Abstract
Background
Depression is prevalent in long-term dialysis patients and is associated with death and hospitalization. Whether depression is present through all chronic kidney disease (CKD) stages or appears after dialysis therapy initiation is not clear. We determined the prevalence of a major depressive episode and other psychiatric illnesses by using a structured gold-standard clinical interview and demographic and clinical variables associated with major depressive episode in patients with CKD.
Study Design
Observational cross-sectional study using a Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)-based structured interview administered by trained persons to 272 consecutive participants. Multivariable logistic regression was used to determine demographic and clinical variables associated with major depressive episode.
Setting & Participants
Patients with stages 2 to 5 CKD not treated by using dialysis were consecutively approached and enrolled from a Veterans Affairs CKD clinic.
Predictors
Demographic and clinical variables.
Outcome
Major depressive episode diagnosed by using a structured Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)-based interview, the Mini International Neuropsychiatric Interview.
Results
The cohort had a mean age of 64.5 ± 12.0 years. Thirty-eight percent were African American, and 55% had diabetes mellitus. Percentages of patients with stages 2, 3, 4, and 5 CKD were 6%, 38%, 41%, and 14%, respectively. Mean hemoglobin level was 12.5 ± 2.0 g/dL. The prevalence of a major depressive episode was 21% and did not vary significantly among different CKD stages. Variables associated with a major depressive episode were diabetes mellitus, comorbid psychiatric illness, and history of drug or alcohol abuse.
Limitations
Single-center study composed of primarily male veterans.
Conclusions
One in 5 patients with CKD had a major depressive episode. Patients with CKD should be screened routinely for depression given this high prevalence and the independent association of depression with poor outcomes in patients with end-stage renal disease receiving maintenance dialysis.
Keywords: Depression, chronic kidney disease, prevalence, risk factors
Depression is a common,1-3 underrecognized, and undertreated1,4-6 problem in patients with end-stage renal disease (ESRD) on long-term dialysis therapy. It is independently associated with markedly increased risk of both morbidity and mortality.7-12 Patients on longterm hemodialysis therapy with a clinical diagnosis of depression are twice as likely to die or require hospitalization within a year compared with those without depression.7 Depression diagnosis in patients with ESRD is independently associated with a 30% increase in both cumulative hospital days and number of hospitalizations, which in turn contribute to excessive Medicare costs.10 In addition, the burden of chronic kidney disease (CKD) has escalated during the past few years to become an urgent matter of public health priority, resulting in a 106% increase in the prevalent ESRD population.13,14 Because processes of care for patients with CKD are predictive of clinical outcomes in patients with ESRD on long-term dialysis therapy, better recognition and characterization of such disease processes as depression that portend morbidity and mortality could improve treatment outcomes for patients with ESRD.15,16
In contrast to long-term dialysis patients, there is a paucity of data about the prevalence of depression in patients with earlier stages of CKD.17-19 Depressive symptoms assessed by means of such selfreport measures as the Beck Depression Inventory are present at a striking rate of 45% at the time of dialysis therapy initiation,4,20 but the prevalence of a major depressive episode based on a Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) diagnosis is not known in patients with earlier stages of CKD before dialysis therapy initiation. In particular, previous estimates of depression prevalence were based on self-report depression scales that may emphasize such somatic symptoms as lack of appetite, weight loss, sleep disturbance, and fatigue, which may coexist with symptoms of uremia and chronic disease and lead to overestimation of a psychiatric diagnosis of depressive disorder in these participants.1,6,21-23
We conducted a cross-sectional observational cohort study of patients with stages 2 to 5 CKD before dialysis therapy initiation to determine the prevalence of a major depressive episode. This diagnosis was established by using a structured interview based on rigorous DSM-IV criteria. In addition, we investigated demographic and clinical factors (including stage of CKD) associated with a major depressive episode in these participants. Estimating the point prevalence of a major depressive episode based on stringent DSM-IV criteria becomes especially important in determining the feasibility of conducting future clinical trials of depression treatment in this population.
METHODS
Study Participants
This is an observational cross-sectional cohort study of outpatients with CKD recruited consecutively from May 2005 to November 2006 from the Dallas Veterans Affairs Medical Center. The CKD clinic roster was reviewed before the visit to screen for eligibility. Eligible patients were approached and invited to participate while they were waiting in the clinic waiting area to be seen for their appointment. Based on local institutional review board regulations, the purpose of the study, which was to determine how common depression is in patients with CKD, was explained in lay language to eligible patients at recruitment. Given the large numbers of patients seen on a given clinic day, not all those who were eligible could be logistically approached and interviewed on the same day. To avoid the introduction of selection bias and keep recruitment consecutive, every sixth eligible patient (starting at a random number between 1 and 6 for each specific clinic day) was approached consecutively to participate. Therefore, each patient had a 1 in 6 chance of being randomly selected. All English-speaking patients who were able to understand and sign informed consent were eligible. The Dallas Veterans Affairs institutional review board approved the study.
Patients with CKD stages 2 to 5, defined as an estimated glomerular filtration rate (eGFR) less than 90 mL/min/1.73 m2, and not on long-term dialysis therapy were included. Participants with stage 2 (eGFR, 60 to 89 mL/min/1.73 m2) had to also have other evidence of kidney damage, defined by the National Kidney Foundation (NKF),24 manifest by either pathological abnormality of kidney on biopsy or markers of kidney damage. eGFR was determined by using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation.25 Estimates of GFR and other evidence of kidney damage had to be present for at least 3 months, according to the NKF definition, for patients to be categorized as having CKD.24 Exclusion criteria were initiation of renal replacement therapy before enrollment, recipient of a kidney transplant, and no health care power of attorney to sign consent.
Assessment of Depression
Participants were categorized as depressed or nondepressed based on the presence or absence of the Mini International Neuropsychiatric Interview (MINI) diagnosis of a current major depressive episode. The MINI was administered to all participants at the time of enrollment by 1 of 2 trained persons blinded to patient medical history. The MINI is a frequently used semistructured clinical interview for psychiatric evaluation based on DSM-IV criteria and has established reliability and validity.26-29 It was used as the gold standard for the diagnosis of a current major depressive disorder, as well as other psychiatric diagnoses. The MINI takes about 30 to 45 minutes to administer.
Data Collection
Demographic and clinical data were collected from central computerized medical records and confirmed with the patient. Laboratory data also were collected at enrollment. Comorbid medical illness was defined as medical conditions other than CKD and included hypertension, diabetes mellitus, congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral vascular disease, lung disease, liver disease, nonskin malignancy, and infection with human immunodeficiency virus (HIV). Comorbid psychiatric illness was any psychiatric diagnosis other than depression or drug or alcohol abuse identified on MINI examination and included dysthymia, current or past manic episode, current or past hypomanic episode, current panic episode, social phobia, obsessive-compulsive disorder, posttraumatic stress disorder, current psychotic disorder, generalized anxiety disorder, and anorexia or bulimia nervosa. Any current or past history of drug or alcohol abuse was coded as drug or alcohol abuse.
Statistical Analysis
Participants were categorized as depressed or nondepressed based on the presence or absence of an MINI diagnosis of a current major depressive episode. Demographic and clinical characteristics were compared between depressed and nondepressed groups by using Student t test or 1-way analysis of variance for continuous and χ2 or Fisher exact test for categorical variables. Multivariable logistic regression models were constructed with a current major depressive episode by means of MINI as the dependent variable to examine the correlates of depression. Candidate independent variables were included in the multivariable model only if clinically relevant and significantly associated with depression in univariate analyses. The number of events (57 depressed participants) allowed for the inclusion of 6 covariates in the multivariable models (~1 covariate/10 events) to not overfit the model. All statistical tests are 2 sided, conducted at the standard significance level of 0.05, and reported using P values and/or 95% confidence intervals (CIs). All analyses were performed using SAS Enterprise Guide, version 3.0, and SAS, version 9.1.3 (SAS Institute, Cary, NC), software.
RESULTS
Characteristics of the Entire Cohort
Of 388 patients who were approached for the study, 274 (71%) agreed to sign consent and 114 (29%) refused to participate. Two patients did not complete the MINI interview and were excluded. Mean age in the 272 participants was 64.5 ± 12.0 years (Table 1). Two participants were women, and more than half were white. About one-half had diabetes mellitus and one fourth had a history of depression recorded in their computerized medical charts. Sixty-three percent had at least 3 current medical comorbidities, and 41% had at least 4. Prevalences of CKD stages 2, 3, 4, and predialysis stage 5 were 6.3%, 38.4%, 41.3%, and 14.0%, respectively. Figure 1 shows participant distribution by deciles of eGFR, and Table 1 lists the cohort’s mean laboratory values.
Table 1. Characteristics of the Entire Cohort.
| Mean age (y) | 64.5 ± 12.0 |
| Sex | |
| Women | 2 (0.7) |
| Men | 270 (99.3) |
| Race | |
| White | 152 (55.9) |
| African American | 103 (37.9) |
| Other | 17 (6.2) |
| Chronic kidney disease stage | |
| 2 | 17 (6.3) |
| 3 | 104 (38.4) |
| 4 | 112 (41.3) |
| 5 | 38 (14.0) |
| Diabetes mellitus | 149 (55.0) |
| History of depression* | 66 (24.7) |
| Mean hemoglobin (g/dL) | 12.5 ± 2.0 |
| Mean serum potassium (mEq/L) | 4.6 ± 0.7 |
| Mean serum phosphorus (mg/dL) | 3.9 ± 1.0 |
| Mean serum calcium (mg/dL) | 9.3 ± 0.7 |
| Mean serum albumin (g/dL) | 4.0 ± 0.5 |
| Mean serum creatinine (mg/dL) | 3.2 ± 2.4 |
| Mean blood urea nitrogen (mg/dL) | 42.0 ± 20.4 |
| Mean estimated glomerular filtration rate (mL/min)† |
31.4 ± 16.6 |
Note: N = 272. Values expressed as mean ± SD or number (percent). Conversion factors for units: hemoglobin in g/dL to g/L, ×10; serum phosphorus in mg/dL to mmol/L, ×0.3229; serum calcium in mg/dL to mmol/L, ×0.2495; serum albumin in g/dL to g/L, ×10; serum creatinine in mg/dL to /nmol/L, ×88.4; urea nitrogen in mg/dL to mmol/L, ×0.357; estimated glomerular filtration rate in mL/min tomL/s, ×0.01667. No conversion is necessary for serum potassium levels in mEq/L and mmol/L.
As documented in the medical records.
Glomerular filtration rate was estimated by using the 4-variable Modification of Diet in Renal Disease Study equation.
Figure 1.
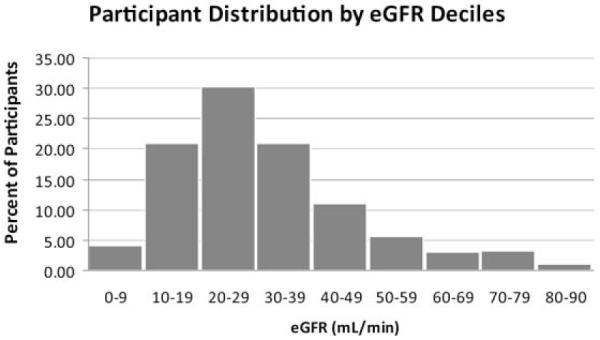
Participant distribution by deciles of estimated glomerular filtration rate (eGFR). GFR was estimated by using the 4-variable Modification of Diet in Renal Disease Study equation.
Prevalence of Major Depressive Episode and Other Psychiatric Disorders
Of 272 participants, 57 (21%) met DSM-IV criteria for a current major depressive episode. Diagnostic frequencies of other psychiatric illnesses assessed by using the DSM-IV-based goldstandard MINI are listed in Table 2. Of the participants, 19.5% also had experienced a recurrent major depressive episode. Three participants (1.1% of the entire cohort) with a current major depressive episode also had a history of a manic episode and thus met criteria for bipolar I disorder. No participant with a current major depressive episode had a history of hypomanic episodes or bipolar II disorder. Fifty participants (18.4%) had an anxiety disorder, including panic disorder, social anxiety disorder, obsessive compulsive disorder, posttraumatic stress disorder, or generalized anxiety disorder. The individual frequency of each anxiety disorder is listed in Table 2.
Table 2. Diagnostic Frequency of Major Depressive Episode and Other Psychiatric Disorders.
| Major depressive episode, current | 57 (21.0) |
| Major depressive episode, recurrent | 53 (19.5) |
| Dysthymia | 5 (1.8) |
| Bipolar I | 3 (1.1) |
| Bipolar II | 0 (0) |
| Depression with psychotic features | 5 (1.8) |
| Psychotic disorder, current | 6 (2.2) |
| Panic disorder, current | 14 (5.1) |
| Social anxiety disorder | 11 (4.0) |
| Obsessive compulsive disorder | 5 (1.8) |
| Posttraumatic stress disorder | 9 (3.3) |
| Generalized anxiety disorder | 11 (4.0) |
| Anorexia nervosa | 0 (0) |
| Bulimia nervosa | 0 (0) |
Note: Values expressed as number (percent).
Comparison of the Cohort Based on a Major Depressive Episode
Participants with a current major depressive episode were significantly younger and twothirds less likely to be employed as those without a current major depressive episode (Table 3). There was a nonsignificant trend toward a greater proportion of participants with a major depressive episode (versus those without) having at least 4 comorbid medical illnesses (51% versus 38%, respectively; P = 0.08). Although diabetes mellitus was significantly more prevalent in depressed than nondepressed participants (68% versus 51% in nondepressed; P = 0.03), the prevalence of other medical comorbidities, including hypertension, coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, and nonskin cancer, was not significantly different (Table 4).
Table 3. Demographics by the Presence or Absence of Major Depressive Episode.
| Demographic | Not Depressed (n = 215) |
Depressed* (n = 57) |
Odds Ratio (95% confidence interval) |
P |
|---|---|---|---|---|
| Mean age (y) | 65.4 ± 11.8 | 61.0 ± 12.2 | 0.97 (0.95-0.99) | 0.01 |
| Women (v men) (%) | 0.5 | 1.8 | 3.82 (0.24-62.05) | 0.3 |
| Race | ||||
| White, referent (%) | 57.2 | 52.6 | 1.00 | 0.6 |
| African American (%) | 36.7 | 38.6 | 1.58 (0.52-4.77) | |
| Other (%) | 6.1 | 8.8 | 1.14 (0.47-1.59) | |
| Education > high school (%) | 60.8 | 57.4 | 0.87 (0.47-1.58) | 0.6 |
| Married (%) | 67.3 | 68.4 | 1.05 (0.56-1.97) | 0.9 |
| Employed (%) | 22.1 | 7.0 | 0.27 (0.09-0.78) | 0.01 |
Note: Values expressed as mean ± SD or percent unless noted otherwise.
Refers to a current major depressive episode diagnosed by using the Mini International Neuropsychiatric Interview.
Table 4. Clinical Characteristics by the Presence or Absence of Major Depressive Episode.
| Clinical Characteristic | Not Depressed (%) (n = 215) |
Depressed (%) (n = 57) |
Odds Ratio (95% confidence interval) |
P |
|---|---|---|---|---|
| Chronic kidney disease stage | 0.9 | |||
| 2 (referent) | 6.1 | 7.0 | 1.00 | |
| 3 | 39.3 | 35.1 | 0.8 (0.2-2.6) | |
| 4 | 41.1 | 42.1 | 0.9 (0.2-3.0) | |
| 5 | 13.6 | 15.8 | 1.01 (0.3-3.9) | |
| Diabetes mellitus | 51.4 | 68.4 | 2.05 (1.1-3.8) | 0.03 |
| Hypertension | 96.7 | 98.3 | 1.9 (0.2-15.6) | 0.6 |
| Coronary artery disease | 37.1 | 45.6 | 1.4 (0.8-2.6) | 0.2 |
| Congestive heart failure | 29.3 | 28.6 | 1.0 (0.5-1.9) | 0.9 |
| Cerebrovascular disease | 23.2 | 28.6 | 1.3 (0.7-2.6) | 0.4 |
| Peripheral vascular disease | 22.3 | 12.3 | 0.5 (0.2-1.2) | 0.1 |
| History of nonskin cancer | 16.1 | 12.3 | 0.7 (0.3-1.7) | 0.5 |
| Comorbid medical illness* | 85.6 | 94.7 | 3.03 (0.9-10.3) | 0.08 |
| History of drug or alcohol abuse | 26.1 | 42.1 | 2.1 (1.1-3.8) | 0.02 |
| Comorbid psychiatric illness† | 4.7 | 42.1 | 14.9 (6.5-33.9) | <0.001 |
| History of depression‡ | 13.3 | 66.7 | 13.0 (6.6-25.7) | <0.001 |
| Antidepressant medication use | 11.6 | 50.9 | 7.9 (4.04-15.3) | <0.001 |
Refers to the presence of 2 or more medical comorbidities in addition to chronic kidney disease.
Refers to the presence of at least 1 psychiatric illness other than depression or drug or alcohol abuse.
As documented in the medical records.
A similar proportion of participants with a major depressive episode (versus without) was in each CKD stage (Table 4), and the mean eGFR was not different based on the presence or absence of a major depressive episode. Laboratory values, including hemoglobin, creatinine, blood urea nitrogen, albumin, and calcium, also did not significantly differ based on the presence or absence of a major depressive episode, except for a greater mean serum phosphorus level in those with compared with those without a major depressive episode (4.2 ± 1.1 and 3.9 ± 1.0 mg/dL, respectively; P = 0.03; Table 5).
Table 5. Laboratory Values by the Presence or Absence of Major Depressive Episode.
| Laboratory Characteristic | Not Depressed (n = 215) |
Depressed (n =57) | Odds Ratio (95% confidence interval) |
P |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 12.5 ± 1.9 | 12.5 ± 2.2 | 1.0 (0.9-1.2) | 0.9 |
| Potassium (mEq/L) | 4.6 ± 0.7 | 4.6 ± 0.7 | 1.02 (0.7-1.6) | 0.9 |
| Phosphorus (mg/dL) | 3.9 ± 1.0 | 4.2 ± 1.1 | 1.3 (1.0-1.7) | 0.03 |
| Calcium (mg/dL) | 9.3 ± 0.6 | 9.3 ± 0.8 | 0.9 (0.6-1.4) | 0.8 |
| Albumin (g/dL) | 4.01 ± 0.4 | 4.0 ± 0.6 | 0.8 (0.4-1.5) | 0.6 |
| Creatinine (mg/dL) | 3.2 ± 2.4 | 3.3 ± 2.4 | 1.02 (0.9-1.1) | 0.8 |
| Blood urea nitrogen (mg/dL) | 42.0 ± 19.8 | 42.1 ± 22.7 | 1.0 (0.99-1.02) | 0.9 |
| Estimated GFR (mL/min)* | 31.5 ± 16.1 | 31.4 ± 18.4 | 1.0 (0.98-1.02) | 0.9 |
Note: Values expressed as mean ± SD unless noted otherwise. Conversion factors for units: hemoglobin in g/dL to g/L, ×10; phosphorus in mg/dL to mmol/L, ×0.3229; calcium in mg/dL to mmol/L, ×0.2495; albumin in g/dL to g/L, ×10; serum creatinine in mg/dL to /nmol/L, ×88.4; urea nitrogen in mg/dL to mmol/L, ×0.357; estimated GFR in mL/min to mL/s, ×0.01667. No conversion is necessary for serum potassium levels in mEq/Land mmol/L.
Abbreviation: GFR, glomerular filtration rate.
GFR estimated by using the 4-variable Modification of Diet in Renal Disease Study equation.
A significantly greater percentage of patients with a current major depressive episode had a history of drug or alcohol abuse, history of lifetime depression, and comorbid psychiatric illness other than depression (Table 4). At enrollment, 50.9% of those with a diagnosis of a major depressive episode and 11.6% of those without were on antidepressant medications.
Variables Associated With a Major Depressive Episode
Table 6 lists the multivariable logistic model to define variables associated with a current major depressive episode. Participants with diabetes were twice as likely to have a major depressive episode compared with those without diabetes (odds ratio, 2.09; 95% CI, 1.01 to 4.33), and employed participants were less likely to have a major depressive episode (odds ratio, 0.20; 95% CI, 0.06 to 0.70). The strongest association with a major depressive episode was observed for the presence of comorbid psychiatric illness. Patients who had a present or past psychiatric illness other than depression were 12 times more likely to have a current major depressive episode as those without such illnesses (odds ratio, 12.03; 95% CI, 4.79 to 30.23). History of drug or alcohol abuse and serum phosphorus level were not significantly associated with a major depressive episode in the full multivariable model (Table 6).
Table 6. Logistic Regression Model for Major Depressive Episode.
| Variable | Odds Ratio (95% confidence interval) |
P |
|---|---|---|
| Age (/1-y increase) | 0.98 (0.94-1.01) | 0.2 |
| Employed | 0.20 (0.06-0.70) | 0.01 |
| Diabetes mellitus | 2.09 (1.01-4.33) | 0.04 |
| Comorbid psychiatric illness* |
12.03 (4.79-30.23) | <0.001 |
| History of drug or alcohol abuse |
1.21 (0.57-2.56) | 0.6 |
| Serum phosphorus (/1-mg/dL increase) |
1.34 (0.98-1.82) | 0.07 |
Note: Refers to a current major depressive episode diagnosed by using the Mini International Neuropsychiatric Interview. The C statistic for the logistic model is 0.797.
Refers to the presence of at least 1 psychiatric illness other than depression or drug or alcohol abuse.
DISCUSSION
The principal new finding in this study is that a major depressive episode is common in patients with earlier stages of CKD, before the onset of ESRD and dialysis therapy. We also found that the prevalence of a major depressive episode does not vary with stage of predialysis CKD. To our knowledge, this is the first study to define the point prevalence of a major depressive episode, as well as other psychiatric illnesses, in a consecutive cohort of participants with CKD by using a DSM-IV-based structured clinical interview before long-term dialysis therapy initiation. Our study contributes to the prior literature in that it included outpatient participants with stages 2 to 5 CKD and assessed blindly and consecutively for a major depressive episode by using a DSM-IV-validated gold standard. The point prevalence by using this method was salient at a rate of 21%.
Overall depression is more common in patients with CKD and ESRD compared with those without CKD. Whereas the point prevalence of depression is 2% to 4% in the general community and 5% to 10% in the primary care setting,30 20% of patients with ESRD experience depression.1,2,31 This prevalence is even greater than those reported for patients with other chronic diseases by using standardized diagnostic interviews, such as 11% for diabetes mellitus,32 14% for congestive heart failure,33 and 16% for coronary artery disease after acute myocardial infarction.34 The prevalence of self-reported depression in deployed US veterans without chronic diseases was 7% to 17%.35,36
We previously used the Structured Clinical Interview for DSM-IV to assess depression in 98 consecutive hemodialysis patients, most of whom were African American, and found a point prevalence of depressive disorder of 26% and of major depressive episode of 17.3%.1 Watnick et al2 found a prevalence of 19% for major depressive disorder by using the same gold-standard interview in a predominantly white sample of hemodialysis patients, and Cukor et al31 reported a prevalence of 19.6% in an urban hemodialysis sample. Reasons that may account for this high prevalence in long-term dialysis patients include loss of bodily function, loss of role at work and in family, loss of sexual function, and burden of comorbid illness.21
However, data regarding the prevalence of depression in patients with earlier stage CKD are scarce and primarily include studies that used the Beck Depression Inventory self-report scale for assessment.4,17,18,20 Two such studies reported that depressive symptoms were present at a striking rate of 45% at dialysis therapy initiation.4,20 These 2 studies provided important information, but lacked data for patients with earlier stages of CKD (stage ≤ 4). Moreover, these studies were subject to confounding by indication in as much as enrolled participants were likely to have uremic symptoms that indicated the need for dialysis therapy initiation. To mitigate this shortcoming, we previously reported a DSM-IV-based interview37 to assess the prevalence of a major depressive disorder in a non-ESRD cohort with creatinine clearances less than 30 mL/min/72 kg.22 The prevalence was 21.6%; however, assessment was performed during hospitalization, which might have led to overestimation of depression.22 In our present study composed of all outpatient participants with CKD, the prevalence of a major depressive episode was still prominent at 21%.
Interestingly, this prevalence was independent of CKD stage, that is, similar proportions of participants from each CKD stage had a diagnosis of a major depressive episode. Similarly, mean eGFR was not different based on the presence or absence of a major depressive episode. This finding is consistent with and confirms the findings of Cohen et al,18 who reported that the mean Beck Depression Inventory score in 92 predialysis participants with stages 1 to 5 CKD did not vary by CKD stage. Similarly, Shidler et al17 did not find a significant correlation between decreasing creatinine clearance and Beck Depression Inventory score, and Perlman et al38 showed that GFR was not statistically associated with the Mental Health Component of the Medical Outcomes Study 36-Item Short-Form Health Survey. Thus, our study provides new and compelling data in a much larger sample indicating that the prevalence of a major depressive episode based on a DSM-IV gold standard is similar in patients with various CKD stages. The reason the prevalence of depression or depressive symptoms may not increase with progressive decrease in renal function in patients with CKD before dialysis therapy initiation may be that there are no clear symptoms of kidney disease for considerable periods during CKD progression, and patients with moderate to advanced CKD often are unaware they have kidney disease.18,38 Only 14% of our cohort had stage 5 CKD, when symptoms of uremia may start to manifest. In addition, the common causes of CKD, such as diabetes, hypertension, and atherosclerotic vascular disease, may impact on depression independently of their negative effect on CKD progression.38
We found that unemployment, comorbid psychiatric illness, and diabetes mellitus were significantly associated with a major depressive episode. Previous studies of non-CKD populations indicate a greater prevalence of depression in patients with diabetes compared with the general population.32,39,40 Golden et al39 recently reported a strong association between baseline depressive symptoms and incident type 2 diabetes and also found that treated patients with diabetes had a greater chance of developing increased depressive symptoms. Explanations for this association could be psychological stress associated with diabetes management or the presence of such diabetic complications as kidney or cardiovascular disease.39,41-44 Whether the prevalence of depression is high in patients with CKD mostly because of the high percentage of patients with CKD with diabetes or whether patients with diabetes who are depressed develop progressive nephropathy because of noncompliance is not a question that can be answered easily in observational studies. However, taken together with previous reports in patients with CKD, we believe patients with CKD should be screened for depression.
Earlier screening of patients with CKD for depression before dialysis therapy initiation becomes particularly important in light of the strong and independent association of depression with poor outcomes reported in multiple studies of patients with ESRD on long-term dialysis therapy.7-12 Long-term hemodialysis patients with a clinical diagnosis of depression are twice as likely to die or require hospitalization compared with their nondepressed counterparts.7 Depression also is associated with peritonitis in long-term peritoneal dialysis patients11 and with increased numbers of hospitalizations and lengths of stay in hemodialysis patients.10 Future controlled clinical trials need to address whether treatment of depression changes outcomes in patients with CKD before and after dialysis therapy initiation.
In contrast to other studies, we did not find a statistically significant association between medical comorbidity and depression in our cohort. Two potential explanations are: (1) the association reported between comorbidity and depression in previous ESRD and non-ESRD cohorts could be caused by the classification of diabetes as one of the medical comorbidities,1,11,41-44 and (2) the large burden of medical comorbidity in our cohort of veterans could have diminished the magnitude of the association based on the presence or absence of a major depressive episode.
Our study has some limitations that are noteworthy. Although this is the first report of the point prevalence of major depressive episodes in patients with CKD before dialysis therapy initiation by using stringent DSM-IV criteria, the majority of participants were men. However, it is encouraging that mean age, eGFR, and serum albumin values were similar to those in a cohort of 92 participants with CKD, 48% of whom were women, studied by the Kimmel group in Washington, DC.18 Because depression is more prevalent in women than in the general population, larger studies that include more women are needed to validate these results. Also, given the high prevalence of such mental illness as posttraumatic stress disorder and depression in veterans, as well as the increased burden of medical comorbidities in this same population,35,36,45,46 it is realized that there may be a limitation in generalizing results to US CKD samples with low burdens of medical and psychiatric comorbidity. In addition, because sample size must be taken into consideration, failure to have found a statistically significant association between depression and certain clinical variables, such as hemoglobin level, eGFR, and medical comorbidity, does not mean that an association would not exist if a larger sample were included. The potential bias introduced by the unavoidable percentage of nonparticipants has been a limitation of several previous studies of depression in patients with ESRD.4,9,47 However, this bias was minimized in our study by the consecutive manner in which patients were approached for participation. Finally, although we report a greater prevalence of major depressive episode in this CKD cohort compared with that previously reported in nonCKD samples, we recognize that a contemporary non-CKD cohort studied simultaneously as a control group would have been ideal.
We showed that a major depressive episode is very common in patients with earlier stages of CKD and as common as previously reported in patients with ESRD on long-term dialysis therapy. Patients with CKD should be screened routinely for depression given the significant and independent association of depression with hospitalization and death reported previously in ESRD samples. Special emphasis should be placed on the depression screening of patients with CKD with diabetes mellitus because diabetic participants with CKD were twice as likely to have a major depressive episode compared with nondiabetic participants with CKD. Future large prospective studies are warranted to investigate whether depression also is related to poor outcomes in patients with CKD as in patients with ESRD on long-term dialysis therapy and whether treatment of depression will make a difference in the quality of life and clinical outcomes of patients with CKD before and after dialysis therapy initiation.
ACKNOWLEDGEMENTS
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Parts of these data were presented in abstract form at the American Society of Nephrology 40th Annual Meeting, San Francisco, CA, November 4, 2007.
Support: Dr Hedayati’s research was supported by grants from the Veterans Integrated Systems Network 17 and the Veterans Affairs North Texas Health Care System Research Corporation. Support was also provided by the University of Texas Southwestern Medical Center O’Brien Kidney Research Core Center (P30DK079328). Support for Dr Rush was provided by the Rosewood Corporation Chair in Biomedical Science. Support for Dr Toto was provided by National Institutes of Health Grant No. 2K24DK002818-0.
Footnotes
Financial Disclosure: None.
REFERENCES
- 1.Hedayati SS, Bosworth HB, Kuchibhatla M, Kimmel PL, Szczech LA. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006;69:1662–1668. doi: 10.1038/sj.ki.5000308. [DOI] [PubMed] [Google Scholar]
- 2.Watnick S, Wang PL, Demadura T, Ganzini L. Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis. 2005;46:919–924. doi: 10.1053/j.ajkd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Craven JL, Rodin GM, Littlefield C. The BDI as a screening device for major depression in renal dialysis patients. Int J Psychiatry Med. 1988;18:365–374. doi: 10.2190/m1tx-v1ej-e43l-rklf. [DOI] [PubMed] [Google Scholar]
- 4.Watnick S, Kirwin P, Mahnensmith R, Concato J. The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis. 2003;41:105–110. doi: 10.1053/ajkd.2003.50029. [DOI] [PubMed] [Google Scholar]
- 5.Lopes AA, Albert JM, Young EW, et al. Screening for depression in hemodialysis patients: Associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int. 2004;66:2047–2053. doi: 10.1111/j.1523-1755.2004.00977.x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SD, Norris L, Acquaviva K, Peterson RA, Kimmel PL. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2007;2:1332–1342. doi: 10.2215/CJN.03951106. [DOI] [PubMed] [Google Scholar]
- 7.Hedayati SS, Bosworth HB, Briley LP, et al. Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int. 2008;74:930–936. doi: 10.1038/ki.2008.311. [DOI] [PubMed] [Google Scholar]
- 8.Kimmel PL, Peterson RA, Weihs KL, et al. Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int. 2000;57:2093–2098. doi: 10.1046/j.1523-1755.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 9.Lopes AA, Bragg J, Young E, et al. Dialysis Outcomes and Practice Patterns Study (DOPPS): Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int. 2002;62:199–207. doi: 10.1046/j.1523-1755.2002.00411.x. [DOI] [PubMed] [Google Scholar]
- 10.Hedayati SS, Grambow SC, Szczech LA, Stechuchak KM, Alan AS, Bosworth HB. Physician-diagnosed depression as a correlate of hospitalizations in patients receiving long-term hemodialysis. Am J Kidney Dis. 2005;46:642–649. doi: 10.1053/j.ajkd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Troidle L, Watnick S, Wuerth DB, Gorban-Brennan N, Kliger AS, Finkelstein FO. Depression and its association with peritonitis in long-term peritoneal dialysis patients. Am J Kidney Dis. 2003;42:350–354. doi: 10.1016/s0272-6386(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 12.Boulware LE, Liu Y, Fink NE, et al. Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease: Contribution of reverse causality. Clin J Am Soc Nephrol. 2006;1:496–504. doi: 10.2215/CJN.00030505. [DOI] [PubMed] [Google Scholar]
- 13.US Renal Data System . USRDS 2006 Annual Report. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2006. [Google Scholar]
- 14.National Institute of Diabetes and Digestive and Kidney Diseases . Healthy People 2010: Chronic Kidney Disease. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2000. [Google Scholar]
- 15.Renal Physicians Association . Appropriate Preparation of the Patient for Renal Replacement Therapy. Renal Physicians Association; Rockville, MD: 2002. Clinical Practice Guideline No 3:4-11. [Google Scholar]
- 16.Owen WF. Patterns of care for patients with chronic kidney disease in the United States: Dying for improvement. J Am Soc Nephrol. 2003;14(suppl 2):S76–S80. doi: 10.1097/01.asn.0000070145.00225.ec. [DOI] [PubMed] [Google Scholar]
- 17.Shidler NR, Peterson RA, Kimmel PL. Quality of life and psychosocial relationships in patients with chronic renal insufficiency. Am J Kidney Dis. 1998;32:557–566. doi: 10.1016/s0272-6386(98)70017-4. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SD, Patel SS, Khetpal P, Peterson RA, Kimmel PL. Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:919–925. doi: 10.2215/CJN.00820207. [DOI] [PubMed] [Google Scholar]
- 19.Kurella M, Luan J, Lash JP, Chertow GM. Self-assessed sleep quality in chronic kidney disease. Int Urol Nephrol. 2005;37:159–165. doi: 10.1007/s11255-004-4654-z. [DOI] [PubMed] [Google Scholar]
- 20.Walters BA, Hays RD, Spritzer KL, Fridman M, Carter WB. Health-related quality of life, depressive symptoms, anemia, and malnutrition at hemodialysis initiation. Am J Kidney Dis. 2002;40:1185–1194. doi: 10.1053/ajkd.2002.36879. [DOI] [PubMed] [Google Scholar]
- 21.Kimmel PL. Depression in patients with chronic renal disease: What we know and what we need to know. J Psychosom Res. 2002;53:951–956. doi: 10.1016/s0022-3999(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 22.Hedayati SS, Jiang W, O’Connor CM, et al. The association between depression and chronic kidney disease and mortality among patients hospitalized with congestive heart failure. Am J Kidney Dis. 2004;44:207–215. doi: 10.1053/j.ajkd.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 23.Cukor D, Cohen SD, Peterson RA, Kimmel PL. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007;18:3042–3055. doi: 10.1681/ASN.2007030345. [DOI] [PubMed] [Google Scholar]
- 24.National Kidney Foundation K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(suppl 1):S17–S242. [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Harnett-Sheehan K, et al. Reliability and validity of the MINI International Neuropsychiatric Interview (M.I.N.I.) according to the SCID-P. Eur Psychiatry. 1997;12:232–241. [Google Scholar]
- 27.Lecrubier Y, Sheehan DV, Weiller E, et al. The MINI International Neuropsychiatric Interview (M.I.N.I.): A short diagnostic structured interview: Reliability and validity according to the CIDI. Eur Psychiatry. 1997;12:224–231. [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):S22–S33. [PubMed] [Google Scholar]
- 29.Amorim P, Lecrubier Y, Weiller E, Hergueta T, Sheehan D. DSM-IH-R Psychotic Disorders: Procedural validity of the Mini International Neuropsychiatric Interview (M.I.N.I.). Concordance and causes for discordance with the CIDI. Eur Psychiatry. 1998;13:26–34. doi: 10.1016/S0924-9338(97)86748-X. [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, Berglund P, Demler O, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 31.Cukor D, Coplan J, Brown C, et al. Anxiety disorders in adults treated by hemodialysis: A single-center study. Am J Kidney Dis. 2008;52:128–136. doi: 10.1053/j.ajkd.2008.02.300. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 33.Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161:1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 34.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- 35.Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 36.The Iowa Persian Gulf Study Group Self-reported illness and health status among Gulf War veterans: A population-based study. JAMA. 1997;277:238–245. [PubMed] [Google Scholar]
- 37.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule, its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 38.Perlman RL, Finkelstein FO, Liu L, et al. Quality of life in chronic kidney disease: A cross-sectional analysis in the Renal Research Institute-CKD Study. Am J Kidney Dis. 2005;45:658–666. doi: 10.1053/j.ajkd.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 39.Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299:2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults: Is there a link? Diabetes Care. 2000;23:1556–1562. doi: 10.2337/diacare.23.10.1556. [DOI] [PubMed] [Google Scholar]
- 41.Watkins LL, Schneiderman N, Blumenthal JA, the ENRICHD Investigators Cognitive and somatic symptoms of depression are associated with medical co-morbidity in patients after acute myocardial infarction. Am Heart J. 2003;146:48–54. doi: 10.1016/S0002-8703(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 42.Robbins J, Hirsch C, Whitmer R, Cauley J, Harris T. The association of bone mineral density and depression in an older population. J Am Geriatr Soc. 2001;49:732–736. doi: 10.1046/j.1532-5415.2001.49149.x. [DOI] [PubMed] [Google Scholar]
- 43.Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. Arch Intern Med. 2000;160:1261–1268. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- 44.Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463–471. doi: 10.1097/00006842-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 45.The Centers for Disease Control Vietnam Experience Group Health status of Vietnam veterans. I. Psychosocial characteristics. JAMA. 1988;259:2701–2707. [PubMed] [Google Scholar]
- 46.Jordan BK, Schlenger WE, Hough R, et al. Lifetime and current prevalence of specific psychiatric disorders among Vietnam veterans and controls. Arch Gen Psychiatry. 1991;48:207–215. doi: 10.1001/archpsyc.1991.01810270019002. [DOI] [PubMed] [Google Scholar]
- 47.Wuerth D, Finkelstein SH, Finkelstein FO. The identification and treatment of depression in patients maintained on dialysis. Semin Dial. 2005;18:142–146. doi: 10.1111/j.1525-139X.2005.18213.x. [DOI] [PubMed] [Google Scholar]


