1995;45:883–888. doi: 10.1212/wnl.45.5.883. [DOI] [PubMed] [Google Scholar]
- 46.Scheltens P, Barkhof F, Valk J, Algra PR, van der Hoop RG, Nauta J, Wolters EC. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer's disease. Evidence for heterogeneity, Brain. 1992;115(Pt 3):735–748. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- 47.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- 48.West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer's disease. Neurobiol Aging. 2004;25:1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Imhof A, Kovari E, von Gunten A, Gold G, Rivara CB, Herrmann FR, Hof PR, Bouras C, Giannakopoulos P. Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci. 2007;257:72–79. doi: 10.1016/j.jns.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 50.White L, Gelber R, Ross W, Masaki K, Uyehara-Lock JH, Launer L, Petrovitch H. Dissonance in diagnoses of dementia type during life with attribution determined at autopsy in the Honolulu-Asia Aging Study; Impact of high prevalence of mixed pathology and specificity/non-specificity of clinical manifestations in the very old. Neurology. 2011 PL01.002. [Google Scholar]
- 51.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 52.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 53.Hof PR, Glannakopoulos P, Bouras C. The neuropathological changes associated with normal brain aging. Histol Histopathol. 1996;11:1075–1088. [PubMed] [Google Scholar]
- 54.Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- 55.Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 56.Duyckaerts C, Hauw JJ. Prevalence, incidence and duration of Braak's stages in the general population: can we know? Neurobiol Aging. 1997;18:362–369. doi: 10.1016/s0197-4580(97)00047-x. discussion 389-392. [DOI] [PubMed] [Google Scholar]
- 57.Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278-284. [DOI] [PubMed] [Google Scholar]
- 58.Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 59.Head E, Corrada MM, Kahle-Wrobleski K, Kim RC, Sarsoza F, Goodus M, Kawas CH. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol Aging. 2009;30:1125–1134. doi: 10.1016/j.neurobiolaging.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 62.Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 63.Polvikoski T, Sulkava R, Myllykangas L, Notkola IL, Niinisto L, Verkkoniemi A, Kainulainen K, Kontula K, Perez-Tur J, Hardy J, Haltia M. Prevalence of Alzheimer's disease in very elderly people: a prospective neuropathological study. Neurology. 2001;56:1690–1696. doi: 10.1212/wnl.56.12.1690. [DOI] [PubMed] [Google Scholar]
- 64.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. "Preclinical" AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 65.Snowdon DA. Healthy aging and dementia: findings from the Nun Study. Ann Intern Med. 2003;139:450–454. doi: 10.7326/0003-4819-139-5_part_2-200309021-00014. [DOI] [PubMed] [Google Scholar]
- 66.Xuereb JH, Brayne C, Dufouil C, Gertz H, Wischik C, Harrington C, Mukaetova-Ladinska E, McGee MA, O'Sullivan A, O'Connor D, Paykel ES, Huppert FA. Neuropathological findings in the very old. Results from the first 101 brains of a population-based longitudinal study of dementing disorders. Ann N Y Acad Sci. 2000;903:490–496. doi: 10.1111/j.1749-6632.2000.tb06404.x. [DOI] [PubMed] [Google Scholar]
- 67.Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O'Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 68.Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phelps ME, Barrio JR. Correlation of brain amyloid with "aerobic glycolysis": A question of assumptions? Proc Natl Acad Sci U S A. 2010;107:17459–17460. doi: 10.1073/pnas.1012684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nelson PT, Abner EL, Scheff SW, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Patel E, Markesbery WR. Alzheimer's-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment. Neurosci Lett. 2009;450:336–339. doi: 10.1016/j.neulet.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 73.Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O'Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 74.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rentz DM, Becker JA, Moran EK, Sardinha M, Manning LN, T.M. S, Mathis CM, DeKosky ST, Klunk WE, Sperling RA, Fischman AJ, Johnson KA. Amyloid imaging with Pittsburgh Compound-B (PIB) in AD, MCI, and highly intelligent older adults. Neurology. 2006;66:A66. [Google Scholar]
- 76.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 79.Delaere P, He Y, Fayet G, Duyckaerts C, Hauw JJ. Beta A4 deposits are constant in the brain of the oldest old: an immunocytochemical study of 20 French centenarians. Neurobiol Aging. 1993;14:191–194. doi: 10.1016/0197-4580(93)90096-t. [DOI] [PubMed] [Google Scholar]
- 80.Berlau DJ, Corrada MM, Head E, Kawas CH. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72:829–834. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mizutani T, Shimada H. Neuropathological background of twenty-seven centenarian brains. J Neurol Sci. 1992;108:168–177. doi: 10.1016/0022-510x(92)90047-o. [DOI] [PubMed] [Google Scholar]
- 82.den Dunnen WF, Brouwer WH, Bijlard E, Kamphuis J, van Linschoten K, Eggens-Meijer E, Holstege G. No disease in the brain of a 115-year-old woman. Neurobiol Aging. 2008;29:1127–1132. doi: 10.1016/j.neurobiolaging.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 83.Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life. Findings from the Nun Study. JAMA. 1996;275:528–532. [PubMed] [Google Scholar]
- 84.Smyth KA, Fritsch T, Cook TB, McClendon MJ, Santillan CE, Friedland RP. Worker functions and traits associated with occupations and the development of AD. Neurology. 2004;63:498–503. doi: 10.1212/01.wnl.0000133007.87028.09. [DOI] [PubMed] [Google Scholar]
- 85.Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68:351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 87.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O'Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza LC, Stern Y, Visser PJ, Scheltens P. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 88.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 89.Jack CR, Jr, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, Bernstein MA, Gunter JL, Pankratz VS, Aisen PS, Weiner MW, Petersen RC, Shaw LM, Trojanowski JQ, Knopman DS. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, Glodzik-Sobanska L, De Santi S, Zinkowski R, Mehta P, Pratico D, Saint Louis LA, Wallin A, Blennow K, de Leon MJ. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging. 2009;30:682–690. doi: 10.1016/j.neurobiolaging.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buchhave P, Blennow K, Zetterberg H, Stomrud E, Londos E, Andreasen N, Minthon L, Hansson O. Longitudinal study of CSF biomarkers in patients with Alzheimer's disease. PLoS One. 2009;4:e6294. doi: 10.1371/journal.pone.0006294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caroli A, Frisoni GB. The dynamics of Alzheimer's disease biomarkers in the Alzheimer's Disease Neuroimaging Initiative cohort. Neurobiol Aging. 2010;31:1263–1274. doi: 10.1016/j.neurobiolaging.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, Rusinek H, Li J, Tsui W, Saint Louis LA, Clark CM, Tarshish C, Li Y, Lair L, Javier E, Rich K, Lesbre P, Mosconi L, Reisberg B, Sadowski M, DeBernadis JF, Kerkman DJ, Hampel H, Wahlund LO, Davies P. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27:394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 94.Rami L, Fortea J, Bosch B, Sole-Padulles C, Llado A, Iranzo A, Sanchez-Valle R, Molinuevo JL. Cerebrospinal fluid biomarkers and memory present distinct associations along the continuum from healthy subjects to AD patients. J Alzheimers Dis. 2011;23:319–326. doi: 10.3233/JAD-2010-101422. [DOI] [PubMed] [Google Scholar]
- 95.Gabuzda D, Busciglio J, Chen LB, Matsudaira P, Yankner BA. Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J Biol Chem. 1994;269:13623–13628. [PubMed] [Google Scholar]
- 96.Gasparini L, Racchi M, Benussi L, Curti D, Binetti G, Bianchetti A, Trabucchi M, Govoni S. Effect of energy shortage and oxidative stress on amyloid precursor protein metabolism in COS cells. Neurosci Lett. 1997;231:113–117. doi: 10.1016/s0304-3940(97)00536-3. [DOI] [PubMed] [Google Scholar]
- 97.Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, Krebs CT, Bennett JC, Parks JK, Swerdlow RH, Parker WD, Jr, Bennett JP., Jr Alzheimer's disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol. 2000;48:148–155. [PubMed] [Google Scholar]
- 98.Webster MT, Pearce BR, Bowen DM, Francis PT. The effects of perturbed energy metabolism on the processing of amyloid precursor protein in PC12 cells. J Neural Transm. 1998;105:839–853. doi: 10.1007/s007020050098. [DOI] [PubMed] [Google Scholar]
- 99.Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci U S A. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 103.Gadaleta MN, Cormio A, Pesce V, Lezza AM, Cantatore P. Aging and mitochondria. Biochimie. 1998;80:863–870. doi: 10.1016/s0300-9084(00)88881-1. [DOI] [PubMed] [Google Scholar]
- 104.Wallace DC. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992;256:628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- 105.Linnane AW, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989;1:642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- 106.Lenaz G, D'Aurelio M, Merlo Pich M, Genova ML, Ventura B, Bovina C, Formiggini G, Parenti Castelli G. Mitochondrial bioenergetics in aging. Biochim Biophys Acta. 2000;1459:397–404. doi: 10.1016/s0005-2728(00)00177-8. [DOI] [PubMed] [Google Scholar]
- 107.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–C686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- 108.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 109.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 110.Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer's disease brain. Hum Mol Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- 111.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pellerin L, Magistretti PJ. Food for thought: challenging the dogmas. J Cereb Blood Flow Metab. 2003;23:1282–1286. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- 115.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet. 1992;2:324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- 116.Barrientos A, Casademont J, Cardellach F, Estivill X, Urbano-Marquez A, Nunes V. Reduced steady-state levels of mitochondrial RNA and increased mitochondrial DNA amount in human brain with aging. Brain Res Mol Brain Res. 1997;52:284–289. doi: 10.1016/s0169-328x(97)00278-7. [DOI] [PubMed] [Google Scholar]
- 117.Boveris A, Navarro A. Brain mitochondrial dysfunction in aging. IUBMB Life. 2008;60:308–314. doi: 10.1002/iub.46. [DOI] [PubMed] [Google Scholar]
- 118.Minnick DT, Pavlov YI, Kunkel TA. The fidelity of the human leading and lagging strand DNA replication apparatus with 8-oxodeoxyguanosine triphosphate. Nucleic Acids Res. 1994;22:5658–5664. doi: 10.1093/nar/22.25.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ozawa T. Genetic and functional changes in mitochondria associated with aging. Physiol Rev. 1997;77:425–464. doi: 10.1152/physrev.1997.77.2.425. [DOI] [PubMed] [Google Scholar]
- 120.Pavlov YI, Minnick DT, Izuta S, Kunkel TA. DNA replication fidelity with 8-oxodeoxyguanosine triphosphate. Biochemistry. 1994;33:4695–4701. doi: 10.1021/bi00181a029. [DOI] [PubMed] [Google Scholar]
- 121.Mecocci P, MacGarvey U, Kaufman AE, Koontz D, Shoffner JM, Wallace DC, Beal MF. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol. 1993;34:609–616. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- 122.Swerdlow RH, Kish SJ. Mitochondria in Alzheimer's disease. Int Rev Neurobiol. 2002;53:341–385. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- 123.Swerdlow RH. Mitochondria and cell bioenergetics: Increasingly recognized components and a possible etiologic cause of Alzheimer’s disease. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2011.4149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105:855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- 125.Joffe GT, Parks JK, Parker WD., Jr Secondary inhibition of 2-ketoglutarate dehydrogenase complex by MPTP. Neuroreport. 1998;9:2781–2783. doi: 10.1097/00001756-199808240-00018. [DOI] [PubMed] [Google Scholar]
- 126.Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefano LM, Nobrega JN. Brain cytochrome oxidase in Alzheimer's disease. J Neurochem. 1992;59:776–779. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- 127.Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 128.Curti D, Rognoni F, Gasparini L, Cattaneo A, Paolillo M, Racchi M, Zani L, Bianchetti A, Trabucchi M, Bergamaschi S, Govoni S. Oxidative metabolism in cultured fibroblasts derived from sporadic Alzheimer's disease (AD) patients. Neurosci Lett. 1997;236:13–16. doi: 10.1016/s0304-3940(97)00741-6. [DOI] [PubMed] [Google Scholar]
- 129.Parker WD, Jr, Parks JK. Cytochrome c oxidase in Alzheimer’s disease brain: purification and characterization. Neurology. 1995;45:482–486. doi: 10.1212/wnl.45.3.482. [DOI] [PubMed] [Google Scholar]
- 130.Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, Ahern GL, Baxter L, Alexander G, Walker DG, Reiman EM. Impaired platelet mitochondrial activity in Alzheimer's disease and mild cognitive impairment. Mitochondrion. 2006;6:323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Edland SD, Silverman JM, Peskind ER, Tsuang D, Wijsman E, Morris JC. Increased risk of dementia in mothers of Alzheimer's disease cases: evidence for maternal inheritance. Neurology. 1996;47:254–256. doi: 10.1212/wnl.47.1.254. [DOI] [PubMed] [Google Scholar]
- 132.Mosconi L, de Leon M, Murray J, E L, Lu J, Javier E, McHugh P, Swerdlow RH. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer's disease. JAD. 2011 doi: 10.3233/JAD-2011-110866. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valla J, Yaari R, Wolf AB, Kusne Y, Beach TG, Roher AE, Corneveaux JJ, Huentelman MJ, Caselli RJ, Reiman EM. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer's susceptibility gene. J Alzheimers Dis. 2010;22:307–313. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J Neurosci Res. 2007;85:3416–3428. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- 135.Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- 136.Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, Zipfel GJ, Holtzman DM. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baloyannis SJ. Mitochondrial alterations in Alzheimer's disease. J Alzheimers Dis. 2006;9:119–126. doi: 10.3233/jad-2006-9204. [DOI] [PubMed] [Google Scholar]
- 139.de la Monte SM, Luong T, Neely TR, Robinson D, Wands JR. Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer's disease. Lab Invest. 2000;80:1323–1335. doi: 10.1038/labinvest.3780140. [DOI] [PubMed] [Google Scholar]
- 140.Nagy Z, Esiri MM, LeGris M, Matthews PM. Mitochondrial enzyme expression in the hippocampus in relation to Alzheimer-type pathology. Acta Neuropathol. 1999;97:346–354. doi: 10.1007/s004010050997. [DOI] [PubMed] [Google Scholar]
- 141.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 142.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 143.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 144.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 145.Swerdlow RH. Pathogenesis of Alzheimer's disease. Clin Interv Aging. 2007;2:347–359. [PMC free article] [PubMed] [Google Scholar]
- 146.Swerdlow RH, Burns JM, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20 Suppl 2:S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Swerdlow RH, Khan SM. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 148.Swerdlow RH, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218:308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]