Abstract
Background
The role of circumcision in male HPV acquisition is not clear.
Methods
Male university students (18–20 years of age) were recruited from 2003–2009 and followed tri-annually. Shaft/scrotum, glans, and urine samples were tested for 37 alpha HPV genotypes. Cox proportional hazards methods were used to evaluate the association between circumcision and HPV acquisition. Logistic regression was used to assess whether number of genital sites infected at incident HPV detection or site of incident detection varied by circumcision status.
Results
In 477 men, rates of acquiring clinically-relevant HPV types (high-risk types plus types 6 and 11) did not differ significantly by circumcision status (hazard ratio [HR] for uncircumcised relative to circumcised subjects: 0.9[95%CI:0.7–1.2]). However, compared to circumcised men, uncircumcised men were 10.1 (95%CI:2.9–35.6) times more likely to have the same HPV type detected in all 3 genital specimens than in a single genital specimen and were 2.7 (95%CI:1.6–4.5) times more likely to have an HPV-positive urine or glans specimen at first detection.
Conclusions
While the likelihood of HPV acquisition did not differ by circumcision status, uncircumcised men were more likely than circumcised men to have infections detected at multiple genital sites, which may have implications for HPV transmission.
Keywords: HPV, human papilloma virus, circumcision, epidemiology, risk factors
Introduction
Human papillomavirus (HPV) is sexually-transmitted and the etiologic agent of genital cancers.1, 2 While HPV infections in women are well-characterized, less is known about HPV infections in men. Identified risk factors for male HPV infection include report of new sex partners3, 4 and smoking.4 Recent work suggests that HPV-infected men may be at increased risk of HIV acquisition.5
The role of male circumcision in HPV acquisition has important implications for understanding the distribution and determinants of HPV-related lesions and cancers. Although some studies have found no association between circumcision and prevalent HPV infection in men6–8, others reported negative associations.9–11 The cross-sectional design used in these studies, however, precluded distinguishing whether differences in HPV prevalence between circumcised and uncircumcised males were due to differential acquisition, persistence, or clearance. Recent randomized controlled trials (RCTs) of circumcision in Ugandan men showed decreased HPV acquisition in circumcised versus uncircumcised men.12–14 In most of these trials interpretation of results was limited because only a single genital site (e.g. glans meatus) was tested for HPV.
Recent observational studies of incident HPV infection and circumcision have found no association.15, 16 Our previous work on incident HPV infection among 240 male university students showed no association between circumcision and infection.4 The present study extends this observation with a larger cohort and more detailed assessment of infection sites.
Studies of site-specific HPV infection in males have found that the association between circumcision and HPV infection may vary by genital site. Recent studies demonstrated a negative association between circumcision and HPV infection of the glans/corona,11, 16, 17 but not between circumcision and infection of the scrotum or urine.11, 16 In uncircumcised men, the prevalence of HPV infection across different genital sites has been shown to vary, with higher prevalence in the glans/corona than in the penile shaft.5, 7, 17
In this paper we analyze data from a cohort of 477 male university students to understand the relationship between circumcision and type-specific acquisition of clinically-relevant HPV types.
Materials and Methods
Study subjects and data collection
Recruited subjects were male University of Washington (UW) students between 18–20 years of age at enrollment. Additional eligibility criteria included reporting sexual activity with women and being healthy, immunocompetent (HIV negative and no history of receiving a transplant or chemotherapy or taking immunosuppressant drugs), and a permanent Washington State resident. Participants were asked to commit to a three-year study, including 10 triannual clinic visits occurring at four-month intervals at the student health clinic. Five hundred individuals were recruited between June 2003 and December 2009, and data were collected through April 2010. All subjects gave informed, written consent for participation. Protocols were approved by the (UW) Institutional Review Board.
A male nurse practitioner conducted medical and sexual history interviews and genital examinations. Exfoliated epithelial cells from genital sites were collected by first using emery paper (3M) to loosen cells and then pre-wetted Dacron swabs (E.I. du Pont de Nemours) to collect them; both the paper and swabs were placed into the collection vial.4 Genital cell samples were taken (in order) from the penile shaft, glans/corona, and scrotum; in uncircumcised subjects, shaft samples included cells from the foreskin exterior and glans samples included cells from the foreskin interior. Originally, shaft and scrotum samples were collected separately, but starting in August 2007 were combined into a single vial for testing due to the high probability of auto-innoculation between these sites and the similarity of their epithelial cell type (keratinized epithelium). Cell samples were stored in specimen transport medium (Qiagen, formerly Digene Diagnostics). Subjects also submitted first-void urine samples. All samples were processed for DNA extraction and tested for HPV DNA. Samples were digested with protease K and 400 µl was used to isolate DNA using QIAamp DNA blood mini columns per manufacturer protocol (Qiagen Cat. No. 51104, Gaithersburg, MD). Following wash steps, DNA was eluted from the columns in 50 µl of Tris-EDTA buffer heated to 70°C. The presence of HPV DNA was detected by dot blot hybridization following PCR amplification of genomic DNA isolated from samples with MY09/MY11 primers. The presence of β-globin DNA in the cell samples was used as DNA input control; cell samples that tested negative for β-globin and HPV DNA were deemed insufficient. Samples positive for HPV DNA were genotyped using a Luminex liquid bead microarray assay (LBMA) based on the MY09–MY11–HMB01 system of primers specific for the HPV L1 fragment, detecting 37 HPV genotypes.18
Biweekly, subjects recorded daily sexual activity into a web-based journal. Data collected included frequency of vaginal intercourse, condom use, and new partner acquisition.4
Data analysis
Data were analyzed for 477 subjects (95.4% of the cohort) completing >1 clinic visit by April 2010. Analyses were restricted to 19 oncogenic HPV types, defined in the cervical cancer literature as HPV16/18/26/31/33/35/39/45/51/52/53/56/58/59/66/68/73/82/IS391, 2 and 2 low-risk HPV types (HPV-6 and -11) associated with genital warts.19, 20 The rationale for this selection was the clinical relevance of these types.
Outcomes included acquisition of any clinically-relevant and type-specific HPV. For analyses of any HPV, the at-risk group included men who were negative for all oncogenic types and HPV-6/-11 at all sites at enrollment. For type-specific analyses, the at-risk group included those who were negative for the specific type at all sites at enrollment.
Cumulative incidence of any clinically-relevant HPV infection was estimated for circumcised and uncircumcised subjects. At-risk time began at enrollment. Subjects' data were censored upon incident infection (defined as the first positive test result following observation of negative test results) or last follow-up visit. Number of clinically-relevant HPV types detected at first incident infection was compared by circumcision status and evaluated by t-test.
The incidence of type-specific HPV acquisition (at any genital site) according to circumcision status was assessed by a generalized estimating equation (GEE) version of a marginal Cox proportional hazards model and Wald tests.21 Time-to-event was measured from enrollment to incident infection with each HPV type or to last clinic visit, with each subject contributing at-risk time for each of 21 clinically-relevant HPV types. Analyses were stratified by HPV type, assuming common relative hazards across HPV types while allowing baseline hazards to vary. Robust variance estimates were used to account for correlation between multiple HPV types within subjects. Similar analyses were performed for HPV-16, the alpha-9 HPV species group (HPV 16/31/33/35/52/58), and the alpha-7 HPV species group (HPV 18/39/45/59/68).
To evaluate whether number of genital sites infected at incident detection varied by circumcision status, all incident type-specific clinically-relevant HPV infections with sufficient testing results at all three genital sites (glans, urine, and shaft/scrotum) were included in a logistic regression model with uncircumcised men as cases and circumcised men as controls. The predictor variable was number of infected sites (defined as a three-level categorical variable: 1 [incident detection in the glans only, urine only, or shaft/scrotum only], 2 [incident detection in two of three sites] or 3 [incident detection in all three sites]). A separate model was constructed to evaluate genital detection site as a predictor (defined as a three-level categorical variable: shaft/scrotum only, glans &/or urine only, and glans &/or urine and shaft-scrotum). Robust variance estimates were used to account for within-subject correlation.
For all risk factor analyses, we evaluated several demographic and behavioral characteristics as potential confounders. Each variable was tested in the model individually to see if it changed the univariate association, and variables that changed the point estimate by >10% were considered confounders and entered into the final, multivariate model. Potential confounders were lifetime number of female sex partners, current smoking (yes/no), condom use (number of times a condom was used divided by the number of sex acts during the previous four months), age at clinic visit (continuous), race (White, Asian, other), Hispanic ethnicity (yes/no) alcohol/drug use associated with sexual (yes/no), age at first intercourse (continuous), anal intercourse with a female partner (ever/never; assessed at enrollment), number of sex acts, number of new sex partners in the previous four months (continuous), and time between bathing and sample collection (≥ or < 24 hours).
Finally, to evaluate whether the proportion of incident infections positive at each site (urine, glans, and shaft/scrotum) varied by circumcision status, Wald tests with robust variance estimates were used.
Results
The 477 men were followed for a mean of 25.3 (standard deviation [SD] 13.0) months. The majority (75%) was circumcised. Circumcised men were more likely than uncircumcised men to be white (82.7% versus 73.7%;p=.01,chi-squared test) and to report a higher lifetime number of sex partners at enrollment (2 versus 1,p=.02, Wilcoxon rank-sum test). (Table 1)
Table 1.
Baseline characteristics of the study cohort.a
| Characteristic | Circumcised Subjects n=359 |
Uncircumcised Subjects n=118 |
P-valueb |
|---|---|---|---|
| Age in Years, Mean (SD) | 18.9 (0.7) | 18.9 (0.6) | 0.77c |
| Ever Smoked Cigarettes, No. (%) | 41 (11.4) | 12 (10.2) | 0.71d |
| Age in Years at First Intercourse with a Female Partner, Median (Range) | 17 (12–20) | 18 (12–20) | 0.09e |
| No Prior Vaginal Intercoursef, No. (%) | 28 (7.8) | 11 (9.3) | 0.31d |
| History of sex with male partners g, No. (%) | 11 (3.1) | 4 (3.4) | 0.87d |
| Lifetime Number of Sex Partners, Median (Range) | 2 (0–30) | 1 (0–16) | 0.02e |
| Race, No. (%) | |||
| White | 297 (82.7) | 87 (73.7) | 0.01h |
| Asian | 19 (5.3) | 17 (14.4) | |
| Otheri | 43 (12.0) | 14 (11.9) | |
| Hispanic ethnicity, No. (%) | 13 (3.6) | 5 (4.2) | 0.76d |
| Condom use in the previous 4 months, No.j (%) | 0.63h | ||
| Always | 87 (32.3) | 34 (38.6) | |
| Most of the time | 69 (25.7) | 19 (21.6) | |
| Sometimes | 59 (21.9) | 16 (18.2) | |
| Never | 54 (20.1) | 19 (21.6) | |
| Number of new sex partners in the previous 4 months, No. (%) | 0.13 h | ||
| 0 | 224 (62.4) | 68 (57.6) | |
| 1 | 80 (22.3) | 38 (32.2) | |
| 2 | 39 (10.9) | 9 (7.6) | |
| 3+ | 16 (4.5) | 3 (2.5) | |
The 477 subjects were University of Washington students, 18–20 years of age, who were recruited between June 2003 and December 2009, and completed at least two study clinic visits by April 2010.
P-value for the statistical comparison between circumcised and uncircumcised subjects.
Compared by two sample t-test with equal variances.
Compared by two sample test of proportions.
Compared by two sample Wilcoxon rank sum test.
Subjects reporting previous sexual activity without vaginal intercourse; includes men reporting sexual activity with men (4 from the circumcised group, 3 from the uncircumcised group).
Includes men reporting prior vaginal intercourse with female partners (7 from the circumcised group, 1 from the uncircumcised group).
Compared by Pearson chi2 test.
Includes men who reported more than one race.
Excludes 104 men (77 circumcised and 27 uncircumcised) who did not report vaginal intercourse in the 4 months prior to enrollment and 15 men (12 circumcised and 3 uncircumcised) with missing data on condom use.
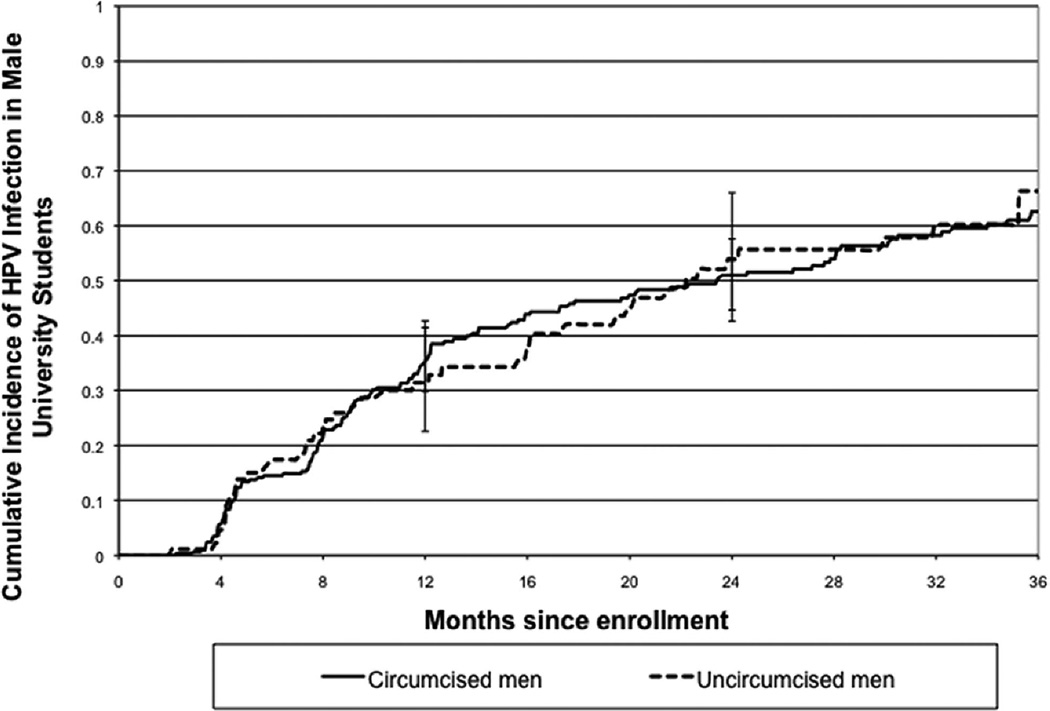
While sample insufficiency was low at all sites, uncircumcised men were more likely than circumcised men to have insufficient glans (1.92% versus 0.74%,p<.01,Z-test) and shaft/scrotum samples (1.28% versus 0.46%,p<.01,Z-test). Urine sample insufficiency was comparable for circumcised and uncircumcised men (0.49% versus 0.37%,p=.13,Z-test). Insufficiency was 0% when results from all three sites were pooled. The baseline prevalence of clinically-relevant HPV was 18.6% among circumcised and 24.4% among uncircumcised men (p=0.17,Z-test). By two years, over half of the 381 men who were negative at enrollment became HPV positive (Figure 1). The 36-month cumulative incidence did not differ between circumcised (62.6%,95%CI:55.8–69.4) and uncircumcised (66.2%,95%CI:53.3–78.7) subjects (Table 2) (p= 0.97,logrank test). The mean number of types detected at first incident detection was 1.9 (SD 0.1) for circumcised and 2.0 (SD 0.2) for uncircumcised subjects (p=0.56,T-test).
Fig. 1.
Cumulative incidence of infection with clinically-relevant (CR) HPV types in circumcised (N= 292) and uncircumcised (N= 89) men. Incident infection was defined as the first positive CR HPV detection in a subject negative for CR HPV at enrollment.
Table 2.
36-month cumulative incidence of HPV among the cohort of 477 male university students, by circumcision status.
| HPV Type | Cumulative Incidence at 36 Months Among Circumcised Subjects (95% CI) |
No. Infections/ No. Person- years at Risk Among Circumcised Subjects |
Cumulative Incidence at 36 Months Among Uncircumcised Subjects (95% CI) |
No. Infections/ No. Person- years at Risk Among Uncircumcised Subjects |
|---|---|---|---|---|
| CRa | 62.6 (56.0, 70.0) | 143/ 403 | 66.2 (53.3, 78.7) | 45/ 124 |
| Any HRb | 61.6 (54.8, 68.4) | 142/ 412 | 66.2 (53.1, 78.8) | 45/ 126 |
| 6 | 14.2 (10.2, 19.6) | 34/ 646 | 13.9 (7.6, 24.7) | 11/ 218 |
| 11 | 2.7 (1.2, 5.7) | 7/ 688 | 0 | 0/ 230 |
| 16 | 26.3 (21.2, 32.2) | 71/ 579 | 35.4 (25.1, 48.3) | 29/ 187 |
| 18 | 19.4 (14.8, 25.4) | 46/ 603 | 14.4 (8.1, 24.9) | 11/ 203 |
| 26 | 0 | 0/ 699 | 1.3 (0.2, 9.1) | 1/ 233 |
| 31 | 8.1 (5.1, 12.6) | 19/ 658 | 10.3 (5.1, 20.3) | 8/ 214 |
| 33 | 7.7 (4.9, 12.2) | 18/ 678 | 7.2 (3.5, 14.7) | 7/ 223 |
| 35 | 1.8 (0.7, 4.8) | 4/ 695 | 5.2 (1.7, 15.4) | 3/ 233 |
| 39 | 9.8 (6.7, 14.4) | 25/ 653 | 10.5 (5.3, 20.5) | 8/ 224 |
| 45 | 7.5 (4.7, 12.0) | 17/680 | 6.0 (2.5, 14.0) | 5/ 226 |
| 51 | 25.5 (20.0, 32.2) | 57/ 620 | 18.6 (11.0, 30.4) | 14/ 204 |
| 52 | 6.1 (3.5, 10.2) | 13/ 677 | 10.3 (5.2, 20.0) | 8/ 223 |
| 53 | 13.3 (9.5, 18.7) | 31/ 656 | 14.3 (7.8, 25.5) | 11/ 221 |
| 56 | 14.3 (10.4, 19.7) | 34/ 655 | 13.9 (7.4, 25.6) | 9/ 223 |
| 58 | 4.1 (2.1, 7.9) | 9/ 682 | 6.5 (2.5, 16.4) | 4/ 232 |
| 59 | 6.7 (4.0, 11.0) | 15/ 669 | 2.7 (0.7, 10.5) | 2/ 232 |
| 66 | 13.1 (9.1, 18.6) | 29/ 658 | 11.2 (5.3, 22.6) | 7/ 224 |
| 68 | 1.7 (0.5, 5.2) | 3/ 697 | 0 | 0/ 235 |
| 73 | 6.2 (3.6, 10.3) | 14/ 686 | 6.7 (2.6, 16.4) | 5/ 222 |
| 82 | 4.4 (2.4, 7.8) | 11/ 685 | 6.1 (2.5, 14.2) | 5/ 229 |
| IS39 | 4.7 (2.5, 8.7) | 10/ 686 | 5.8 (2.2, 14.8) | 4/ 229 |
| Combinationsc | ||||
| 6/11 | 16.1 (11.9, 21.8) | 39/ 635 | 12.7 (6.7, 23.4) | 10/ 214 |
| 16/18 | 37.0 (31.2, 43.6) | 95/ 516 | 42.7 (31.5, 56.0) | 34/ 170 |
| 6/11/16/18 | 43.1 (36.7, 50.0) | 106/ 485 | 47.6 (35.9, 61.0) | 37/ 162 |
| alpha-9d | 38.1 (32.1, 44.9) | 95/ 532 | 44.7 (33.6, 57.7) | 35/ 164 |
| alpha-7e | 34.1 (28.1, 40.9) | 79/ 552 | 31.4 (21.8, 44.0) | 23/ 185 |
Clinically-relevant types were defined as high or probable high-risk types plus HPV6 and HPV11.
High or probable high risk types were defined as HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and IS39, as defined in the cervical cancer literature.
First infection with any type in the combined group.
Alpha 9 group (HPV16, 31, 33, 35, 52, and 58; excluding low risk type HPV70).
Alpha 7 group (HPV18, 39, 45, 59, and 68; excluding low risk type HPV67).
When type-specific acquisition was the outcome of interest, no risk differences were found by circumcision status when considering all clinically-relevant types, high-risk types, high-risk alpha-9 types, or HPV-16 (Table 3). Uncircumcised men were slightly less likely to acquire high-risk alpha-7 types than circumcised men (hazard ratio=0.7;95%CI:0.5–1.04). There was no apparent confounding of the relationship between HPV acquisition and circumcision by any measured demographic or behavioral characteristics.
Table 3.
Hazard ratios for risk of type-specifica HPV acquisition at any genital site by circumcision status.
| Clinically-relevant HPVb |
High-risk HPVc | HPV 16 | Alpha 9d | Alpha 7e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) |
No. Infections/ No. Person- years at Risk |
Hazard Ratio (95% CI) |
No. Infections/ No. Person- years at Risk |
Hazard Ratio (95% CI) |
No. Infections/ No. Person- years at Risk |
Hazard Ratio (95% CI) |
No. Infections/ No. Person- years at Risk |
Hazard Ratio (95% CI) |
No. Infections/ No. Person- years at Risk |
|
| Circumcisedf | 1.0 | 500/14777 | 1.0 | 458/13364 | 1.0 | 75/610 | 1.0 | 143/4203 | 1.0 | 118/3499 |
| Uncircumcised | 0.9 (0.7, 1.2) | 165/5031 | 0.9 (0.7, 1.3) | 153/4547 | 1.2 (0.8, 1.9) | 31/ 200 | 1.3 (0.9, 1.9) | 64/1413 | 0.7 (0.5, 1.04) | 30/1208 |
Type-specific acquisition is defined as the first detection of an HPV type at any genital site.
Clinically-relevant types were defined as high or probable high-risk types plus HPV6 and HPV11.
High or probable high risk types were defined as HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and IS39, as defined in the cervical cancer literature.
Alpha 9 group (HPV16, 31, 33, 35, 52, and 58; excluding low risk type HPV70).
Alpha 7 group (HPV18, 39, 45, 59, and 68; excluding low risk type HPV67).
The circumcised group was the referent group.
A total of 665 incident type-specific infections with clinically-relevant HPV types were detected (Table 4). While the likelihood of first detecting HPV at two sites versus one did not differ significantly by circumcision status (OR=1.3;95%CI:0.8–2.0), infections detected at all three sites versus one were more common in uncircumcised than circumcised men (OR=10.4;95%CI:2.9–36.8) after adjusting for number of new sex partners in the last four months (Table 5). No confounding was evident by other evaluated variables. Similar associations were observed across HPV-type subgroups (Table 5).
Table 4.
Site of incident detection of type-specific infections with clinically-relevant HPV types,a by circumcision status.
| Site of Incident HPV Detection |
No. Infections Among Circumsized Subjects (% of Total Incident Infections Among Circumsized Subjects) |
No. Infections Among Uncircumsized Subjects (% of Total Incident Infections Among Uncircumsized Subjects) |
No. Infections Among All Subjects (% of Total Incident Infections Among All Subjects) |
|---|---|---|---|
| Urine Only | 21 (4.2) | 7 (4.2) | 28 (4.2) |
| Glans Only | 100 (20.0) | 51 (30.9) | 151 (22.7) |
| Shaft/Scrotum Only | 251 (50.2) | 49 (29.7) | 300 (45.1) |
| Urine & Glans | 1 (0.2) | 0 | 1 (0.2) |
| Urine & Shaft/Scrotum | 2 (0.4) | 0 | 2 (0.3) |
| Glans & Shaft/Scrotum | 120 (24.0) | 45 (27.3) | 165 (24.8) |
| All Sites | 5 (1.0) | 13 (7.9) | 18 (2.7) |
| Total Incident Infections | 500 (100) | 165 (100) | 665 (100) |
Clinically-relevant types were defined as high or probable high-risk types (HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and IS39, as defined in the cervical cancer literature) plus HPV6 and HPV11.
Table 5.
Odds ratios (ORs) for the associations between number of sites positive or site of infection at the time of incident type-specific HPV detection and circumcision status (with circumcised men as the control group and uncircumcised men as the case group).
| Variable | Clinically-relevant HPVa | High-risk HPVb | HPV 16 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) |
Adjusted ORc (95% CI) |
No. of Infections in Uncirc./No. of Infections in Circ. |
Unadjusted OR (95% CI) |
Adjusted ORc (95% CI) |
No. of Infections in Uncirc./No. of Infections in Circ. |
Unadjusted OR (95% CI) |
Adjusted ORc (95% CI) |
No. of Infections in Uncirc./ No. of Infections in Circ. |
|
| One Site | 1.0 | 1.0 | 101/358 | 1.0 | 1.0 | 94/330 | 1.0 | 1.0 | 18/57 |
| Two Sites | 1.3 (0.8, 2.0) | 1.3 (0.8, 2.0) | 44/120 | 1.3 (0.8, 2.0) | 1.3 (0.8, 2.0) | 40/106 | 1.3 (0.4, 3.8) | 1.0 (0.4, 3.8) | 6/15 |
| Three Sites | 9.2 (2.7, 31.1) | 10.4 (2.9, 36.8) | 13/5 | 8.9 (2.6, 30.2) | 10.1 (2.9, 36.1) | 13/5 | 12.7 (1.3, 122.1) | 12.8 (1.3, 126.9) | 4/1 |
| Shaft/Scrotum Only | 1.0 | 1.0 | 49/251 | 1.0 | 1.0 | 45/228 | 1.0 | 1.0 | 10/32 |
| Glans &/or Urine Only | 2.4 (1.5, 4.0) | 2.7 (1.6, 4.5) | 58/122 | 2.4 (1.4, 3.9) | 2.6 (1.5, 4.3) | 55/117 | 1.4 (0.5, 3.7) | 1.4 (0.5, 3.8) | 11/26 |
| Glans &/or Urine + Shaft/Scrotum | 2.3 (1.5, 3.8) | 2.4 (1.5, 3.9) | 58/127 | 2.4 (1.5, 3.9) | 2.4 (1.5, 4.0) | 53/113 | 1.9 (0.7, 5.4) | 1.9 (0.6, 5.6) | 10/17 |
| Variable | Alpha 9e | Alpha 7f | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) |
Adjusted ORc (95% CI) |
No. of Infections in Uncirc./No. of Infections in Circ. |
Unadjusted OR (95% CI) |
Adjusted ORc (95% CI) |
No. of Infections in Uncirc./No. of Infections in Circ. |
|
| Number of Sites Infected at First Incident Detectiond | ||||||
| One Site | 1.0 | 1.0 | 42/ 114 | 1.0 | 1.0 | 23/87 |
| Two Sites | 1.1 (0.5, 2.4) | 1.1 (0.5, 2.4) | 9/23 | 0.7 (0.2, 1.9) | 0.7 (0.2, 1.9) | 5/29 |
| Three Sites | 9.5 (1.9, 47.8) | 10.7 (2.0, 57.0) | 7/2 | *** | *** | 2/0 |
| Site of First Detected Infection | ||||||
| Shaft/Scrotum Only | 1.0 | 1.0 | 19/65 | 1.0 | 1.0 | 11/59 |
| Glans &/or Urine Only | 1.9 (0.8, 4.1) | 2.0 (0.9, 4.4) | 29/53 | 2.2 (0.9, 5.6) | 2.2 (0.9, 5.6) | 12/29 |
| Glans &/or Urine + Shaft/Scrotum | 2.2 (0.98, 4.9) | 2.3 (1.01, 5.3) | 16/25 | 1.3 (0.5, 3.4) | 1.3 (0.5, 3.4) | 7/30 |
Clinically-relevant types were defined as high or probable high-risk types plus HPV6 and HPV11.
High or probable high risk types were defined as HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and IS39, as defined in the cervical cancer literature.
Adjusted for number of sex partners in the previous 4 months, as this variable confounded the associations between circumcision and number of sites infected and site of detection.
Sites of detection were urine, glans, and shaft/scrotum.
Alpha 9 group (HPV16, 31, 33, 35, 52, and 58; excluding low risk type HPV70).
Alpha 7 group (HPV18, 39, 45, 59, and 68; excluding low risk type HPV67).
OR not estimable due to zero alpha-7 triple-site infections in the circumcised group.
Site of incident HPV detection varied by circumcision status (Tables 4 and 5). Incident type-specific infections first detected in the glans and/or urine only were 2.7 (95%CI:1.6–4.5) times more likely to occur in uncircumcised men than those detected in the shaft/scrotum only. Incident infections detected in both the shaft-scrotum and the glans and/or urine were 2.4 (95%CI:1.5–3.9) times more likely to occur in uncircumcised men than infections detected in the shaft/scrotum only. These analyses were adjusted for number of new sex partners in the last four months. No confounding was evident by other evaluated variables. Similar associations were observed across different HPV-type subgroups (Table 5).
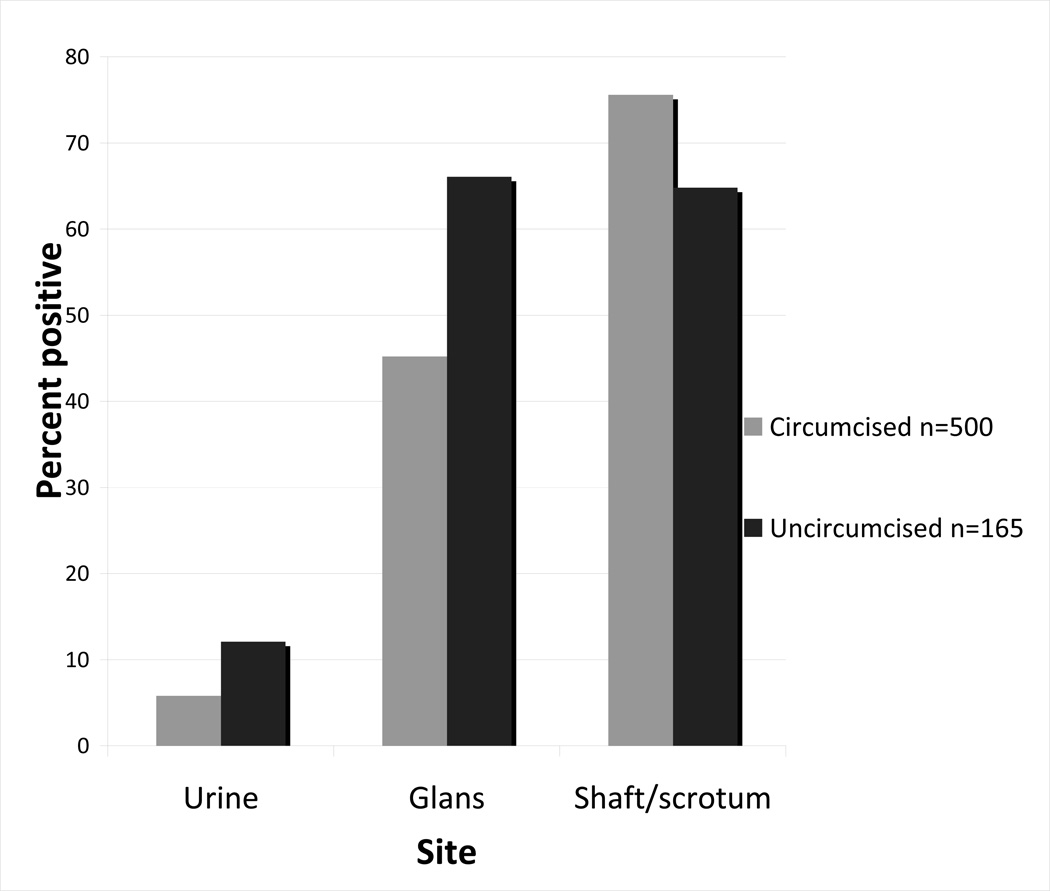
Site-specific differences by circumcision status were also observed in the proportion of incident type-specific HPV infections detected at each genital site (Figure 2). At incident detection, uncircumcised men were more likely than circumcised men to have HPV-positive glans specimens (p=<.001) and more likely to have HPV-positive urine specimens (p=.035), but less likely to have HPV-positive shaft-scrotum specimens (p=.021).
Figure 2.
Proportion of incident type-specific HPV infections detected at each genital site, by circumcision status (n=665 incident type-specific infections).
Discussion
We found no differences by circumcision status in overall HPV acquisition or in number of HPV types acquired. Findings held for all clinically-relevant HPV types, as well as for the subgroups of high-risk types, high-risk alpha-9 types, and HPV-16. This observation is consistent with findings from other longitudinal studies.15, 22 It does, however, differ from recently reported RCTs of circumcision for prevention of HIV infection in Africa where residual specimens collected from the glans were tested for HPV DNA.12–14
In the Ugandan trial, a decrease in oncogenic HPV detection was observed among HIV-positive and HIV-negative men in the circumcision treatment group relative to the control group (adjusted RR=0.67,95%CI:0.51–0.89). When single versus multiple-type oncogenic HPV infections were evaluated as separate outcomes, however, the protective associations were restricted to multiple-type infections.12–14
Several factors may contribute to differences in observed effects between RCTs and longitudinal studies. In our cohort, all but one circumcised man underwent the procedure shortly after birth. The circumcision intervention in the RCTs was performed on adult subjects. This intervention may have altered exposure for a period of time after the procedure was performed. Additionally, the RCTs collected data on HPV infection for only one genital site (glans). Due to higher numbers of shaft infections detected in circumcised men relative to glans infections, basing estimates on glans infections alone would inflate the estimate of risk reduction afforded by circumcision. Work by Weaver et al7 suggests that sampling the glans only (and not the shaft) will underestimate HPV prevalence in circumcised men (whereas the overall HPV prevalence was similar for circumcised and uncircumcised men in that study, sampling the glans only would have underestimated HPV prevalence by 51% in circumcised men, versus only 11% in uncircumcised men). It is likely that differences in site sampling contribute to the different results observed in previous RCTs and cohort studies.
We observed site-specific differences in HPV acquisition by circumcision status. While incident infection at all 3 genital sites was uncommon, simultaneous detection in all 3 genital sites (versus one site only) was 10-fold more common in uncircumcised versus circumcised men. Uncircumcised men were also more likely to have HPV-positive glans and urine specimens than circumcised men while circumcised men were more likely to be positive in the shaft/scrotum. The observed difference in number of sites infected between circumcised and uncircumcised men was driven by these site-specific differences. Other studies have shown a similar difference in infection of the glans/corona based on circumcision status,11, 16, 17 but not in urine specimens.11, 16 Methodological differences in urine collection methods could account for inter-study variations in results. In our study, we collected first-void urine; we did not collect data about retraction of the foreskin prior to urine collection by uncircumcised subjects. If the foreskin was not retracted prior to urine void, it could have led to contamination of the urine with any HPV present in the interior foreskin, which would bias the results toward a higher incidence of HPV detection in the urine of uncircumcised subjects.
It is possible that lack of circumcision could prolong contact between the virus and the epithelial cells of the glans and inner foreskin, increasing the risk of HPV acquisition in the glans/corona in uncircumcised men. Alternative mechanistic hypotheses from the field of HIV research may be relevant for HPV transmission. The keratin layer of the interior foreskin in uncircumcised men was shown by some studies to be thinner than that of the outer foreskin or glans, suggesting an increased susceptibility to viral infection.23, 24 Other studies, however, have found either no difference or a thicker keratin layer in the inner foreskin;25, 26 the issue remains controversial.27
The observed difference in number and anatomic location of infected sites by circumcision status might have potential implications for increased transmissibility to sex partners. This study, however, was not designed to evaluate male to female HPV transmission. The role of male circumcision on female acquisition of HPV has not been consistent in previous studies. Recent work in Uganda showed a reduction of HPV infection in female partners of HIV-negative men receiving the circumcision intervention in a RCT of male circumcision and HPV.28 A longitudinal study of HPV acquisition in female university students found no association between circumcision status of the male partner and risk of HPV acquisition by the female subject.29 Studies of male circumcision’s effect on cervical cancer risk have likewise yielded conflicting results.30 A multi-center case-control study found an association between circumcision and reduced cervical cancer risk, but only among women with male partners who exhibited high-risk sexual behavior (e.g. multiple lifetime partners and/or history of sex with prostitutes).31 The study had a methodological limitation in that it focused on the current male partner of women with cervical cancer, while transmission of the oncogenic HPV may have occurred with an earlier partner.30 Moreover, the glans was the only site tested for HPV in that study.
Differences in viral load at various male genital sites might also affect the relationship between circumcision and HPV transmission. Evidence suggests a higher viral load in the penile shaft than the glans/corona;32 a higher viral load implies a higher risk of transmission to a sex partner. While incident HPV infection was detected more frequently in shaft/scrotum specimens than in glans or urine specimens of both circumcised and uncircumcised men in our study, the incidence of shaft/scrotum infections was higher in circumcised relative to uncircumcised men. Finally, the risk of transmission is also related to infection duration.33 While this manuscript focuses solely on HPV acquisition, in the same cohort, we found that circumcision status had no effect on the likelihood of detecting a persistent versus transient HPV infection over an 8-month time period (Laura Koutsky, personal communication).
Limitations of our study include the potential for misclassification of prevalent infection as incident, which would inflate our estimates of cumulative incidence. Based on findings in a similar cohort study of young female university students (where similar rates of HPV acquisition were observed for those who did and did not report sexual activity prior to enrolment), this potential misclassification is likely to be minimal.29 Also our results may not be generalizable to other populations of men; we studied a cohort of heterosexual university students with a narrow age range, and their HPV histories may not be similar to that of older populations or to men who have sex with men. Finally, there may have been some unmeasured confounding due to religious or cultural practices related to circumcision that we were not able to assess.
Further research is needed to determine how circumcision impacts HPV transmission and whether the number of genital sites infected in the male affects female risk of HPV acquisition. While circumcision does not appear to affect male acquisition of HPV overall, it is important to fully investigate the potential public health implications of the site-specific differences in acquisition attributable to circumcision status, in order to help reduce the burden of HPV-related cancers and genital warts.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01 CA105181 to LAK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflicts of interest.
References
- 1.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24S3:S1–S10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 3.Kjaer SK, Munk C, Winther JF, Jorgensen HO, Meijer CJ, van den Brule AJ. Acquisition and persistence of human papillomavirus infection in younger men: a prospective follow-up study among Danish soldiers. Cancer Epidemiol Biomarkers Prev. 2005;14:1528–1533. doi: 10.1158/1055-9965.EPI-04-0754. [DOI] [PubMed] [Google Scholar]
- 4.Partridge JM, Hughes JP, Feng Q, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007;196:1128–1136. doi: 10.1086/521192. [DOI] [PubMed] [Google Scholar]
- 5.Smith JS, Moses S, Hudgens MG, et al. Increased risk of HIV acquisition among Kenyan men with human papillomavirus infection. J Infect Dis. 2010;201:1677–1685. doi: 10.1086/652408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin HR, Franceschi S, Vaccarella S, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004;190:468–476. doi: 10.1086/421279. [DOI] [PubMed] [Google Scholar]
- 7.Weaver BA, Feng Q, Holmes KK, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis. 2004;189:677–685. doi: 10.1086/381395. [DOI] [PubMed] [Google Scholar]
- 8.Vardas E, Giuliano AR, Goldstone S, et al. External genital human papillomavirus prevalence and associated factors among heterosexual men on 5 continents. J Infect Dis. 2011;203:58–65. doi: 10.1093/infdis/jiq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auvert B, Sobngwi-Tambekou J, Cutler E, et al. Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: results of a randomized controlled trial conducted in Orange Farm, South Africa. J Infect Dis. 2009;199:14–19. doi: 10.1086/595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliano AR, Lazcano E, Villa LL, et al. Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer. 2009;124:1251–1257. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielson CM, Schiaffino MK, Dunne EF, Salemi JL, Giuliano AR. Associations between male anogenital human papillomavirus infection and circumcision by anatomic site sampled and lifetime number of female sex partners. J Infect Dis. 2009;199:7–13. doi: 10.1086/595567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray RH, Serwadda D, Kong X, et al. Male circumcision decreases acquisition and increases clearance of high-risk human papillomavirus in HIV-negative men: a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1455–1462. doi: 10.1086/652184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serwadda D, Wawer MJ, Makumbi F, et al. Circumcision of HIV-infected men: effects on high-risk human papillomavirus infections in a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1463–1469. doi: 10.1086/652185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360:1298–1309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez BY, Shvetsov YB, Goodman MT, et al. Reduced clearance of penile human papillomavirus infection in uncircumcised men. J Infect Dis. 2010;201:1340–1343. doi: 10.1086/651607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez BY, Wilkens LR, Zhu X, et al. Circumcision and human papillomavirus infection in men: a site-specific comparison. J Infect Dis. 2008;197:787–794. doi: 10.1086/528379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobian AA, Kong X, Gravitt PE, et al. Male circumcision and anatomic sites of penile high-risk human papillomavirus in Rakai, Uganda. Int J Cancer. 2011 doi: 10.1002/ijc.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Q, Cherne S, Winer RL, et al. Development and evaluation of a liquid bead microarray assay for genotyping genital human papillomaviruses. J Clin Microbiol. 2009;47:547–553. doi: 10.1128/JCM.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown DR, Schroeder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in Condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37:3316–3322. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greer CE, Wheeler CM, Ladner MB, et al. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol. 1995;33:2058–2063. doi: 10.1128/jcm.33.8.2058-2063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994;13:2233–2247. doi: 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]
- 22.Lu B, Wu Y, Nielson CM, et al. Factors associated with acquisition and clearance of human papillomavirus infection in a cohort of US men: a prospective study. J Infect Dis. 2009;199:362–371. doi: 10.1086/596050. [DOI] [PubMed] [Google Scholar]
- 23.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–1495. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 24.Patterson BK, Landay A, Siegel JN, et al. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinh MH, McRaven MD, Kelley Z, Penugonda S, Hope TJ. Keratinization of the adult male foreskin and implications for male circumcision. AIDS. 2010;24:899–906. doi: 10.1097/QAD.0b013e3283367779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin Q, Zheng XY, Wang YY, Shen HF, Sun F, Ding W. Langerhans' cell density and degree of keratinization in foreskins of Chinese preschool boys and adults. Int Urol Nephrol. 2009;41:747–753. doi: 10.1007/s11255-008-9521-x. [DOI] [PubMed] [Google Scholar]
- 27.Templeton DJ. Male circumcision to reduce sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:344–349. doi: 10.1097/COH.0b013e32833a46d3. [DOI] [PubMed] [Google Scholar]
- 28.Wawer MJ, Tobian AA, Kigozi G, et al. Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet. 2011;377:209–218. doi: 10.1016/S0140-6736(10)61967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Incident infection with genital human papillomavirus: rates and risk factors among a cohort of female university students. Am J Epidemiol. 2003;157:218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 30.Adami HO, Trichopoulos D. Cervical cancer and the elusive male factor. N Engl J Med. 2002;346:1160–1161. doi: 10.1056/NEJM200204113461511. [DOI] [PubMed] [Google Scholar]
- 31.Castellsague X, Bosch FX, Munoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–1112. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 32.Flores R, Lu B, Nielson C, et al. Correlates of human papillomavirus viral load with infection site in asymptomatic men. Cancer Epidemiol Biomarkers Prev. 2008;17:3573–3576. doi: 10.1158/1055-9965.EPI-08-0467. [DOI] [PubMed] [Google Scholar]
- 33.Garnett GP. The geographical and temporal evolution of sexually transmitted disease epidemics. Sex Transm Infect. 2002;78 Suppl 1:i14–i19. doi: 10.1136/sti.78.suppl_1.i14. [DOI] [PMC free article] [PubMed] [Google Scholar]